Abstract
The increasing use of single-cell immune profiling and advanced microscopic imaging technologies has deepened our understanding of the cardiac immune system, confirming that the heart contains a broad repertoire of innate and adaptive immune cells. Leucocytes found in the healthy heart participate in essential functions to preserve cardiac homeostasis, not only by defending against pathogens but also by maintaining normal organ function. In pathophysiological conditions, cardiac inflammation is implicated in healing responses after ischaemic or non-ischaemic cardiac injury. The aim of this review is to provide a concise overview of novel methodological advancements to the non-expert readership and summarize novel findings on immune cell heterogeneity and functions in cardiac disease with a focus on myocardial infarction as a prototypic example. In addition, we will briefly discuss how biological sex modulate the cardiac immune response. Finally, we will highlight emerging concepts for novel therapeutic applications, such as targeting immunometabolism and nanomedicine.
Keywords: Heart failure, Sexual dimorphism, Single-cell RNA sequencing, Bioimaging, Immunometabolism, Nanomedicine
Graphical Abstract
Introduction
The immune system plays an important role in the heart, not only under physiological conditions such as development and homeostatic function, but also in cardiac disease.1 All major types of immune cells can be found in the healthy heart, contributing to immunosurveillance to protect against pathogens, toxic insults, hypoxia, or other injury. Furthermore, resident macrophages play important roles, e.g. in electrical coupling, mechanosensing, and maintenance of cardiomyocyte homeostasis. A major cause of cardiac injury is myocardial infarction (MI), which elicits an acute immune response to repair the ischaemic infarct area. Although immune cells are integral key players of cardiac healing, an unbalanced or unresolved immune reaction after MI aggravates tissue damage that triggers maladaptive remodelling and heart failure (HF). There is emerging evidence for a metabolic crosstalk between immune cells and cardiomyocytes, which crucially regulates immune cell reprogramming and resolution of inflammation.2 Non-ischaemic causes for pathological cardiac remodelling and HF involve, among others, pressure or volume overload, metabolic diseases such as diabetes, and ageing.3 HF refers to the inability of the heart to transport sufficient amounts of blood to meet the high energy demands of our body. Notably, some sex-specific disparity seems to exist in HF, since men are predisposed to HF with reduced ejection fraction, whereas women more frequently develop HF with preserved ejection fraction.3 , 4 Besides differences in cardiovascular structure, risk factors, comorbidities and distinct immunological responses may contribute to the sex-specific HF manifestations. In general, females exhibit stronger innate and adaptive immune responses compared with males,5 suggesting that the immune response in cardiac disease might differ between men and women. Yet, most experimental studies do not consider the biological sex as a variable when studying molecular pathways in animal models of cardiovascular disease (CVD). Likewise, women are under-represented in clinical studies, which is related to the fact that women generally develop CVD at advanced age.
In the following, we will provide an overview on emerging high throughput and imaging technologies for investigating the cardiac immune system and provide an update on novel insights about cardiac immune cell functions in steady state and disease. New concepts to target immunometabolism and nanomedicine will also be covered (Graphical Abstract).
Graphical Abstract.
State of the art technologies to study cardiac immunology include single cell RNA sequencing and advanced microscopic imaging, revealing an unprecedented immune cell heterogeneity of the heart.
New insights into cardiac immune cell heterogeneity at single-cell resolution
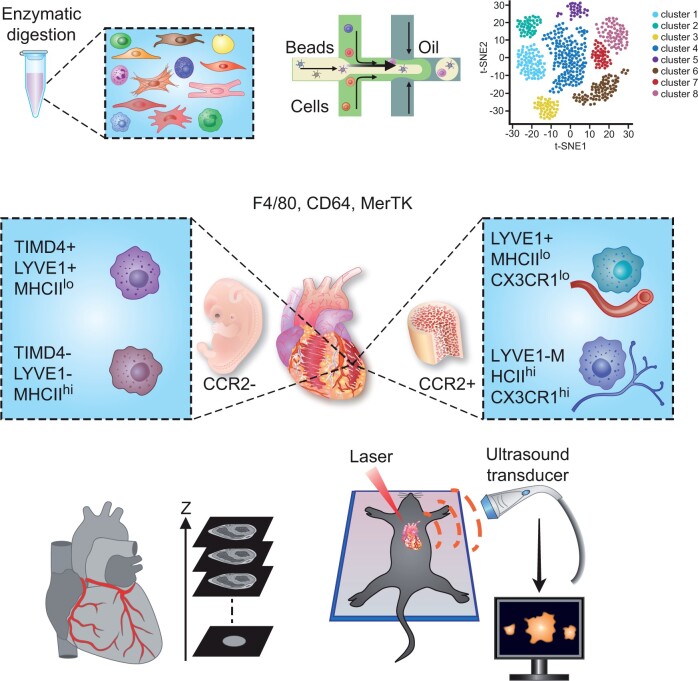
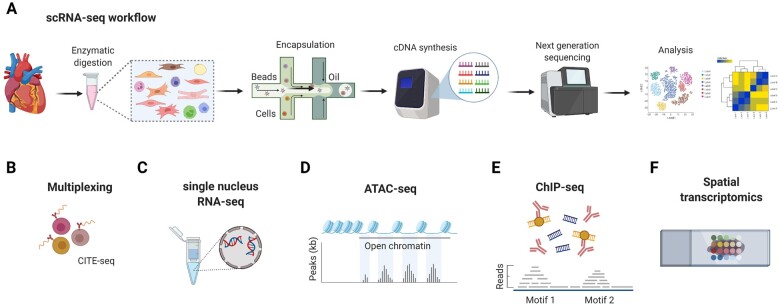
Recent studies using single-cell RNA sequencing (scRNA-seq) approaches of the murine and human heart have deepened our understanding of the cardiac cellular composition.6 The principal experimental workflow of this technology is outlined in Figure 1A. Although the method is certainly a valuable tool for studying cardiac immune cell heterogeneity, it also has its limitations and requires tissue- and cell-specific optimization of the experimental protocol. For example, any type of cardiac tissue processing for generating single-cell suspensions may inherently cause some cell damage and possibly changes in gene expression.13 Consequently, the relative distribution of cell populations and transcriptomic information may vary between individual studies. Due to limitations in the sequencing depth, less abundantly expressed genes are often not detectable in scRNA-seq databases and may require more sensitive detection methods such as quantitative polymerase chain reaction (PCR) or digital droplet PCR. Prior to scRNA-seq, cells can be marked with cell surface labels, e.g. to increase the sensitivity for rare cells or to enable sample multiplexing (Figure 1B). An alternative to scRNA-seq is the single nucleus RNA-seq (snRNA-seq) method, which is useful for larger cells such as cardiomyocytes (Figure 1C). Integrated scRNA-seq and snRNA-seq analysis has been applied for studying the single-cell transcriptomic landscape of the human healthy and failing heart.14–16 Although snRNA-seq is useful as it facilitates the inclusion of myocytes, a disadvantage that needs to be considered is that less transcripts are detected, since only the nuclear fraction is included.17 Other technical advancements involve the combination of scRNA-seq with epigenetic profiling7 (Figure 1D and E) or spatial transcriptomics,12 in order to integrate spatial information and (bulk) transcriptomic data (Figure 1F).
