Abstract
Sordarin derivatives represent a new class of antifungal agents that act as potent inhibitors of fungal protein synthesis and possess a broad spectrum of activity. The in vivo activity of GM193663 and GM237354 was studied in mouse models of disseminated candidiasis and aspergillosis and in a rat model of pneumocystosis. The pharmacokinetic behavior of both sordarin derivatives was studied in mice and rats. In all studies, compounds were administered by the subcutaneous route. After a subcutaneous dose of 50 mg/kg of body weight to mice, the maximum level in serum, area under the concentration-time curve, half-life, and clearance for GM193663 and GM237354 were 51.8 and 23 μg/ml, 79.5 and 46 μg · h/ml, 0.8 and 0.85 h, and 21 and 25 ml/h, respectively. Systemic candidiasis and aspergillosis were established in CD-1 male mice infected with Candida albicans or Aspergillus fumigatus. For systemic candidiasis, compounds were given three times per day for seven consecutive days at 15, 30, 60, or 120 mg/kg/day. GM193663 and GM237354 showed dose-related efficacy against C. albicans, with 50% effective doses, 1 month after infection, of 25.2 and 10.7 mg/kg/dose, respectively. In experimental infections with A. fumigatus, GM237354 was given three times per day at 30, 60, or 120 mg/kg/day for five consecutive days. Animals treated with GM237354 demonstrated irregular responses. The survival of animals treated with GM237354 was 0, 30, and 0% at 30, 60, and 120 mg/kg/day, respectively. The therapeutic efficacy of GM193663 and GM237354 against Pneumocystis carinii was studied in an experimental P. carinii pneumonia (PCP) rat model. After a subcutaneous dose of 10 mg/kg given to rats, the maximum level in serum, area under the concentration-time curve, half-life, and clearance for GM193663 and GM237354 were 6.6 and 7.2 μg/ml, 8.5 and 11.8 μg · h/ml, 0.7 and 0.8 h, and 230 and 133 ml/h, respectively. To induce spontaneous PCP, rats were chronically immunosuppressed with dexamethasone. Infected animals were treated twice daily for 10 days at 0.2, 2, or 10 mg/kg/day. The therapeutic effect was estimated by the reduction in the number of cysts in the lungs of treated versus untreated animals. GM193663 and GM237354 significantly reduced the mean (± standard deviation) log number of cysts from 7.6 ± 0.2 in the untreated group to 4.7 ± 0.2 and 4.6 ± 0.1, respectively, when the drugs were administered at a dose of 2 mg/kg/day. The log number of cysts was also reduced in infected animals given lower doses of the compounds (0.2 mg/kg/day). In summary, GM193663 and GM237354 are new sordarin derivatives that may potentially play a major role in the treatment of candidiasis and PCP. Further testing with Aspergillus in other animal models is warranted.
Systemic fungal infections represent a growing challenge due to the increase in the number and life expectancy of immunosuppressed patients (16). Immunosuppression is frequently found in neutropenic patients, bone marrow and solid-organ transplant recipients, and human immunodeficiency virus-infected individuals (8, 22).
Candida spp. and especially Candida albicans constitute one of the most frequent causes of invasive fungal infection in neutropenic and solid-organ transplant patients (23). On the other hand, C. albicans is also the most frequently implicated species in oral candidiasis (4), which occurs most frequently in human immunodeficiency virus-infected patients (13). Aspergillus fumigatus causes life-threatening infections in immunocompromised patients, especially in those with hematological malignancies or aplastic anaemia or in those undergoing bone marrow transplantation (9). Pneumocystis carinii pneumonia (PCP) remains a serious opportunistic infection in patients with AIDS (14). However, treatments for fungal infections are still limited to a few agents. This situation has created a critical need for new, safe, and effective antifungal agents (5).
Sordarin derivatives are a new class of antifungal agents that target the yeast protein elongation cycle (6, 10, 11). Previously reported sordarin derivatives have demonstrated potent broad-spectrum antifungal activity in several in vitro studies (18), and earlier research indicated that sordarin derivatives possess promising activity in several animal models of infection (15).
In order to better understand the potential use of this novel class of compounds, we investigated the pharmacokinetic behavior and therapeutic properties of GM193663 and GM237354 as representatives of sordarin derivatives. To this effect, the in vivo efficacy of these new compounds has been evaluated in systemic candidiasis and aspergillosis in mice and in a pneumocystosis model in rats.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999 [A. Martinez, E. Jimenez, P. Aviles, J. Caballero, F. Gomez de las Heras, and D. Gargallo-Viola, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. abstr. 294, 1999].)
