Abstract
Study objective
SARS-CoV-2 represents an occupational risk to paramedics, who work in uncontrolled environments. We sought to identify the occupation-specific risk to paramedics by comparing their seroprevalence of SARS-CoV-2 infection-specific antibodies to that of blood donors in Canada.
Methods
In this prospective cohort study, we performed serology testing (Elecsys Anti-SARS-CoV-2 nucleocapsid assay) on samples from paramedics and blood donors (January to July 2021) in Canada. Paramedic samples were compared to blood donor samples through 1:1-matched (based on age, sex, location, date of blood collection, and vaccination status) and raking weighted comparisons. We compared the seroprevalence with a risk difference (and 95% confidence interval [CI]) and performed secondary analyses within subgroups defined by vaccination status.
Results
The 1:1 match included 1,627 cases per group; in both groups, 723 (44%) were women, with a median age of 38. The raking weighted comparison included 1,713 paramedic samples and 19,515 blood donor samples, with similar characteristics. In the 1:1 match, the seroprevalence was similar (difference 1.2; 95% CI –0.20 to 2.7) between paramedics (5.2%) and blood donors (3.9%). The raking weighted comparison was consistent (difference 0.97; 95% CI –0.10 to 2.0). The unvaccinated paramedic samples, in comparison to the blood donor samples, demonstrated a higher seroprevalence in the 1:1 (difference 5.9; 95% CI 1.8 to 10) and weighted (difference 6.5; 95% CI 1.8 to 10) comparisons. Among vaccinated cases, the between-group seroprevalence was similar.
Conclusion
Overall, paramedics demonstrated similar evidence of prior SARS-CoV-2 infection to that of blood donors. However, among unvaccinated individuals, evidence of prior infection was higher among paramedics compared to blood donors.
Editor’s Capsule Summary.
What is already known on this topic
The occupational risk of COVID-19 infection to out-of-hospital clinicians is unknown.
What question this study addressed
What is the incremental risk of COVID-19 in paramedics compared to persons from the general community and does vaccination aid?
What this study adds to our knowledge
In a prospective matched cohort of 3254 equally sized groups, paramedics had a similar seroprevalence of COVID-19 antibodies as a sample of blood donors. Unvaccinated paramedics had three-fold higher seroprevalence compared to unvaccinated blood donors.
How this is relevant to clinical practice
This study did not detect a higher risk of COVID-19 to paramedics, compared to others. Vaccination may provide enhanced protection.
Introduction
Background
SARS-CoV-2, the virus that causes COVID-19, was identified as the cause of an international pandemic by the World Health Organization on March 20, 2020.1 Occupational groups that routinely and directly interact with the public in settings that are difficult to control may face an increased risk of SARS-CoV-2 infection.
Importance
Paramedics, who work in unpredictable and uncontrolled environments, are called to manage patients with medical emergencies—some of which may be COVID-19 related—in the out-of-hospital setting and, thus, represent an occupation with substantial potential risk of SARS-CoV-2 exposure. However, there are no previous data quantifying the COVID-19–related occupational risk to paramedics. Such data would inform occupational health policy.
Goals of This Investigation
We sought to estimate the incremental risk of COVID-19 incurred by paramedics due to occupational exposures by comparing the evidence of preceding SARS-CoV-2 infections among paramedics to that among individuals from the general population. We examined the seroprevalence of SARS-CoV-2 antibodies among paramedics working in Canada during the COVID-19 pandemic in comparison to a control group of Canadian blood donors with similar characteristics.
Methods
Study Design and Setting
In this prospective, observational cohort study, we compared the serologic results from samples obtained from paramedics and those obtained from the general public for blood donation, both within Canada. Canada is divided into 141 geographic regions (called forward sortation areas), shown by the first 2 digits of the postal codes.2 There were no vaccine mandates in effect in Canada at the time of this study. We designed this study and wrote the manuscript according to the “Strengthening the Reporting of Observational studies in Epidemiology” guidelines.