Figure 1.
Overview of scRNA-seq and related technological advancements to study the cardiac transcriptome. (A) The technologies used for scRNA-seq mostly employ microfluidics platforms for single-cell encapsulation as initial step of sample preparation, combined with next-generation sequencing approaches for entire transcriptome measurements.7 (B) To improve the precision of immune cell subset identification, which might be relevant for rare cell populations, oligonucleotide-barcoded antibody labelling, or lipid-tagged indices for barcoding of cells prior to their processing for scRNA-seq can be used.8 , 9 This multimodal measurement of cell surface labels in combination with transcript expression allows combining several biological samples in one scRNA-seq library, which is referred to as multiplexing. This reduces inter-sample technical bias and facilitates exclusion of cellular doublets, thereby limiting analytical artefacts. (C) An alternative to scRNA-seq is the single nucleus RNA-seq (snRNA-seq) method, which might be preferable when working with tissues that are difficult to dissociate or frozen and large cells such as cardiomyocytes.10 , 11 Other technical advancements involve combined methods for epigenetic profiling with scRNA-seq, such as (D) assay for transposase-accessible chromatin using sequencing (ATAC-seq) or (E) chromatin immunoprecipitation followed by sequencing (ChIP–seq).7 ATAC-seq allows sequencing of the regions of the genome with open or accessible chromatin, while ChIP-seq enables genome-wide profiling of DNA-binding proteins and histone modifications. (F) Novel developments combine transcriptomics with spatial information, in order to overcome the issue that the information on cellular distribution gets lost due to the generation of single-cell suspensions.12
The emerging human and mouse scRNA-seq data consistently document a high frequency of non-myocyte stromal cell types in the healthy heart, including endothelial cells and fibroblasts, as well as the presence of all major immune cells, such as macrophages, dendritic cells, granulocytes, B- and T cells, as well as NK cells.15 , 16 , 18–21 The combined analysis of male and female hearts revealed overall highly similar clustering patterns for female and male cells, while in individual cell types, several genes appeared to exhibit sexual dimorphism.21 Interestingly, male-up-regulated genes appear to play a role in responding to foreign antigens, whereas, female-up-regulated genes in macrophages were enriched for processes involving response to stress and the electron transport chain. It should be taken into account that in the specific study, only two male and two female hearts were included into the analysis, which emphasizes the preliminary nature of these assumptions and need for further investigation of sex-specific dimorphic transcriptomic immune cell phenotypes. In addition, the causal factors, which may involve sex hormones, the chromosomal genotype, and possibly additional factors,22 remain to be explored. Combining scRNA-seq with fate mapping strategies further delineated the functional heterogeneity of the cardiac macrophage compartment, as discussed in the following sections.23
In summary, scRNA-seq has recently emerged as a powerful method in cardiovascular research for elucidating biological mechanisms at the cellular level. This area of research is still rapidly advancing, e.g. in view of methods that combine transcriptomics with spatial information, in order to overcome the issue that the information on cellular distribution gets lost due to the generation of single-cell suspensions (Figure 1F).12 Undoubtedly, the possibility to implement spatial transcriptomics to the heart, e.g. for infarct and border zone sequencing, will be a valuable tool to complement existing scRNA-seq data. Of particular interest are recent efforts to create open resource platforms in order to facilitate the data accessibility for the broad research community, without need to have expert knowledge for exploring the transcriptional landscape of healthy and diseased murine hearts and aorta. The available platforms are Reference for the Heart failure Transcriptome (ReHeaT; https://saezlab.shinyapps.io/reheat/), which provides a transcriptomic consensus signature and is based on meta-analysis of 16 published bulk sequencing studies of left ventricular samples from a total of 263 healthy and 653 failing human hearts, and CardiovascuLAR Atlas (CLARA; https://clara.baker.edu.au/), which integrates 5 murine and 2 human scRNA-seq data sets.24 , 25
New insights into the cardiac immune system from advanced imaging technologies
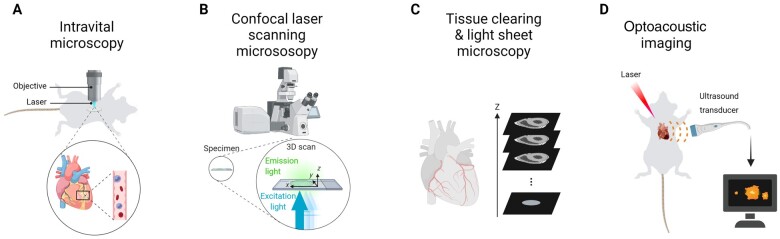
Other important technological innovations, which have greatly advanced our understanding of cardiac immunology, include microscopic developments for multidimensional imaging of cells and tissues with subcellular resolution.26 While real-time imaging of leucocyte behaviour in the beating heart by intravital microscopy remains challenging (Figure 2A),27 , 31 other technologies such as confocal laser scanning microscopy and light sheet microscopy of optically cleared tissue (Figure 2B and C)32 or optoacoustic imaging (Figure 2D)33 may be more easily applied to study pathophysiological mechanisms in cardiac disease. So far, there are only few reports that used intravital microscopy to visualize leucocyte recruitment in rodent models of ischaemia-reperfusion and permanent left anterior descending artery (LAD) ligation.34–36
Figure 2.
Overview of advanced imaging technologies. (A) Intravital microscopy of the beating mouse heart is an invasive method that involves thoracic surgery and cardiac tissue stabilizers.27 In addition, it requires acquisition gating algorithms to avoid moving artefacts caused by the cardiac and respiratory cycles. Due to these technical requirements and need for highly specialized knowledge, the methodology has not been broadly applied to study immune cell behaviour in cardiac disease models. (B) Confocal laser scanning microscopy allows scanning multiple layers of a sample and subsequent 3-dimensional image reconstruction. (C) For tissue clearing, the heart is processed by organic solvent- or aqueous-based clearing or hydrogel embedding tissue clearing.28 When combined with diverse labelling methods (such as immunolabelling or use of reporter mice) and high-throughput optical sectioning light sheet microscopy,29 tissue clearing enables whole-body and whole-organ imaging at cellular or subcellular resolution. (D) Optoacoustic imaging is a technology that uses ultrasound sensors to visualize structures based on the optoacoustic effect, which refers to the generation of acoustic waves from endogenous chromophores in biological tissues (e.g. haem) following pulsed-light illumination.26 The light absorption by these endogenous components results in thermoelastic expansion of the tissue and generation of mechanical waves, which are resistant to scattering, thereby enabling higher spatial resolution and greater penetration depths than emitted light in optical imaging. In addition to label-free imaging of endogenous structures such as blood vessels, optoacoustic imaging can also be used to track transplanted immune cells in entire living animals, which is achieved by near-infrared fluorescent optoacoustic probes.30
A breakthrough development for advanced imaging was certainly the development of protocols for tissue clearing in order to minimize light scattering and light absorption (Figure 2C), thus facilitating deep imaging of large volume samples.37 Whole mouse heart imaging after tissue clearing has been achieved by light sheet microscopy, thereby visualizing ischaemia-reperfusion damage zones and neutrophil infiltrates in response to transient LAD ligation32 and resident macrophages under steady state.38 Another interesting novel application in the cardiovascular research field is optoacoustic imaging, which uses ultrasound sensors to visualize structures based on the generation of acoustic waves from endogenous chromophores in biological tissues.26 As a proof-of-principle, optoacoustic imaging of the beating heart has been successfully applied in an isolated murine Langendorff-perfused heart.33 Although this ex vivo set up is certainly not useful to study leucocyte recruitment, it offers a high spatio-temporal resolution of the heart at centimetre-scale imaging depths.