MATERIALS AND METHODS
Antifungal agents.
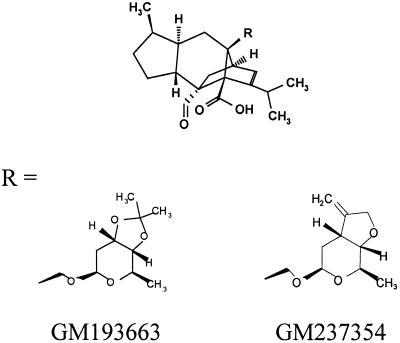
GM193663 and GM237354 (Fig. 1) were synthesized at the Glaxo Wellcome Research Centre in Madrid, Spain, and were provided as sodium salt powders. Immediately before each experiment, compounds were dissolved in sterile deionized water to reach the appropriate concentrations.
FIG. 1.
Chemical structures of sordarin derivatives.
Microorganisms.
C. albicans 4711E and A. fumigatus 48238E obtained from the Glaxo Wellcome culture collection (Glaxo Wellcome Laboratories, Greenford, United Kingdom) were used to produce lethal systemic infections in mice. PCP was induced with immunosuppression in spontaneously infected Wistar rats, as described below.
Animals.
Male CD-1 mice (age, 6 weeks; weight, approximately 25 g; Charles River France Inc., Lyon, France) were used in the pharmacokinetic studies and in the mouse protection tests. Female Wistar rats (age, 6 weeks; weight, approximately 150 g; Iffa-Credo France Inc., Lyon, France) were used in the PCP studies. These animals develop spontaneous P. carinii infection after corticosteroid treatment (1). Mice and rats were housed in cages of 10 and 5 animals per group, respectively, with food and water available ad libitum. The research complied with European legislation and with company policy on the care and use of animals and with related codes of practice.
Pharmacokinetic studies.
GM193663 and GM237354 were administered once subcutaneously at a dose of 50 and 10 mg/kg of body weight to mice and rats, respectively. In the case of mice, blood samples were taken by cardiac puncture at 0, 0.25, 0.5, 0.75, 1.5, 2, 2.5, and 3 h postadministration. Three animals were sacrificed at each sampling time by cervical dislocation. Groups of three rats each were sampled from the end of the tail (19) at 0, 0.25, 0.5, 0.75, 1.5, 2, 2.5, and 3 h postadministration. Blood samples were allowed to clot for at least 2 h, then centrifuged to obtain the serum, and finally frozen at −70°C until analysis. Concentrations of sordarin derivatives in serum were determined by the agar diffusion bioassay method, using C. albicans 2005 as the indicator organism. The medium for the bioassay was prepared by supplementing yeast nitrogen base agar (Difco, Detroit, Mich.) with 10% d-glucose (Sigma-Aldrich S.A., Madrid, Spain) and 6% sodium citrate (Merck, Darmstadt, Germany). Then, C. albicans 2005 was added to yield a final concentration of 5 × 105 CFU/ml. Supplemented yeast nitrogen base agar (100 ml) with microorganisms was poured into square plastic Nunc (Nalge Nunc International) bioassay plates (245 by 245 mm). The agar was allowed to settle to room temperature for 1 h, and 5-mm-diameter wells were cut using a 36-well template. Wells were loaded with 20 μl of fluids. Standard curves were generated from pooled mouse or rat serum using concentrations of 0.625, 1.25, 2.5, 5, and 10 μg/ml. Each standard sample was assayed in triplicate, while unknown samples were loaded in duplicate. Plates were incubated overnight at 35°C, and the inhibition zone was measured with a digital caliper (Mitutoyo Ltd., London, United Kingdom). The lower limit of detection was <0.625 μg/ml. Finally, samples were quantitatively analyzed and pharmacokinetic parameters were derived for a one-compartment model using WinNonlin version 1.1 software (Scientific Consulting, Inc., North Carolina).
In vivo antifungal activities.
Therapeutic efficacy tests were performed with the most important fungal opportunistic pathogens: C. albicans, A. fumigatus, and P. carinii.
(i) Systemic infections in mice.