Paramedic samples
Paramedics’ blood samples were obtained from the COVID-19 Occupational Risks, Seroprevalence and Immunity among Paramedics in Canada study, a prospective, observational cohort of adult paramedics in Canada (enrollment commenced January 2021), approved by the University of British Columbia (H20-03620) and University of Toronto (#40435) Research Ethics Boards. The COVID-19 Occupational Risks, Seroprevalence and Immunity among Paramedics in Canada study was designed with the primary aim of measuring infection-induced SARS-CoV-2 seroprevalence among Canadian paramedics and estimating the occupation-related risks of COVID-19. Adult individuals (≥19 years) working as paramedics in the Canadian provinces of British Columbia, Alberta, Saskatchewan, Manitoba, and Ontario were eligible. Paramedics were invited to participate through study communications from their employers or unions and through social media and enrolled and consented on an online platform. Participants completed web-based sociodemographic and health questionnaires, including vaccination status and history of nucleic acid amplification test-confirmed SARS-CoV-2 infection, and provided blood samples. Paramedic participants who had provided blood samples at the time of this analysis were eligible for this study; we excluded any sample that was collected within 3 weeks of a COVID-19 diagnosis.
Blood donor samples
Blood samples from each routine blood donation were collected by Canadian Blood Services in order to create a prospective, observational cohort, as approved by the Canadian Blood Services Research Ethics Board (2021.024). Canadian Blood Services is the single blood donation operator in Canada for all provinces except Quebec. Individuals are eligible to donate blood if they are well and do not have risk factors for blood-transmissible infections, such as HIV. Donors provided sociodemographic information at registration for donation by completing a health history questionnaire including SARS-CoV-2 infections and vaccination. Blood donations were deferred from individuals with SARS-CoV-2 infections until a 2-week asymptomatic period was complete or 3 weeks after hospitalization. All blood donation samples from January to July 2021 were eligible for this analysis.
Selection of Participants
For this analysis, we considered all samples that were collected between January and July 2021 (inclusive). Cases missing data that were required for matching were excluded. We performed 2 separate comparisons. First, for a 1:1-matched comparison, we selected participants by matching blood donor control samples to paramedic samples using 1:1 matching (without replacement). Optimal matches were obtained using exact matching according to sex, age, forward sortation area region, blood collection month, and vaccination status. Second, for a raking weighted comparison, blood donor control samples were chosen in order to achieve the same characteristic frequencies as paramedic participants with respect to age, sex, forward sortation area region, month of blood collection, and vaccination status.3 In the primary analysis, we matched samples, not participants; thus, participants who provided more than one sample (collected at different time junctures during the study) were not restricted from being represented in the analysis more than once; a sensitivity analysis examined only samples from unique participants.
Measurements
All plasma samples from both groups were tested using the Elecsys Anti-SARS-CoV-2 nucleocapsid (Roche) assay, which has demonstrated 97.2% (95% confidence interval [CI] 95.4 to 98.4) sensitivity and 99.8% (95% CI 99.3 to 100) specificity for classifying preceding SARS-CoV-2 infections.4 Nucleocapsid antibodies are produced as part of the immune response to COVID-19 but not from COVID-19 vaccinations currently available in Canada. Testing for all samples was undertaken as per manufacturer instructions at a Canadian Blood Services laboratory facility by medical laboratory technologists.
Outcomes
The primary outcome measure was a reactive Elecsys nucleocapsid test (binary outcome).
Analysis
We performed analyses using SAS (SAS Institute Inc). In our primary analysis, we compared the proportion of reactive Elecsys nucleocapsid tests between groups in the 1:1-matched comparison and calculated the risk difference and corresponding 95% CI. We performed several a priori-planned secondary and sensitivity analyses. First, we compared the outcomes between paramedic samples and a raking weighted control group of blood donors.3 We then repeated these analyses within subgroups defined by whether the sample was taken from an individual who had been previously vaccinated.
Whereas in the primary analysis, we allowed a participant to be represented by more than one sample, in a sensitivity analysis, we repeated the primary and secondary analyses after excluding samples that resulted in participants being represented more than once.