In summary, advanced imaging technologies for studying leucocyte dynamic behaviour in cardiac disease have emerged as a valuable tool in preclinical research. Examples of cardiac immune cell functions in the heart studied with the help of advanced imaging modalities will be discussed in the following sections. Depending on the specific question, resolution and imaging depth needed, different technologies ranging from nano- to macroscale may be useful. In particular, the ability to visualize fast and dynamic behaviours of leucocyte sub-types may help, e.g. to study patrolling behaviour of non-classical monocytes and unravel cellular interactions possibly involved in immune cell subset activation or phenotypic switch during inflammation resolution.
Immune cell function in cardiac homeostasis
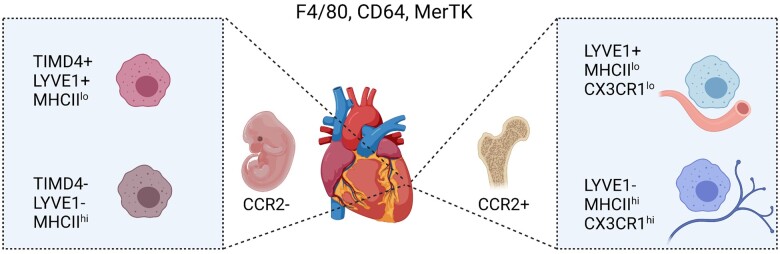
Fate mapping mouse models have been instrumental to clarify the developmental origin of cardiac leucocytes residing in the heart1 (Figure 3). Adult resident cardiac macrophages are mostly chemokine receptor CCR2-negative (CCR2–) and primarily of foetal origin, seeding the heart during embryogenesis and maintaining themselves by local proliferation. Conversely, the monocyte-derived CCR2+ macrophage subset is a minor population in the healthy adult heart, but expands after tissue injury and is thought to display more pro-inflammatory functions and promote cardiac disease, whereas CCR2– resident macrophages promote angiogenesis and repair.1 , 41 A seminal study of interstitial macrophages in lung, heart, and other peripheral tissues employing scRNA-seq in comparison to existing bulk RNA seq transcriptomic signatures and genetic fate mapping models adds to the concept of cardiac macrophage heterogeneity. Two independent populations of monocyte-derived interstitial macrophages were identified: LYVE1loMHCIIhi and LYVE1hiMHCIIlo.40 Remarkably, these distinct populations reside within separate locations, with LYVE1loMHCIIhi mostly located adjacent to nerves, while LYVE1hiMHCIIlo reside next to blood vessels. Functionally, LYVE1loMHCIIhi macrophages had superior antigen-presentation capacity and ability to induce antigen-specific T cell activation and played an antifibrotic role in isoprenaline-induced heart fibrosis. In a separate study, Dick et al.23 combined genetic fate mapping with scRNA-seq analysis to identify the phosphatidylserine receptor TIMD4 as a marker to track a subset of resident CCR2– macrophages (Figure 3).
Figure 3.
Cardiac resident macrophage heterogeneity during steady state. Macrophages in the healthy adult heart are highly heterogeneous, which is related to their distinct ontogeny (embryonic vs. adult bone marrow monocyte progenitor-derived) and tissue microenvironment. A common set of markers to identify macrophages includes F4/80, CD64, and MerTK. CCR2– macrophages largely originate from embryonic (yolk sac and foetal liver) origin and can be further subdivided into LYVE1+ MHCIIlo and LYVE1– MHCIIhi. TIMD4 recently emerged as additional marker of CCR2– MHCIIlo resident macrophages that is fully maintained independent of blood monocytes, whereas CCR2– MHCIIhi TIMD4– resident macrophages are partially replaced.23 CCR2+ macrophages are derived from bone marrow haematopoiesis, giving raise to CCR2+ blood monocytes that enter the adult heart.39 Another dimension of cardiac bone marrow monocyte-derived macrophage heterogeneity involves the separation into LYVE1+ MHCIIlo CX3CR1lo and LYVE1– MHCIIhi CX3CR1hi tissue macrophage subsets with distinct localization and functional specializations.40
Two more recent studies employing bulk or scRNAs-seq, respectively, shed further light on the role of CCR2– resident macrophages in cardiac homeostasis and adaptive remodelling. In a murine experimental model of angiotensin II-induced hypertension, the release of insulin-like growth factor-1 (IGF-1) by resident CCR2– macrophages was identified as a crucial driver of cardiomyocyte growth.42 Using a genetic model of dilated cardiomyopathy, resident CCR2– macrophages were shown to interact with cardiomyocytes via focal adhesion complexes.43 Mechanical stimuli were sensed by these resident macrophages via TRPV4 and resulted in IGF-1 release. This further supports the physiological implication of resident cardiac macrophages in mechanosensing, as initially reported by Sager et al.44
Despite the intense mechanical stress and high metabolic demand, cardiomyocytes in the adult heart have only a minimal turnover rate. Hence, it may not be surprising that cardiomyocytes rely on the interaction of non-myocytes, including immune cells, in maintaining cardiac tissue homeostasis. Recent studies have elucidated important housekeeping functions of resident cardiac macrophages for maintaining cardiac homeostasis. Light sheet microscopy imaging of cardiac resident macrophages with the help of reporter mice expressing GFP under the CX3CR1 promoter revealed their ubiquitous distribution throughout both ventricles.38 Confocal imaging further revealed that cardiac macrophages where generally localized in direct contact to cardiomyocytes, suggesting a cellular crosstalk between both cell types. Using a multitude of experimental models and imaging modalities, the same study elegantly demonstrated that cardiac resident macrophages take up cardiomyocyte cellular content, including mitochondria-carrying particles. The particle formation is driven by the cardiomyocyte autophagy machinery, while the uptake by cardiac macrophages is phagocytosis receptor MerTK-dependent. This cellular crosstalk seems to play an important role in maintaining cardiac homeostasis, since its blockage, e.g. by cardiomyocyte autophagy ablation, resulted in cardiometabolic dysfunction. These novel insights strengthen previously reviewed findings on crucial functions of macrophages in the heart, including electrical conduction driven by gap junction-dependent electrical coupling between atrial cardiomyocytes and macrophages.39 , 45
Less is known about adaptive immune cells in cardiac homeostasis, although insights from other chronic disease conditions such as rheumatoid arthritis suggest that maladaptive T cell ageing may promote chronic inflammatory disorders, as protective T cell functions decline and pro-inflammatory effector cells are enriched.46 , 47 Hence, maladaptive T cell responses may render the heart susceptible to tissue-damaging chronic inflammation. A potential role for B cells in cardiac homeostasis was suggested after tracking circulating B cells in murine hearts, revealing that a subset of B cells transiently arrested on the cardiac microvascular endothelium during their transit through the heart.48 The vast majority of myocardial B cells remained intravascular, whereas < 5% extravasated into myocardial tissue. B cell-deficient animals had a reduced myocardial mass, increased left ventricular ejection fraction, and faster relaxation as well as an increased cardiac T cell composition. However, the precise mechanisms how B cells might affect cardiac growth and contractility remain to be investigated.