For inoculation in mice, C. albicans or A. fumigatus was grown on Sabouraud dextrose agar (Difco) plates at 30°C for 48 h or on agar slants at 30°C for 5 days, respectively. After incubation, cells or conidia were harvested, washed in sterile saline, and suspended and adjusted in sterile saline to a final concentration of 107 cells per ml. The inoculum size was verified by quantitative culture of serial 10-fold dilutions on Sabouraud dextrose agar plates. Animals were infected by injection of 200 μl of the suspension into a lateral tail vein. After infection, the mice were randomized in groups of 10 for controls or for treatment with the antifungals. Compounds were administered subcutaneously three times per day (t.i.d.), starting 1 h postinfection. For systemic candidiasis, GM193663 and GM237354 were administered at doses of 15, 30, 60, and 120 mg/kg/day for seven consecutive days. For systemic aspergillosis, GM237354 was administered subcutaneously t.i.d, at doses of 30, 60, and 120 mg/kg/day for five consecutive days. Control animals received subcutaneous injections of sterile water. Morbidity and mortality in each group following infection and treatment were monitored daily for up to 30 or 14 days after challenge with C. albicans or A. fumigatus, respectively.
(ii) Pneumocystosis in rats.
PCP was established according to a previously described method (1). Briefly, animals were immunosuppressed with dexamethasone (Fortecortin; Merck Laboratories, Spain) at a concentration of 2 mg/liter in the drinking water for 9 weeks. These animals develop spontaneous P. carinii infection after corticosteroid treatment. Tetracycline (Terramicine; Pfizer Laboratories, Spain) at 1 g/liter also was added to the drinking water to minimize bacterial infections. All animals remained on immunosuppressive therapy with dexamethasone throughout the study. Before the start of treatment, two animals were sacrificed to microscopically confirm the presence of acute PCP, as previously described (20). The sordarin derivatives GM193663 and GM237354 were administered at doses of 0.2, 2, and 10 mg/kg/day by the subcutaneous route. The drugs were given twice a day for 10 consecutive days. Control animals were dosed with sterile water. Twenty-four hours after the last dose, all animals were sacrificed by an overdose of sodium pentobarbital (Euthalender; Normon, Spain). Lungs were aseptically removed and weighed. Parasite extractions were performed by means of a previously described method (1), with slight modifications. Lungs were cut into small pieces in sterile phosphate-buffered saline solution and homogenized using a Stomacher 400 blender (Pacisa S.A., Spain). Cell debris were removed by filtering the homogenate through sterile gauze. The filtrate was centrifuged at 2.900 × g for 10 min, and the pellet was resuspended in phosphate-buffered saline. Quantification of P. carinii cystic forms was performed with toluidine blue-O (Sigma-Aldrich) staining. The number of cysts was determined by visual assessment under a light microscope (20 microscopic fields). Drug efficacy against P. carinii was determined by comparing the P. carinii cyst burden of lungs in the treatment groups with those in the controls. All results were expressed as the log10 number of cysts per gram of lung.
Statistical analysis. (i) Systemic candidiasis and aspergillosis in mice.
Statistical evaluation of differences in the survival rates (Kaplan-Meier plot) for mice with invasive candidiasis or invasive aspergillosis were performed by the log rank test. This test examines the decrease in survival rates over time as well as the final percentage of survival. P values of <0.05 were considered significant in these analyses. Also, cumulative mortality was used to calculate by probit analysis the amount of drug, in milligrams per kilogram of body weight per dose, required to prevent 50% of the lethality in infected mice at the end of the experiment (ED50).
(ii) PCP in rats.
The mean log number of cysts per gram of lung in treatment groups was compared with that in the lungs of untreated controls by the Student-Newman-Keuls multiple comparison procedure. A P value of <0.05 was accepted as statistically significant.
RESULTS
The molecular structures of the new sordarin derivatives are displayed in Fig. 1. These compounds are structurally related and have different types of fused rings at position C-3′–C-4′ of the sugar moiety of the sordarin molecule. GM193663 contains a 3′, 4′-fused dioxolane ring, while GM237354 contains a 3′,4′-fused tetrahydrofurane ring with an exomethylene group.
Pharmacokinetic studies.