For our sample size calculation, we sought to identify a seroprevalence difference of 2.0% or higher. In order to detect this difference, assuming a 3.0% seroprevalence of the general population and a 5.0% seroprevalence of the paramedic population, we required 1,506 individuals per group.5 , 6
Results
Characteristics of Study Subjects
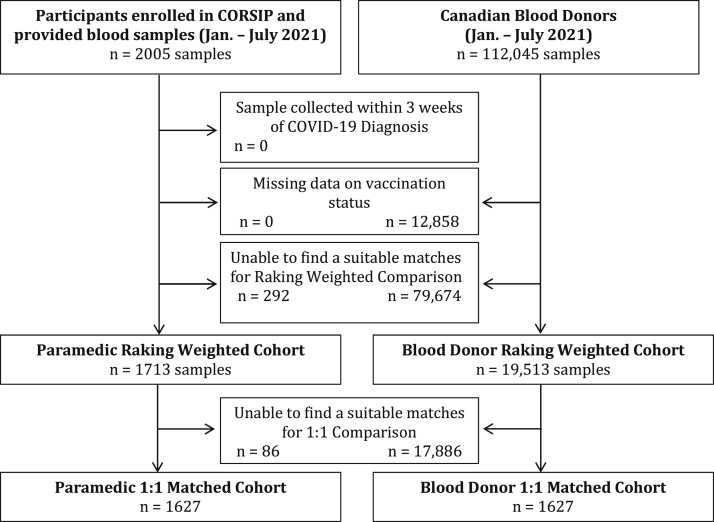
There were 2,005 paramedic samples (1,459 unique participants provided first samples [32 from Alberta, 661 from British Columbia, 52 from Manitoba, and 714 from Ontario], 532 also provided second samples, and 14 also provided third samples). Of 112,045 blood donor samples, 12,858 were excluded due to missing data on vaccination and 99,187 were assessed for matching (Figure ).
Figure.
Study flow. CORSIP, COVID-19 Occupational Risks, Seroprevalence and Immunity among Paramedics; n, number.
For the 1:1-matched comparison, we matched 1,627 paramedic samples to blood donor samples, and for the ranking weighted comparison, we compared 1,713 paramedic samples to 19,513 blood donor samples. Table 1 shows the participant characteristics and dates of blood collection, which were similar between groups. Approximately half of the cases were women, and more than 90% were between the ages of 25 and 59.
Table 1.
Participant characteristics.
| Participant Characteristic | 1:1 Comparison |
Raking Weighted Comparison |
||
|---|---|---|---|---|
| Paramedic Samples (n=1627) | Blood Donor Samples (n=1627) | Paramedic Samples (n=1713) | Blood Donor Samples (n=19,513) | |
| Women, n (%) | 723 (44) | 723 (44) | 743 (43) | 84634 (43) |
| Age category, n (%) | ||||
| 17-24 years | 48 (3.0) | 48 (3.0) | 49 (2.9) | 558 (2.9) |
| 25-39 years | 848 (52) | 848 (52) | 910 (53) | 10,366 (53) |
| 40-59 years | 672 (41) | 672 (41) | 694 (41) | 7,905 (41) |
| 60 years | 59 (3.6) | 59 (3.6) | 60 (3.5) | 683 (3.5) |
| Location, n (%) | ||||
| British Columbia | 710 (44) | 710 (44) | 773 (45) | 8,806 (40) |
| Alberta | 45 (2.8) | 45 (2.8) | 45 (2.6) | 513 (2.6) |
| Saskatchewan | 0 | 0 | 0 | 0 |
| Manitoba | 49 (3.0) | 49 (3.0) | 49 (2.9) | 558 (2.9) |
| Ontario | 823 (51) | 823 (51) | 846 (49) | 9,638 (49) |
| Blood collection month, n (%) | ||||
| January-February | 199 (12) | 199 (12) | 230 (13) | 2,620 (13) |
| March-April | 559 (34) | 559 (34) | 596 (35) | 6,789 (35) |
| May-June | 288 (18) | 288 (18) | 289 (17) | 3,292 (17) |
| July | 581 (36) | 581 (36) | 598 (35) | 6,812 (35) |
| COVID-19 vaccinated, n (%) | 1,340 (82) | 1,340 (82) | 1,423 (83) | 16,210 (83) |
Main Results