Innate and adaptive immunity in ischaemic cardiac disease
A major cause of cardiac disease is MI, which represents the leading cause of hospitalization and mortality in Europe.49 MI induces a massive loss of cardiac tissue, which subsequently elicits an inflammatory response and repair process.50 Immune cells including neutrophils and monocyte-derived macrophages are recruited to the heart and promote the clearance of dead cardiomyocytes. Neutrophils and macrophages in the ischaemic myocardium are involved in both, inflammatory as well as reparative processes.51–54 Although these immune cells are integral key players of cardiac healing, an unbalanced or unresolved immune reaction after MI aggravates tissue damage that triggers maladaptive remodelling and HF.50 , 55–58 Hence, stimulating the resolution of post-ischaemic inflammation has been considered as an attractive therapeutic strategy to improve infarct repair. In this context, experimental studies have focused on local regulators and signalling pathways in the cardiac microenvironment promoting the resolution of inflammation, highlighting among other, the relevance of Wnt signalling in this process.59
The distinct functional properties of myeloid cells during the post-MI inflammatory and resolution stage suggests the involvement of phenotypically distinct subsets. Indeed, there is substantial evidence that cardiac macrophages exhibit high phenotypic diversity with distinct origins and functions, as reviewed elsewhere.39 More recent mechanistic insight on how monocyte-derived CCR2+ macrophages shape the inflammatory responses in the ischaemic myocardium revealed an involvement of the cGAS-STING signalling pathway, which is activated by extracellular DNA released from dying cells (Figure 4).60 This leads to an activation of the transcription factor interferon regulatory factor 3 (IRF3), which, in turn, induces Type I interferon (IFN) release and further monocyte recruitment. A more in-depth scRNA-seq-based transcriptional profiling of myeloid cells after MI in mice revealed that the IRF3-dependent IFN gene expression signature is already acquired in bone marrow myeloid cell precursors, and negatively regulated by Tet methylcytosine dioxygenase 2 (TET2).67 Genetic loss of TET2 in humans is one of the most common mutations linked to clonal haematopoiesis. This is in agreement with previous findings showing that Tet2 deficiency in haematopoietic cells is associated with greater cardiac dysfunction in murine models of HF, as a result of elevated interleukin (IL)-1β signalling.68 In CCR2– cardiac resident macrophages, the IFN response is counteracted by transcription factor nuclear factor erythroid-derived 2-like 2 activation.67 The IFN response signature was also confirmed in humans, using scRNA-seq analysis of blood cells collected from a patient with non-ST-elevation MI 48 h after onset of chest pain.
Figure 4.
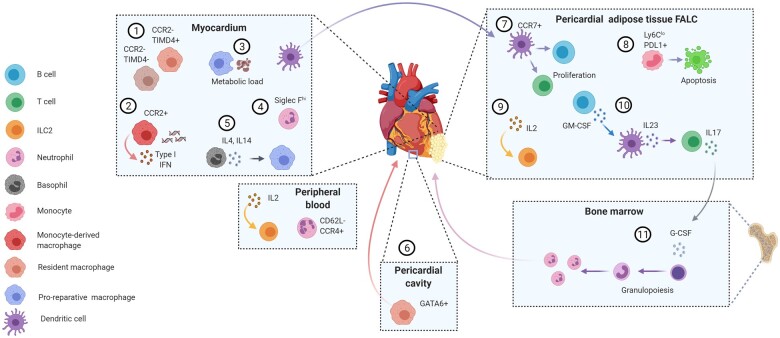
New insights into immune cell functions and phenotypes in ischaemic cardiac injury. (1) Cardiac macrophages in the healthy heart are mostly CCR2– and of foetal origin, as opposed to monocyte-derived CCR2 expressing macrophages, which infiltrate the heart after MI.1 Ischaemic injury reduces CCR2– TIMD4+ and TIMD4– resident macrophage abundance,23 whereas CCR2+ monocyte-derived macrophages expand and adopt heterogeneous phenotypes within infarcted tissue. (2) The cGAS-STING signalling pathway is activated in CCR2+ macrophages by extracellular DNA released from dying cells, thereby promoting an IRF3-dependent type I IFN release and monocyte recruitment.60 (3) Engulfment of dying cells by macrophages within the infarcted myocardium results in elevated macrophage fatty acid content, which stimulates mitochondrial respiration.61 This metabolic signalling pathway promotes an anti-inflammatory macrophage response during wound healing after MI. (4) The different post-MI healing stages are also accompanied by diverse neutrophil subsets, with an aged neutrophil phenotype accumulating in the circulation over time. Conversely, heart infiltrating neutrophils acquire a high expression level of the surface lectin SiglecF, resembling an activated phenotype previously observed in cancer.62 (5) Basophil-derived IL4 and IL14 production plays an essential role in the infarcted heart, by balancing the ratio of proinflammatory monocytes to reparative Ly6Clo macrophages.63 (6) A new macrophage phenotype expressing GATA6 has been identified in the pericardial cavity fluid, which is recruited to the heart in response to MI and plays a protective role by preventing excessive cardiac fibrosis. (7) The pericardial adipose tissue contains lymphoid clusters [fat-associated lymphoid clusters (FALCs)], which expand after MI.64 Activated dendritic cells migrate from the infarcted myocardium to pericardial adipose tissue FALCs and induce B and T cell proliferation. (8) Moreover, an increasing number of patrolling Ly6Clo monocytes, which express PDL1, interacts with T cells within pericardial FALCs and induces their apoptosis, possibly as an inhibitory mechanism to prevent sustained lymphocyte activation.65 (9) IL2 promotes ILC2 expansion in the circulation and the pericardial adipose tissue after MI, and is associated with an improved cardiac function, while genetic depletion of ILC2s worsened cardiac recovery post-MI.66 (10) An innate-like B cell subset within pericardial FALCs expresses high amounts of GM-CSF in response to MI, which induces IL23 and IL17 cytokine release by other pericardial immune cells.64 (11) The systemic cytokine increase triggers the production of the granulocytic factor G-CSF, which, in turn promotes de novo production of neutrophils in the bone marrow, thereby maintaining the neutrophil supply to the ischaemic heart.64
A new macrophage phenotype was recently identified in the pericardial cavity of mice and humans, which is transcriptionally distinct from neighbouring cardiac macrophages.69 This subset expresses GATA6, which is also a marker of peritoneal macrophages (Figure 4). Bulk seq transcriptional pathway analysis in comparison to cardiac macrophages revealed enrichment for expression of genes related to protein and nucleic acid metabolism, whereas the expression profile was rather similar to peritoneal macrophages. In response to cardiac ischaemic injury, pericardial cavity macrophages were recruited to the myocardium between Days 3 and 7, which was independent of the chemokine receptor CCR2. This suggests that pericardial cavity macrophages do not depend on monocyte influx. Targeted genetic deletion of GATA6+ pericardial macrophages in mice subjected to MI resulted in detrimental cardiac interstitial fibrosis in the remote myocardium and consequently increased ventricular stiffness. The finding that such macrophages were also found in human pericardial fluid supports a possible relevance of these cavity cells in human cardiac tissue homeostasis and immunosurveillance. In murine models of MI, however, it is challenging to study their pathophysiological role, as the pericardium is usually disrupted by the surgical procedure. Using non-invasive models such as the recently described echocardiography-assisted technique for MI induction in mice might be preferable.70 Moreover, it might be interesting to study their function in other models of cardiac disease, e.g. using a Cre-inducible diphtheria toxin receptor mouse model for targeted necrosis of cardiomyocytes or angiotensin II infusion-induced remodelling.