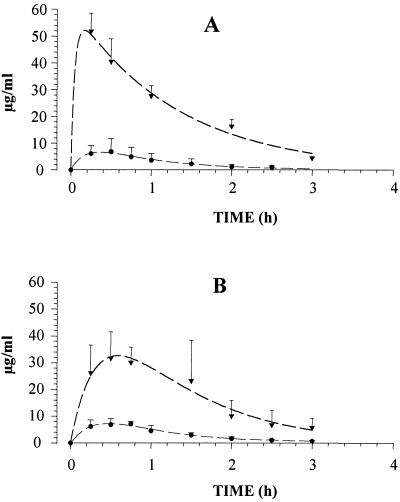
Concentrations of GM193663 and GM237354 in the serum of mice and corticosteroid-treated rats administered a single subcutaneous dose of 50 and 10 mg/kg, respectively, are shown in Fig. 2. The maximum concentration in serum (Cmax), area under the concentration-time curve for serum (AUC), elimination half-life (t1/2), and clearance (CL) for sordarin derivative compounds are shown in Table 1. In mice, the peak concentration of GM193663 (51.8 μg/ml) was twofold higher than that of GM237354 (23.0 μg/ml). The AUC was also twofold greater for GM193663 (79.5 μg · h/ml) than for GM237354 (46.0 μg · h/ml). However, similar t1/2 and CL values were obtained for both sordarin derivatives. After subcutaneous administration to immunosuppressed rats, the Cmax (6.6 and 7.2 μg/ml), AUC (8.5 and 11.8 μg · h/ml), and t1/2 (0.7 and 0.8 h) values were similar for both compounds. However, the CL of GM193663 (230 ml/h) was significantly higher than that of GM237354 (133 ml/h).
FIG. 2.
Serum profiles of GM193663 (A) and GM237354 (B) after subcutaneous administration of 50 and 10 mg of sordarin derivative per kg to mice (▾) and rats (●), respectively.
TABLE 1.
Pharmacokinetic parameters after subcutaneous administration of GM193663 and GM237354 to mice and ratsa
| Species | Compound | Dose (mg/kg) | Cmax (μg/ml) | AUC (μg · h/ml) | t1/2 (h) | CL (ml/h) |
|---|---|---|---|---|---|---|
| Mouse | GM193663 | 50 | 51.8 | 79.5 | 0.8 | 21 |
| GM237354 | 50 | 23 | 46 | 0.85 | 25 | |
| Rat | GM193663 | 10 | 6.6 | 8.5 | 0.7 | 230 |
| GM237354 | 10 | 7.2 | 11.8 | 0.8 | 133 |
Values are means for three animals.
In vivo antifungal activities.
The therapeutic efficacy of GM193663 and GM237354 was studied in mouse models of disseminated candidiasis and aspergillosis and in a rat model of pneumocystosis.
(i) Systemic candidiasis in mice.
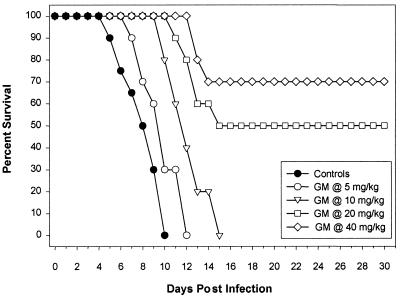
MICs of GM193663 and GM237354 for C. albicans 4711E were 0.015 and 0.001 μg/ml, respectively (E. Herreros, personal communication). C. albicans 4711E infection was lethal, with untreated control mice dying by days 5 to 10. A summary of MICs, mean and median survival times, and P values, from comparisons to the control group, for GM193663 and GM237354 against C. albicans is provided in Table 2. There was a significant improvement in survival in mice treated with sordarin derivatives at any administered dose compared to survival of untreated controls. The ED50s of sordarin derivatives were calculated from the survival rates at the end of the experiment (30 days after infection). Infections caused by C. albicans were more effectively treated with GM237354 than with GM193663. The ED50 of GM237354 was 10.7 mg/kg/dose and was at least twofold more effective than GM193663 (ED50, 25.2 mg/kg/dose). When GM237354 was administered at 60 and 120 mg/kg/day, 90 and 100% of the treated mice survived for 30 days postinfection, respectively (Fig. 3 and 4).
TABLE 2.