Overall, we did not detect a significant difference in the nucleocapsid antibody seroprevalence between paramedic participants and blood donors in the 1:1 (5.2% versus 3.9%; risk difference 1.2; 95% CI –0.20 to 2.7) or raking weighted (5.0% versus 4.1%; risk difference 0.97; 95% CI –0.10 to 2.0) comparisons (Table 2 ). Unvaccinated paramedic participants, in comparison to blood donors, demonstrated a higher seroprevalence for nucleocapsid antibodies in the 1:1 (9.8% versus 3.8%; risk difference 5.9; 95% CI 1.8 to 10) and raking weighted (10% versus 3.5%; risk difference 6.5; 95% CI 1.8 to 10) comparisons. Among the vaccinated participants, we did not detect a between-group difference in the 1:1 (4.2% versus 4.0%; risk difference 0.22; 95% CI –1.3 to 1.7) or raking weighted (4.0% versus 4.2%; risk difference –0.16; 95% CI –1.2 to 0.91) comparisons.
Table 2.
Comparison of nucleocapsid serology results, overall and stratified by vaccination status.
| Nucleocapside Assay Results | 1:1-Matched Comparison |
Raking Weighted Comparison |
||||
|---|---|---|---|---|---|---|
| Paramedic Samples | Blood Donor Samples | Risk Difference (95% CI) | Paramedic Samples | Blood Donor Samples | Risk Difference (95% CI) | |
| Reactive nucleocapsid assay, n (%) | 84/1,627 (5.2) | 64/1,627 (3.9) | 1.2 (–0.20 to 2.7) | 86/1,713 (5) | 790/19,513 (4.1) | 0.97 (–0.10 to 2.0) |
| Vaccinated, n (%) | 56/1,340 (4.2) | 53/1,340 (4.0) | 0.22 (–1.3 to 1.7) | 57/1,421 (4) | 672/16,188 (4.2) | –0.16 (–1.2 to 0.91) |
| Unvaccinated, n (%) | 28/287 (9.8) | 11/287 (3.8) | 5.9 (1.8–10)∗ | 29/290 (10) | 116/3,303 (3.5) | 6.5 (1.8–10)∗ |
Indicates a significant risk difference.
The sensitivity analyses including only unique participants demonstrated results similar to those of the primary and secondary analyses (Table 3 ).
Table 3.
Comparison of nucleocapsid serology results among unique participants, overall and stratified by vaccination status.
| Nucleocapside Assay Results | 1:1-Matched Comparison |
Raking Weighted Comparison |
||||
|---|---|---|---|---|---|---|
| Paramedic Samples | Blood Donor Samples | Risk Difference (95% CI) | Paramedic Samples | Blood Donor Samples | Risk Difference (95% CI) | |
| Reactive nucleocapsid assay, n (%) | 64/1,183 (5.4) | 47/1,183 (4.0) | 1.4 (–0.27 to 3.1) | 66/1,256 (5.3) | 643/15,415 (4.2) | –1.0 (–0.19 to 2.4) |
| Vaccinated, n (%) | 36/901 (4.0) | 37/901 (4.1) | –0.11 (–1.9 to 1.7) | 38/971 (3.9) | 508/11,914 (4.3) | –0.35 (–1.6 to 0.92) |
| Unvaccinated, n (%) | 28/282 (9.9) | 10/282 (3.5) | 6.4 (2.3–10)∗ | 28/285 (9.8) | 135/3,501 (3.9) | 5.98 (2.5-9.5)∗ |
Indicates a significant risk difference.