Temporal changes of neutrophil markers have been initially described by gene expression profiling of isolated cardiac neutrophils using a limited number of predefined markers.71 Interestingly, experimental data also indicate sex-specific differences in neutrophil phenotypes post-MI, which strengthens the relevance of sex-specific aspects in cardiac inflammation.72 More recently, the detailed temporal transcriptomic analysis of murine neutrophils at single-cell resolution highlighted the diversity of neutrophils during ischaemic cardiac inflammation. While circulating neutrophils acquired an aged phenotype characterized by loss of surface CD62L and up-regulation of CXCR4, heart infiltrating neutrophils acquired a unique SiglecFhi signature (Figure 1).62 A similar cardiac neutrophil subset appearing after MI was validated by scRNA-seq data from Kevin King’s lab.73 Siglec F is a cell surface lectin, and a few experimental studies have reported pro-tumourigenic and antimicrobial functions of SiglecFhi neutrophils. SiglecFhi neutrophils have an activated phenotype, produce reactive oxygen species, and have higher phagocytic activity than SiglecFlo neutrophils and a prolonged lifespan.74–76 However, the functional role of this subset in post-ischaemic cardiac healing remains to be investigated. Another fundamental question is whether heterogeneity originates from the release of bone marrow neutrophils into the circulation at different maturation stage or local priming of neutrophils in the cardiac tissue microenvironment or both. The fact that the SiglecFhi neutrophil subset was neither found in the bone marrow nor spleen suggests a local priming in the inflammatory microenvironment.62 In line with this concept, latest assay for transposase-accessible chromatin using sequencing data unveiled differential chromatin remodelling and transcription factor networks in neutrophils, depending on their developmental stage, activation state, and tissue microenvironment.77 In particular, RELB, IRF5, and JUNB have been linked to neutrophil effector responses in inflamed tissue, and RFX2 and RELB to increased neutrophil survival. Nevertheless, it is also conceivable that during emergency granulopoiesis post-MI, neutrophils with different maturation stages are mobilized and contribute to cardiac neutrophil heterogeneity, as already described upon microbial or tumourigenic stress, both in mice and human patients.78 , 79
While basophils have been largely ignored as innate immune subset in the context of post-MI inflammation, recent experimental data now highlight an essential role for basophil-derived IL-4 and IL-14 production in the heart, by balancing the ratio of proinflammatory monocytes to reparative Ly6Clo macrophages in the infarcted heart (Figure 1).63 A possible clinical relevance of basophils was supported by associations between initial low blood basophil counts and worse 1-year outcome in MI patients. Likewise, innate lymphoid cells (ILCs) have not been studied much in the context of CVD so far. Despite being part of the lymphoid lineage, they do not contribute to antigen-specific responses. Latest published findings highlighted their contribution in post-MI repair.66 Remarkably, ILC Type 2 (ILC2s) expand in the pericardial adipose tissue after experimental MI, while genetic depletion of ILC2s worsened cardiac recovery post-MI (Figure 4). Treatment with low-dose IL-2 to expand ILC2s improved cardiac function in mice. In view of a translational perspective, IL-2 infusion in patients with acute coronary syndromes expanded circulating ILC2s. Although still quite speculative, it is suggested by the authors that activation of ILC2s by low-dose IL-2 could be a novel therapeutic strategy to improve post-MI outcome.
Adaptive immune cells also contribute to the immune response after MI, although the underlying mechanisms are still incompletely understood. The cardiac B and T lymphocyte numbers are relatively low and peak around day 7 after MI in murine models of permanent ligation.80–83 Zouggari et al.80 demonstrated that B cells contribute to MI injury and unfavourable remodelling by promoting the release of Ly6Chi monocytes from the bone marrow via secretion of CCL7. Furthermore, experimental data have identified a substantial amount of B cells residing in clusters within the pericardial adipose tissue.84 Pericardial B and T cells were activated and expanded by proliferation in response to MI.64 The interaction with dendritic cells and patrolling non-classical monocytes might play a relevant role in controlling the pericardial lymphocyte fate in this context (Figure 4).64 , 65 , 85 Mechanistic in vivo experiments further demonstrated that the pericardial lymphocyte response enhanced bone marrow granulopoiesis and thereby neutrophil infiltration into the infarcted myocardium via pericardial granulocyte-macrophage colony-stimulating factor and IL-17 release, which, in turn, triggered granulocyte colony-stimulating factor production.
In addition to cytokine release and cell–cell contact, B cells exhibit humoral immune functions. A recent experimental study investigated the contribution of autoantibody producing B cells in an experimental model of MI-accelerated atherosclerosis.86 Alarmins released from the infarcted heart were proposed as B cell activating factors in a mechanism involving Toll-like receptor adaptor protein MyD88. The B cell activation resulted in enhanced splenic plasma cell immunostaining, accompanied with increased deposits of immunoglobulin G antibodies in atherosclerotic plaques. These findings support the concept that atherosclerosis shares features of autoimmune disease, with autoreactive clones producing antibodies raised against circulating or plaque epitopes.87 Increased production of autoantibodies after MI is proposed as a causal mechanism for accelerated atherosclerosis progression in this mouse model.86 Although this concept is intriguing and may open new perspectives for prognosis and therapy, further studies are needed to strengthen these findings. For example, the study did not assess circulating antibody titres in the mouse plasma, and no flow cytometric immunoprofiling of the splenic B cell repertoire was performed. Circulating immunoglobulins could be easily monitored in human MI patients with or without secondary coronary events, in order to clarify a potential relevance for human pathophysiology. A recent study from Heinrichs et al.88 employed scRNA-seq and B cell receptor sequencing to study B cell responses after MI in the murine heart and draining lymph nodes. While the post-MI cardiac B cell repertoire was polyclonal without indication for antigen-specific clonal expansion, a specific subset of activated cardiac B cells expressing CD69, CCR7, and CXCR5 was identified. The cardiac B cell response was accompanied by a moderate antigen-specific germinal centre reaction in the draining lymph nodes.