Efficacy of sordarin derivatives against systemic candidiasis in mice
| Compounda (MIC [μg/ml]) | Dose (mg/kg) | No. of survivors/ total no. of mice | Survival (days)b
|
||
|---|---|---|---|---|---|
| Mean ± SE | Median | Range | |||
| Control | 0 /20 | 8.1 ± 0.40 | 8.0 | 5–10 | |
| GM193663 (0.015) | 5 | 0 /10 | 9.8 ± 0.57 | 10.0 | 7–12 |
| 10 | 0 /10 | 12.2 ± 0.57 | 12.0 | 10–15 | |
| 20 | 5 /10 | 21.4 ± 2.74 | 15.0 | 11–30 | |
| 40 | 7 /10 | 25.0 ± 2.42 | 30.0 | 13–30 | |
| GM237354 (0.001) | 5 | 0 /10 | 10.1 ± 0.59 | 11.0 | 7–12 |
| 10 | 5 /10 | 20.6 ± 2.99 | 14.0 | 9–30 | |
| 20 | 9 /10 | 28.4 ± 1.52 | 30.0 | 14–30 | |
| 40 | 10 /10 | 30.0 ± 0.0 | 30.0 | 30–30 | |
Sordarin derivatives were administered subcutaneously every 8 h (t.i.d.) for seven consecutive days.
P values, comparing survival in treated and control groups by log rank test, were all <0.05.
FIG. 3.
Cumulative survival of mice infected with C. albicans and either untreated or treated with GM193663 every 8 h (t.i.d.) at 5, 10, 20, or 40 mg/kg/dose for seven consecutive days.
FIG. 4.
Cumulative survival of mice infected with C. albicans and either untreated or treated with GM237354 every 8 h (t.i.d.) at 5, 10, 20, or 40 mg/kg/dose for seven consecutive days.
(ii) Systemic aspergillosis in mice.
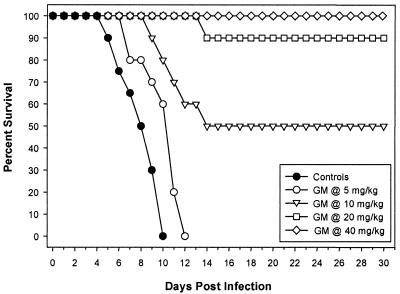
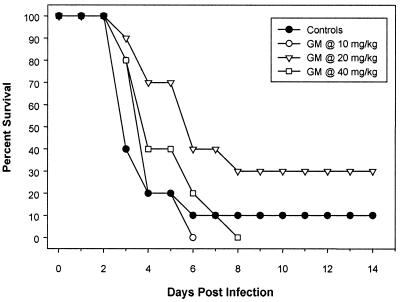
GM237354 demonstrated limited in vitro activity against A. fumigatus, and the MIC for A. fumigatus 48238E was 64 μg/ml (18). With the exception of one mouse, all mice inoculated with A. fumigatus 48238E and untreated died by day 6 after infection (Fig. 5). The survival rates of animals treated with GM237354 at 30, 60, and 120 mg/kg/day were 0, 30, and 0%, respectively. Mice treated with GM237354 at 60 mg/kg/day showed a significant improvement in survival in comparison to untreated control mice (P = 0.04); however, this was not true for mice treated with GM237354 at 30 or 120 mg/kg/day (Table 3).
FIG. 5.
Cumulative survival of mice infected with A. fumigatus and either untreated or treated with GM237354 every 8 h (t.i.d.) at 10, 20, or 40 mg/kg/dose for five consecutive days.
TABLE 3.
Efficacy of sordarin derivatives against systemic aspergillosis in mice
| Compounda (MIC [μg/ml]) | Dose (mg/kg) | No. of survivors/ total no. of mice | Survival (days)b
|
||
|---|---|---|---|---|---|
| Mean ± SE | Median | Range | |||
| Control | 1 /10 | 4.7 ± 1.12 | 3.0 | 3–15 | |
| GM237354 (64) | 10 | 0 /10 | 4.0 ± 0.21 | 4.0 | 3–5 |
| 20 | 3 /10 | 8.2 ± 1.47 | 6.0 | 3–15 | |
| 40 | 0 /10 | 4.9 ± 0.55 | 4.0 | 3–8 | |
GM237354 was administered subcutaneously every 8 h (t.i.d.) for five consecutive days.
P values, comparing survival in treated and control groups by log rank test, were 0.82, 0.04, and 0.49 for 10, 20, and 40 mg/kg, respectively.
(iii) PCP in rats.