Limitations
We sought to compare the seroprevalence of SARS-CoV-2 among paramedics to that among similar individuals from the general population using a large national serosurvey of blood donors. However, blood donors may be healthier than the general population and may be less representative of socioeconomically deprived populations at higher risk of COVID-19, thereby posing a risk of type I error in our analysis; in fact, however, we did not detect an overall seroprevalence difference. Blood donors have been used to monitor SARS-CoV-2 seroprevalence around the world and have demonstrated seroprevalence rates similar to those in studies with other general population samples.7, 8, 9 In Canada, a national serosurvey reported seroprevalence results similar to Canadian Blood Services data.6 , 10 , 11 Paramedics in Canada use personal protective equipment and follow occupational safety guidelines as protection from SARS-CoV-2; results may have differed if we had compared the seroprevalence in paramedics working without these protocols. Criteria for blood donation eligibility are not the same as those for eligibility for paramedic employment, which may have affected our results.
We did not have data on the occupational characteristics of blood donors; it is possible that paramedics, as well as other health care professionals, were also represented within the blood donor group; however, this would be expected in a sampling of the general population. We used serologic testing for this analysis, which has advantages of identifying asymptomatic infections; however, it is unable to determine dates of COVID-19 and their relation to vaccination. It is possible that participants (from both groups) with history of COVID-19 may have delayed vaccination. We did not have data on the dates of vaccination for blood donors, and it is possible that there were between-group imbalances with vaccination timing. It is possible that vaccinated blood donors may belong to high-risk groups for contracting COVID-19, such as health care workers, and thus may have been prioritized for vaccination in 2021. If so, this may have increased the risk of type II error in our vaccinated subgroup comparison. This study was performed in the first half of 2021; the data may not be applicable to variants prevalent during other time periods. Our power calculation used the full cohort, and, thus, the subgroup analyses may have been underpowered. The Elecsys Anti-SARS-CoV-2 nucleocapsid assay has demonstrated high sensitivity (97.2%) and specificity (99.8%) in identifying previous SARS-CoV-2 infection4; although some cases may have been misclassified, the proportions would likely have been similar between groups. Our results may differ from other communities with variable prevalence of SARS-CoV-2.
Discussion
In this prospective cohort study, we compared serology samples from paramedics working during the COVID-19 pandemic to those from blood donors in Canada. Overall, we found that paramedics had similar seroprevalence to comparable blood donors from the general population. However, among unvaccinated individuals, the proportion of paramedics with evidence of COVID-19 was nearly 3 times that of blood donors. These study results are among the first to provide seroprevalence estimates in paramedics, a group with a presumed high occupational risk of COVID-19. Vaccination may mitigate this risk; significantly higher seroprevalence estimates were found in unvaccinated paramedics compared with matched unvaccinated blood donors.
The transmission of SARS-CoV-2 occurs through close contact with infected people, through infectious respiratory aerosols and droplets. Paramedics provide out-of-hospital medical assessment and treatment, often with incomplete information on patient medical histories and previous testing, regularly in small private residences or in difficult-to-control public settings, and transport patients in confined spaces. Beyond the assessment of stable patients, paramedics often treat unstable patients with cardiac and respiratory interventions that may further increase their risk through procedures that may lead to aerosol generation, such as airway maneuvers and chest compressions. The International Liaison Committee on Resuscitation has identified the risks to rescuers providing out-of-hospital and resuscitative interventions in the COVID-19 pandemic as a key knowledge gap, with data needed to inform international guidelines. Although we did not specifically examine medical procedures in this study, overall, we did not find that paramedics had significantly higher seroprevalence of SARS-CoV-2 than matched blood donors.
The higher rate of seroprevalence of COVID-19–specific antibodies among unvaccinated paramedics in comparison to unvaccinated blood donors suggests that—despite infection-prevention and control measures—paramedics face increased occupational COVID-19–related risk. While this appears to be mitigated or eliminated with vaccination, there are implications for future pandemics and for new, vaccine-resistant SARS-CoV-2 strains. These results support the importance of vaccination among paramedics and other health care workers. Currently, particularly in settings without mandatory vaccine policies, unvaccinated paramedics may benefit from more stringent personal protective equipment policies.