Linking metabolism to immune cell repair function in cardiac disease
Heart failure is associated with a metabolic shift and loss of metabolic flexibility.89 Under physiological conditions, the heart is able to use multiple energy substrates for mitochondrial ATP production, including fatty acids, glucose, and ketones. There is also evidence that metabolism in non-myocyte populations, including cardiac fibroblasts, immune cells, and endothelial cells, plays an important role in cardiac remodelling and adaptation to injury.90 For example, endothelial Notch signalling is a critical regulator of cardiac blood vessel formation and fatty acid transport in the heart under homeostatic conditions, while impaired endothelial Notch signalling leads to cardiac hypertrophy and HF.91
During cardiac inflammation such as ischaemic injury, both cardiomyocytes and immune cells undergo metabolic reprogramming, in order to deal with decreased availability of nutrients and oxygen.2 Moreover, there is an accumulation of signalling metabolites such as lactate, which may also affect the immune cell properties and possibly interfere with their reprogramming to a reparative phenotype. Hence, the metabolic crosstalk between immune cells and cardiomyocytes might crucially regulate the balance between resolution of inflammation vs. adverse cardiac remodelling. Existing knowledge about macrophage immunometabolism is mostly based on in vitro studies in bone marrow-derived macrophages, showing that pro-inflammatory polarization promotes glycolysis, whereas anti-inflammatory stimulation shifts the metabolic profile towards oxidative phosphorylation.92 Engulfment of apoptotic bodies in the infarcted myocardium, which is termed efferocytosis, is an essential process in the resolution of inflammation and post-MI repair56 and induces a heterogeneous transcriptional reprogramming in macrophages.93 Moreover, the apoptotic body uptake leads to a metabolic load in the engulfing macrophages, in particular long chain fatty acids, which induces the respiratory chain and is required for their anti-inflammatory polarization.61 In mice with genetically impaired mitochondrial electron transport chain in myeloid cells, the inflammatory response after MI was disturbed, with an increased mortality due to left ventricular rupture and reduced expression of anti-inflammatory and pro-fibrotic cytokines IL-10 and transforming growth factor-β. A key regulator of metabolic adaptation to hypoxia is hypoxia inducible factor (HIF). Latest findings from DeBerge and colleagues94 revealed differential roles of various HIF isoforms in post-ischaemic regulation of cardiac macrophage metabolism. HIF-2α suppressed anti-inflammatory macrophage mitochondrial metabolism, while HIF-1α promoted cleavage of cardioprotective MerTK through glycolytic reprogramming of macrophages, suggesting that both isoforms antagonize post-ischaemic repair. Surprisingly, the combined loss of both isoforms in myeloid cells resulted in enhanced necroptosis, disturbed fibrotic repair, and consequently enhanced cardiac rupture after MI as a consequence of impaired inflammation resolution. It is suggested that the underlying reason for this unexpected finding is an increased hypoxic macrophage death, since both isoforms act together to suppress mitochondrial reactive oxygen species production and necroptosis.
To conclude, a growing body of evidence highlights the close interconnection between macrophage signalling and metabolic reprogramming. Further research is needed to better understand the hierarchy of events leading to the distinct cellular responses, in particular whether failed resolution is a consequence of disturbed metabolic reprogramming or vice versa if excessive proinflammatory activation leads to a metabolic imbalance. The immunometabolic changes in adaptive immune cells in the context of cardiac ischaemia and repair are still unknown and deserve further investigation. For example, it is conceivable that local changes in metabolites within the ischaemic myocardium may have an impact on T cell effector and regulatory functions.92
Immunotherapy for cardiac disease: emerging targets and delivery methods
The concept of immunotherapy has revolutionized the cancer field and other immune diseases, including CVD. The first clinical study testing the effect of anti-inflammatory therapy on cardiovascular clinical outcome was the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial.95 Canakinumab resulted in a significantly lower rate of recurrent cardiovascular events than placebo in patients with previous MI, regardless of lipid-level lowering, although all-cause mortality did not significantly differ. The downside was a small but statistically significant increase in bacterial and viral infections, including fatal infections, in those receiving canakinumab. Clinical benefits in MI patients were also observed in the Colchicine Cardiovascular Outcomes Trial (COLCOT), despite a small, significant increase in pneumonia.96 This emphasizes the need for safer anti-inflammatory therapies by optimizing target specificity and drug delivery, in order to limit potential systemic side effects and preserve the patient’s host defence.
Nanomedicine has emerged as a promising tool for targeted drug delivery with reduced toxicity, e.g. for cancer treatment. It uses nanoparticles, such as lipid-based formulations, as carriers for targeted drug delivery into selective tissues or cell types. A prototypic example for a nanotechnology-based medical application is the new class of COVID-19 mRNA vaccines developed by BioNTech/Pfizer and Moderna, which are composed of messenger ribonucleic acid strands encapsulated in lipid nanoparticles.97 The goal of the nanoparticle delivery is to improve the solubility, biodistribution, and stability of the drug, which is optimized by chemical engineering. Moreover, fluorescent or radiolabelling of nanoparticles allows non-invasive in vivo imaging of the drug dynamics.98 , 99 A few preclinical studies have already used nanoparticles designed for selective immunotherapy of CVD.100 , 101 For example, systemic administration of nanoparticles for selective Ccl2 transcriptional silencing in bone marrow niche endothelial cells efficiently inhibited leucocyte egress and translated into reduced cardiac leucocyte recruitment upon MI.102 In order to identify nanoparticles for selective immune cell targeting in CVD, an in vivo screening approach with a nanoparticle library has been established, which can be useful to optimize drug delivery for selected compounds.103
Moreover, nanotracers have been successfully used for studying myeloid cell behaviour in mouse models of atherosclerosis and MI. In particular, a radiolabelled high-density lipoprotein-derived nanotracer was developed for non-invasive tracking of myeloid cell egress from the spleen and bone marrow and subsequent accumulation within the plaque and infarcted heart.104 In the future, it is conceivable to implement such imaging modalities together with RNA-seq transcriptomic profiling as additional readout in clinical trials, e.g. in order to monitor haematopoietic bone marrow niche activity in MI patients that receive anti-inflammatory medications.105 , 106
Conclusions
In the past few years, we have encountered an impressive technological revolution, which was accompanied by collective efforts to disambiguate the nature of immune cell diversity in health and disease. The available datasets will be instrumental for upcoming studies aimed at better understanding the pathophysiological role of the diverse subsets and emerging new markers in cardiac disease. More efforts are needed to consider the impact of the biological sex on cardiac disease outcome in preclinical studies. There is emerging evidence that the immune response in cardiac disease differs between men and women, which may require sex-specific testing of novel anti-inflammatory cardiovascular drugs. Finally, with the globally ageing population, age-related changes in immune cell functions, referred to as inflammageing, may become more important in the future. To this end, the preclinical testing of novel immunotherapies should also include animals at advanced age and models of age-related cardiac dysfunction.
Acknowledgements
The figures were created with BioRender.com.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (STE-1053/6-1, STE-1053/8-1 and SFB 1123) and the German Ministry of Research and Education (DZHK FKZ 81Z0600205) to S.S., a grant from Incyte and funds from Ministro dell’Istruzione, Università e Ricerca Scientifica (549901_2020_Madonna: Ateneo) to R.M., and the National Institutes of Health grants HL142494, NS108419, HL139598, HL125428 and the MGH Research Scholar program to M.N.
Conflict of interest: M.N. has received funds or material research support from Lilly, Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis, and Pfizer, as well as consulting fees from Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, and Verseau Therapeutics. The other authors report no conflicts of interest.