GM193663 and GM237354 proved to be highly potent inhibitors of P. carinii protein synthesis, and both compounds showed 50% inhibitory concentrations of <0.008 μg/ml. A concentration of 0.008 μg of GM193663 or GM237354 per ml produced an inhibition of protein synthesis of 70 and 95%, respectively (18). Corticosteroid-treated rats showed physical signs of PCP (e.g., loss of weight, cyanosis, etc.) immediately before starting the antifungal treatment; however, no mortality was recorded in the control or treated groups throughout the experiment. Untreated animals showed high P. carinii infection levels before and after the treatment period, with a mean (± standard deviation) log number of cysts per gram of lung tissue of 7.6 ± 0.4 at the end of the experiment. The therapeutic effect of GM193663 and GM237354 was estimated by the reduction in the number of cysts from the lungs of treated versus untreated rats. GM193663 administered at a dose of 2 mg/kg/day significantly reduced the log number of cysts per gram to 4.7 ± 0.2 (99.9% reduction of lung cyst burden, compared to untreated control group). The therapeutic efficacy of GM193663 administered at 10 mg/kg/day (log 4.8 ± 0.3 cysts/g) was similar to that obtained with 2 mg/kg/day. A reduction in the number of cysts was also observed in infected animals treated with 0.2 mg of GM193663/kg/day, although the results were not statistically significant. GM237354 was more potent than GM193663 and showed dose-related efficacy against PCP. At a dose of 0.2 mg/kg/day, GM237354 reduced the log number of cystic forms of P. carinii to 5.8 ± 0.9 per gram (99.8% reduction). In rats administered GM237354 at 2 and 10 mg/kg/day, the cyst levels were reduced 99.98 and 99.99%, respectively, relative to those in untreated control animals. Table 4 shows the results obtained after treatment with the sordarin derivatives.
TABLE 4.
Efficacy of sordarin derivatives against pneumocystosis in rats
| Compounda | Dose (mg/kg) | Log no. of cysts/g of lungb | Reduction in log | % Reduction |
|---|---|---|---|---|
| Control | 7.6 ± 0.2 | |||
| GM193663 | 0.1 | 6.7 ± 0.9 | 0.9 | 89.81 |
| 1.0 | 4.7 ± 0.2* | 2.9 | 99.90 | |
| 5.0 | 4.8 ± 0.3* | 2.8 | 99.86 | |
| GM237354 | 0.1 | 5.8 ± 0.9* | 1.8 | 99.82 |
| 1.0 | 4.6 ± 0.1* | 3.0 | 99.98 | |
| 5.0 | 3.4 ± 0.2* | 4.2 | 99.99 |
Sordarin derivatives were administered subcutaneously every 12 h for 10 consecutive days.
*, P value of <0.05 for mean log of cysts in treated versus control group by Student-Newman Keuls multiple comparison procedure. Values are means ± standard deviations.
DISCUSSION
The growing population of immunocompromised patients receiving immunosuppressive or anticancer therapy has resulted in an increased incidence of opportunistic mycoses. Deep-seated infections due to C. albicans are an important cause of infection in the immunocompromised population, and treatment for these infections is still limited to a few agents, including several liposomal amphotericin B formulations and, mainly, azole derivative compounds (2). Invasive aspergillosis is a life-threatening infection increasingly recognized in immunocompromised patients (8, 21), and pulmonary pneumocystosis has also become problematic in certain clinical settings (14).
Sordarin derivatives belong to a new family of antifungal compounds characterized by a novel mechanism of action. Dominguez et al. have identified elongation factor 2 of C. albicans as the primary target of this new class of antifungals (10, 11). Recently, Herreros et al. demonstrated the in vitro activity of several members of this new family against a wide range of pathogenic yeasts and filamentous fungi, including P. carinii (18). Moreover, in their article, Herreros et al. reported that modifications at position 19 resulted in a marked effect on the in vitro activity of sordarin derivatives (18).
The therapeutic potential of this new family of antifungal agents has been assayed on the basis of in vitro activities, pharmacokinetic behavior, and in vivo activity, as it is well known that the final outcome of any anti-infective treatment is a consequence of in vitro activity and pharmacokinetic properties (12). GM193663 showed a MIC of 0.015 μg/ml for the C. albicans strain used in the murine model, which was 1 order of magnitude higher than the MIC of GM237354, 0.001 μg/ml. After subcutaneous administration of 50 mg/kg, pharmacokinetic studies in mice showed that GM193663 reached higher concentrations in serum than did GM237354 (51.8 and 23 μg/ml, respectively). In addition, the AUC of GM193663 was twofold higher than the AUC of GM237354 (79.5 and 46 μg · h/ml, respectively). In mice with systemic infection caused by C. albicans 4711E, the therapeutic efficacies (ED50s) of GM193663 and GM237354 were 25.2 and 10.7 mg/kg/dose, respectively. These results were consistent with the in vitro data obtained and with the different pharmacokinetic profiles of GM193663 and GM237354. The results of these studies clearly demonstrate that sordarins show in vitro and in vivo activity against C. albicans.
Sordarins also have been evaluated in invasive aspergillosis in mice. GM237354 has demonstrated limited in vitro activity against Aspergillus spp. (18) and, consequently, limited therapeutic efficacy in treating systemic aspergillosis in mice. In addition, animals treated with GM237354 demonstrated an irregular response (the survival of animals treated with GM237354 was 0, 30, and 0% at 30, 60, and 120 mg/kg/day, respectively). However, these results are consistent with results obtained in other studies, such as those obtained by Oakley et al. in a murine temporary-neutropenia model of invasive aspergillosis. The survival rates of animals treated with GM237354 in that experiment were 0, 10, 40, and 0% for animals treated with 20, 40, 80, and 160 mg/kg/day (K. L. Oakley, P. E. Verweij, G. Morrissey, J. Morrissey, and D. W. Denning, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-61, 1997). In spite of the limited anti-Aspergillus in vivo activity displayed by GM237354, sordarin derivatives have demonstrated in vitro and in vivo activities against other filamentous fungi. Clemons and Stevens recently demonstrated that sordarins (GM193663, GM211676, and GM237354) were equivalent or superior to fluconazole in the treatment of experimental systemic coccidioidomycosis in mice (7). In addition, Graybill et al. demonstrated that sordarins were effective at doses as low as 2 mg/kg in a murine model of histoplasmosis (17). Moreover, these authors noted that on a milligram-for-milligram basis, sordarins may be less potent than amphotericin B but they are more potent than fluconazole. Furthermore, sordarins can be given orally, unlike amphotericin B (17).
P. carinii remains an important pathogen in AIDS patients and other immunocompromised individuals (14). Although the combination of trimethoprim and sulfamethoxazole has been used for prophylaxis and treatment of PCP for 25 years, the high frequency of adverse reactions to these drugs and a lack of efficacy in some patients have emphasized the need for new, safe, and effective drugs. The sordarin derivatives tested, GM193663 and GM237354, were very effective in the treatment of experimental PCP in rats (2 mg/kg/day). The therapeutic efficacy showed by sordarins against P. carinii may be related to the observed high in vitro activity and pharmacokinetic properties. GM193663 and GM237354 proved to be highly potent inhibitors of P. carinii protein synthesis, with both compounds having 50% inhibitory concentrations of <0.008 μg/ml. Furthermore, good agreement between in vitro parameters and in vivo outcome has been demonstrated recently, when PCP in rats was treated with sordarin derivatives (3). In addition, the two sordarins evaluated achieved significantly higher serum drug concentrations. Subcutaneous absorption of GM193663 and GM237354 was rapid, reaching peak concentrations in serum of 6.6 and 7.2 μg/ml, respectively, with half-lives of 0.7 and 0.8 h, respectively. The activity displayed a dose-related behavior, with the highest reduction obtained when higher doses were administered.
In addition to the above considerations, the in vitro toxicity profiles of the sordarin derivatives demonstrated the low toxicity of this new family of antifungals. In fact, these results have been confirmed by preliminary rodent toxicity tests demonstrating the favorable therapeutic index of these compounds (15).
We conclude that sordarins are effective in the treatment of lethal systemic candidiasis in mice and PCP in rats and showed a limited protective effect in a murine model of lethal disseminated aspergillosis. The protective effect shown by GM193663 and GM237354 against a variety of experimental infections may be explained by integrating their in vitro antifungal activities and pharmacokinetic behaviors. Further studies to more accurately investigate the relationships between the in vitro and in vivo activities are in progress.
ACKNOWLEDGMENTS
We thank Esperanza Herreros for providing all the in vitro data, Rosaura San Román and Centro de Investigacion Farmacologica for their excellent technical assistance, and members of the Organic Chemistry Group for compound synthesis.