Contributor Information
Sabine Steffens, Institute for Cardiovascular Prevention (IPEK), Ludwig-Maximilians-Universität, Pettenkoferstraße 9, Munich 80336, Germany; Munich Heart Alliance, DZHK Partner Site, Munich, Germany.
Matthias Nahrendorf, Center for Systems Biology, Massachusetts General Hospital, Harvard Medical School, 185 Cambridge Street, 8.228 Boston, MA 02114, USA; Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Rosalinda Madonna, Department of Internal Medicine, McGovern School of Medicine, The University of Texas Health Science Center at Houston, Houston, TX, USA; Department of Pathology, Cardiology Division, University of Pisa, c/o Ospedale di Cisanello Via Paradisa, 2, 56124 Pisa, Italy.
References
- 1. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733–744. [DOI] [PubMed] [Google Scholar]
- 2. Marelli-Berg FM, Aksentijevic D. Immunometabolic cross-talk in the inflamed heart. Cell Stress 2019;3:240–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007–2018. [DOI] [PubMed] [Google Scholar]
- 4. Lam CSP, Arnott C, Beale AL et al. Sex differences in heart failure. Eur Heart J 2019;40:3859c–3868c. [DOI] [PubMed] [Google Scholar]
- 5. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 6. Yamada S, Nomura S. Review of single-cell RNA sequencing in the heart. Int J Mol Sci 2020;21:8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 2018;18:35–45. [DOI] [PubMed] [Google Scholar]
- 8. McGinnis CS, Patterson DM, Winkler J et al. MULTI-seq: sample multiplexing for single-cell RNA sequencing using lipid-tagged indices. Nat Methods 2019;16:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stoeckius M, Hafemeister C, Stephenson W et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 2017;14:865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grindberg RV, Yee-Greenbaum JL, McConnell MJ et al. RNA-sequencing from single nuclei. Proc Natl Acad Sci 2013;110:19802–19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slyper M, Porter CBM, Ashenberg O et al. A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat Med 2020;26:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson L, Frisén J, Lundeberg J. Spatially resolved transcriptomics adds a new dimension to genomics. Nat Methods 2021;18:15–18. [DOI] [PubMed] [Google Scholar]
- 13. Ziegenhain C, Vieth B, Parekh S, Hellmann I, Enard W. Quantitative single-cell transcriptomics. Brief Funct Genomics 2018;17:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koenig AL, Shchukina I, Andhey PS et al. Single cell transcriptomics reveals cell type specific diversification in human heart failure. bioRxiv, doi: 10.1101/2021.07.06.451312. [DOI] [PMC free article] [PubMed]
- 15. Litviňuková M, Talavera-López C, Maatz H et al. Cells of the adult human heart. Nature 2020;588:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tucker NR, Chaffin M, Fleming SJ et al. Transcriptional and cellular diversity of the human heart. Circulation 2020;142:466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakken TE, Hodge RD, Miller JA et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS One 2018;13:e0209648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farbehi N, Patrick R, Dorison A et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife 2019;8:e43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martini E, Kunderfranco P, Peano C et al. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation 2019;140:2089–2107. [DOI] [PubMed] [Google Scholar]
- 20. Gladka MM, Molenaar B, de Ruiter H et al. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation 2018;138:166–180. [DOI] [PubMed] [Google Scholar]
- 21. Skelly DA, Squiers GT, McLellan MA et al. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep 2018;22:600–610. [DOI] [PubMed] [Google Scholar]
- 22. Walker CJ, Schroeder ME, Aguado BA, Anseth KS, Leinwand LA. Matters of the heart: cellular sex differences. J Mol Cell Cardiol 2021;160:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dick SA, Macklin JA, Nejat S et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flores ROR, Lanzer JD, Holland CH et al. Consensus transcriptional landscape of human end-stage heart failure. J Am Heart Assoc 2021;10:e019667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dona MSI, Hsu I, Rathnayake TS et al. CLARA: A web portal for interactive exploration of the cardiovascular cellular landscape in health and disease. bioRxiv, doi: 10.1101/2021.07.18.452862. [DOI]
- 27. Vinegoni C, Aguirre AD, Lee S, Weissleder R. Imaging the beating heart in the mouse using intravital microscopy techniques. Nat Protoc 2015;10:1802–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods 2010;7:603–614. [DOI] [PubMed] [Google Scholar]
- 28. Tian T, Yang Z, Li X. Tissue clearing technique: recent progress and biomedical applications. J Anat 2021;238:489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power RM, Huisken J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods 2017;14:360–373. [DOI] [PubMed] [Google Scholar]
- 30. Tzoumas S, Zaremba A, Klemm U, Nunes A, Schaefer K, Ntziachristos V. Immune cell imaging using multi-spectral optoacoustic tomography. Opt Lett 2014;39:3523–3526. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Nava RG, Bribriesco AC et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J Clin Invest 2012;122:2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merz SF, Korste S, Bornemann L et al. Contemporaneous 3D characterization of acute and chronic myocardial I/R injury and response. Nat Commun 2019;10:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin HA, Déan-Ben XL, Reiss M et al. Ultrafast volumetric optoacoustic imaging of whole isolated beating mouse heart. Sci Rep 2018;8:14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuura R, Miyagawa S, Fukushima S et al. Intravital imaging with two-photon microscopy reveals cellular dynamics in the ischeamia-reperfused rat heart. Sci Rep 2018;8:15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung K, Kim P, Leuschner F et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res 2013;112:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kavanagh DPJ, Lokman AB, Neag G, Colley A, Kalia N. Imaging the injured beating heart intravitally and the vasculoprotection afforded by haematopoietic stem cells. Cardiovasc Res 2019;115:1918–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell 2015;162:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 2020;183:94.e23–109.e23. [DOI] [PubMed] [Google Scholar]
- 39. Leuschner F, Nahrendorf M. Novel functions of macrophages in the heart: insights into electrical conduction, stress, and diastolic dysfunction. Eur Heart J 2020;41:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakarov S, Lim HY, Tan L et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019;363:eaau0964. [DOI] [PubMed] [Google Scholar]
- 41. Lavine KJ, Epelman S, Uchida K et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A 2014;111:16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaman R, Hamidzada H, Kantores C et al. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress. Immunity 2021;54:2057.e6–2071.e6. [DOI] [PubMed] [Google Scholar]
- 43. Wong NR, Mohan J, Kopecky BJ et al. Resident cardiac macrophages mediate adaptive myocardial remodeling. Immunity 2021;54:2072.e7–2088.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sager HB, Hulsmans M, Lavine KJ et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hulsmans M, Clauss S, Xiao L et al. Macrophages facilitate electrical conduction in the heart. Cell 2017;169:510.e20–522.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:583–588. [DOI] [PubMed] [Google Scholar]
- 47. Shirakawa K, Sano M. T cell immunosenescence in aging, obesity, and cardiovascular disease. Cells 2021;10:2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adamo L, Rocha-Resende C, Lin C-Y et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight 2020;5:e134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hausenloy DJ, Garcia-Dorado D, Botker HE et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 50. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 2014;102:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018;24:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alard JE, Ortega-Gomez A, Wichapong K et al. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci Transl Med 2015;7:317ra196. [DOI] [PubMed] [Google Scholar]
- 54. Horckmans M, Ring L, Duchene J et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
- 55. Heymans S, Luttun A, Nuyens D et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 1999;5:1135–1142. [DOI] [PubMed] [Google Scholar]