REFERENCES
- 1.Aliouat E, Martinez A, Jimenez E, Dei-Cas E, Mullet C, Delcourt P, Gargallo-Viola D. Development of pneumocystosis animal models: corticosteroid-treated Wistar rat; SCID mouse and nude rat. J Eukaryot Microbiol. 1997;44:41S–42S. doi: 10.1111/j.1550-7408.1997.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Therapy of deep fungal infection in haematological malignancy. Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 1997;40:779–788. doi: 10.1093/jac/40.6.779. [DOI] [PubMed] [Google Scholar]
- 3.Aviles P, Aliouat E M, Martinez A, Dei-Cas E, Herreros E, Dujardin L, Gargallo-Viola D. In vitro pharmacodynamic parameters of sordarin derivatives in comparison with those of marketed compounds against Pneumocystis carinii isolated from rats. Antimicrob Agents Chemother. 2000;44:1284–1290. doi: 10.1128/aac.44.5.1284-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi F, Najvar L, Luther M, Scalise G, Rinaldi M, Graybill J. Variation in fluconazole efficacy for Candida albicans strains sequentially isolated from oral cavities of patients with AIDS in an experimental murine candidiasis model. Antimicrob Agents Chemother. 1996;40:1317–1320. doi: 10.1128/aac.40.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett M S, Current W L, Goheen M P, Boylan C J, Lee C H, Shaw M M, Queener S F, Smith J W. Semisynthetic echinocandins affect cell wall deposition of Pneumocystis carinii in vitro and in vivo. Antimicrob Agents Chemother. 1996;40:1811–1816. doi: 10.1128/aac.40.8.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capa L, Mendoza A, Lavandera J L, Gomez de las Heras F, García-Bustos J F. Translation elongation factor 2 is part of the target for a new family of antifungals. Antimicrob Agents Chemother. 1998;42:2694–2699. doi: 10.1128/aac.42.10.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemons K V, Stevens D A. Efficacies of sordarin derivatives GM193663, GM211676, or GM237354 in a murine model of systemic coccidioidomycosis. Antimicrob Agents Chemother. 2000;44:1874–1877. doi: 10.1128/aac.44.7.1874-1877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 9.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez J M, Kelly V A, Kinsman O S, Marriott M S, Gómez de las Heras F, Martín J J. Sordarins: a new class of antifungal with selective inhibition of the protein synthesis elongation cycle in yeast. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domínguez J M, Martín J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drusano G L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont B, Dromer F, Improvisi L. The problem of azole resistance in candida. J Mycol Med. 1996;6:12–19. [Google Scholar]
- 14.Fishman J A. Prevention of infection due to Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:995–1004. doi: 10.1128/aac.42.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargallo-Viola D. Sordarins as antifungical compounds. Curr Opin Anti-Infect Invest Drugs. 1999;1:297–305. [Google Scholar]
- 16.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graybill J R, Najvar L, Fothergill A, Bocanegra R, Gomez de las Heras F. Activities of sordarins in murine histoplasmosis. Antimicrob Agents Chemother. 1999;43:1716–1718. doi: 10.1128/aac.43.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herreros E, Martinez C M, Almela M J, Marriot M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivates against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Vadgama J, Diaz T, Henry J P. Tail sectioning: a rapid and simple method for repeated blood sampling of the rat for cortisone determination. Lab Anim Sci. 1996;46:243–245. [PubMed] [Google Scholar]
- 20.Soulez B, Dei-Cas E, Palluault F, Camus D. Morphological evaluation of Pneumocystis carinii after extraction from infected lung. J Parasitol. 1991;77:449–453. [PubMed] [Google Scholar]
- 21.Stevens D A, Kan V L, Judson M A, Morrison V A, Dummer S, Denning D W, Bennett J E, Walsh T J, Patterson T F, Pankey G A. Practice guidelines for diseases caused by Aspergillus Clin. Infect Dis. 2000;30:696–709. doi: 10.1086/313756. [DOI] [PubMed] [Google Scholar]
- 22.Summers K K, Hardin T C, Gore S J, Graybill J R. Therapeutic drug monitoring of systemic antifungal therapy. J Antimicrob Chemother. 1997;40:753–764. doi: 10.1093/jac/40.6.753. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J L, Anaissie E, Bruining H, Demajo W, el-Ebiary M, Haber J, Hiramatsu Y, Nitenberg G, Nystrom P O, Pittet D, Rogers T, Sandven P, Sganga G, Schaller M D, Solomkin J. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med. 1998;24:206–216. doi: 10.1007/s001340050552. [DOI] [PubMed] [Google Scholar]