- 56. Wan E, Yeap XY, Dehn S et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 2013;113:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schloss MJ, Horckmans M, Nitz K et al. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 2016;8:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meyer IS, Jungmann A, Dieterich C et al. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. EMBO Mol Med 2017;9:1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. King KR, Aguirre AD, Ye YX et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 2017;23:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang S, Weinberg S, DeBerge M et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab 2019;29:443.e5–456.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vafadarnejad E, Rizzo G, Krampert L et al. Dynamics of cardiac neutrophil diversity in murine myocardial infarction. Circ Res 2020;127:e232–e249. [DOI] [PubMed] [Google Scholar]
- 63. Sicklinger F, Meyer IS, Li X et al. Basophils balance healing after myocardial infarction via IL-4/IL-13. J Clin Invest 2021;131:e136778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Horckmans M, Bianchini M, Santovito D et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation 2018;137:948–960. [DOI] [PubMed] [Google Scholar]
- 65. Bianchini M, Duchene J, Santovito D et al. PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci Immunol 2019;4:eaar3054. [DOI] [PubMed] [Google Scholar]
- 66. Yu X, Newland SA, Zhao TX et al. Innate lymphoid cells promote recovery of ventricular function after myocardial infarction. J Am Coll Cardiol 2021;78:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Calcagno DM, Ng RP Jr, Toomu A et al. The myeloid type I interferon response to myocardial infarction begins in bone marrow and is regulated by Nrf2-activated macrophages. Sci Immunol 2020;5:eaaz1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sano S, Oshima K, Wang Y et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deniset JF, Belke D, Lee WY et al. Gata6(+) pericardial cavity macrophages relocate to the injured heart and prevent cardiac fibrosis. Immunity 2019;51:131.e5–140.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sicklinger F, Zhang Y, Lavine KJ et al. A minimal-invasive approach for standardized induction of myocardial infarction in mice. Circ Res 2020;127:1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma Y, Yabluchanskiy A, Iyer RP et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 2016;110:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Puhl SL, Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution? Front Cardiovasc Med 2019;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Calcagno DM, Zhang C, Toomu A et al. SiglecF(HI) marks late-stage neutrophils of the infarcted heart: a single-cell transcriptomic analysis of neutrophil diversification. J Am Heart Assoc 2021;10:e019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pfirschke C, Engblom C, Gungabeesoon J et al. Tumor-promoting Ly-6G(+) SiglecF(high) cells are mature and long-lived neutrophils. Cell Rep 2020;32:108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Engblom C, Pfirschke C, Zilionis R et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 2017;358:eaal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Matsui M, Nagakubo D, Satooka H, Hirata T. A novel Siglec-F(+) neutrophil subset in the mouse nasal mucosa exhibits an activated phenotype and is increased in an allergic rhinitis model. Biochem Biophys Res Commun 2020;526:599–606. [DOI] [PubMed] [Google Scholar]
- 77. Khoyratty TE, Ai Z, Ballesteros I et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat Immunol 2021;22:1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Evrard M, Kwok IWH, Chong SZ et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 2018;48:364.e8–379.e8. [DOI] [PubMed] [Google Scholar]
- 79. Dinh HQ, Eggert T, Meyer MA et al. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity 2020;53:319.e6–334.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zouggari Y, Ait-Oufella H, Bonnin P et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yan X, Anzai A, Katsumata Y et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 2013;62:24–35. [DOI] [PubMed] [Google Scholar]
- 82. Anzai A, Anzai T, Nagai S et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation 2012;125:1234–1245. [DOI] [PubMed] [Google Scholar]
- 83. Hofmann U, Beyersdorf N, Weirather J et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 2012;125:1652–1663. [DOI] [PubMed] [Google Scholar]
- 84. Benezech C, Luu NT, Walker JA et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol 2015;16:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tahir S, Steffens S. Nonclassical monocytes in cardiovascular physiology and disease. Am J Physiol Cell Physiol 2021;320:C761–C770. [DOI] [PubMed] [Google Scholar]
- 86. Kyaw T, Loveland P, Kanellakis P et al. Alarmin-activated B cells accelerate murine atherosclerosis after myocardial infarction via plasma cell-immunoglobulin-dependent mechanisms. Eur Heart J 2021;42:938–947. [DOI] [PubMed] [Google Scholar]
- 87. Lorenzo C, Delgado P, Busse CE et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature 2021;589:287–292. [DOI] [PubMed] [Google Scholar]
- 88. Heinrichs M, Ashour D, Siegel J et al. The healing myocardium mobilises a distinct B-cell subset through a CXCL13-CXCR5-dependent mechanism. Cardiovasc Res 2021;117:2664–2676. [DOI] [PubMed] [Google Scholar]
- 89. Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 2018;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mouton AJ, Hall JE. Novel roles of immunometabolism and nonmyocyte metabolism in cardiac remodeling and injury. Am J Physiol Regul Integr Comp Physiol 2020;319:R476–R484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jabs M, Rose AJ, Lehmann LH et al. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018;137:2592–2608. [DOI] [PubMed] [Google Scholar]
- 92. O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016;16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lantz C, Radmanesh B, Liu E, Thorp EB, Lin J. Single-cell RNA sequencing uncovers heterogenous transcriptional signatures in macrophages during efferocytosis. Sci Rep 2020;10:14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DeBerge M, Lantz C, Dehn S et al. Hypoxia-inducible factors individually facilitate inflammatory myeloid metabolism and inefficient cardiac repair. J Exp Med 2021;218:e20200667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ridker PM, Everett BM, Thuren T et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 96. Tardif JC, Kouz S, Waters DD et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 97. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021;21:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Priem B, Tian C, Tang J, Zhao Y, Mulder WJ. Fluorescent nanoparticles for the accurate detection of drug delivery. Expert Opin Drug Deliv 2015;12:1881–1894. [DOI] [PubMed] [Google Scholar]
- 99. Tang J, Pérez-Medina C, Zhao Y, Sadique A, Mulder WJM, Reiner T. A comprehensive procedure to evaluate the in vivo performance of cancer nanomedicines. J Vis Exp 2017;121:55271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Duivenvoorden R, Senders ML, van Leent MMT et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat Rev Cardiol 2019;16:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Calcagno C, Pérez-Medina C, Mulder WJM, Fayad ZA. Whole-body atherosclerosis imaging by positron emission tomography/magnetic resonance imaging: from mice to nonhuman primates. Arterioscl Thromb Vasc Biol 2020;40:1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Krohn-Grimberghe M, Mitchell MJ, Schloss MJ et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat Biomed Eng 2020;4:1076–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tang J, Baxter S, Menon A et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci 2016;113:E6731–E6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Senders ML, Meerwaldt AE, van Leent MMT et al. Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging. Nat Nanotechnol 2020;15:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nahrendorf M, Frantz S, Swirski FK et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 2015;65:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stiekema LCA, Willemsen L, Kaiser Y et al. Impact of cholesterol on proinflammatory monocyte production by the bone marrow. Eur Heart J 2021;42:4309–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]