With just less than one quarter of our paramedic cohort remaining unvaccinated at the time of data collection, our study raises questions of vaccine hesitancy. While we did not explore reasons for nonvaccination, the proportion of unvaccinated individuals was similar to that reported in the United States.12 , 13 A study from Germany reported that 57% of emergency medical services personnel voiced “willingness to be vaccinated.”14 Our data showing that the COVID-19 incidence tended to be higher among unvaccinated compared to vaccinated paramedics may be affected by other non–vaccine-related behavioral factors, such as other COVID-19–related precautions in nonoccupational settings. However, assuming that paramedics and nonparamedics would be affected by this in similar ways, one would then expect nonparamedics to also demonstrate higher proportions of COVID-19 among the unvaccinated, which was not apparent.
To our knowledge, no previous studies have compared the risk of COVID-19 among paramedics to that among the general population. Brown et al15 examined the risk of COVID-19 (using test results from an administrative database) among emergency medical service providers based on occupational COVID-19 exposures and reported a very low overall risk attributed to patient with COVID-19 encounters. A single-site study of 203 emergency medical services personnel in Florida reported seroprevalence results; however, the findings were limited by an assay with poor performance.16 Several studies have examined the correlation between the health care worker occupation and COVID-19 risk. Mutambudzi et al17 examined the association between occupational group and the risk of a hospital-based COVID-19 diagnosis in the United Kingdom. They reported that health care workers had a 7-times–higher risk than “nonessential” workers. This was consistent with the results from a study of the first and second COVID-19 waves in Norway.18 To mitigate the risks of COVID-19, health care workers have relied on increased attention to personal protective equipment and policies to reduce the potential exposure from aerosol-generating procedures. A large study from the United Kingdom and United States found that front-line health care workers had an 11-times–increased risk of a positive COVID-19 test; however, they reported that adequate personal protective equipment likely diminished this risk.19 Our study, which examined participants within the vaccine era, suggests that the COVID-19–related risk to health care workers may be attenuated with vaccination.
In conclusion, overall, paramedics demonstrated similar evidence of prior COVID-19 to that of blood donors. However, among unvaccinated individuals, the evidence of prior COVID-19 was significantly higher among paramedics.
Acknowledgments
We would like to acknowledge the contributions of Qi-Long Yi PhD (Canadian Blood Services) for statistical analyses, all participating paramedics, Tara Martin BSc, Christopher MacDonald MScCH, Dong Vo BEng, Yann Charles Lafontant, Richard Armour MSc MParamedicine AACPara, Scott Haig PCP, Jeff Maxim ACP, Cheryl Cameron MEd ACP, Troy Clifford PCP and Ambulance Paramedics of BC, Dave Deines PCP and the National Paramedics Association of Canada, Justin Yap, Veronica Chow MHA, Sheldon Cheskes MD, Jim Christenson MD, Ryan Sneath RN ACP, Jennifer Bolster ACP, Nechelle Wall ACP, Heba Qazilbash, Brian Twaites ACP, Sandra Jenneson MD, Robert Schlamp CCP, Ashley Curtis BA, Chelsie Osmond PCP, Suzanne Vercauteren MD PhD and the BC Children’s Hospital Biobank, and all participating paramedic services and unions.
Footnotes
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article.
A podcast for this article is available at www.annemergmed.com.
Please see page 39 for the Editor’s Capsule Summary of this article.
Supervising editor: Jane H. Brice, MD, MPH. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: BG, TK, PD, JS, JH, ID, and DG obtained funding for the paramedic observational cohort. SOB and SD obtained funding for the blood donor observational cohort. BG and DG conceived the study idea. BG, DG, and SOB designed the protocol with input from all authors. BG, DG, SOB, TK, PD, SD, and DON collected samples and data. SOB, BG, QLY, and MAB performed statistical analyses. BG drafted the manuscript. All authors contributed to interpretation of data, manuscript revision and final approval. BG takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria:(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fundingandsupport: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org).
SD has acted as a content expert for respiratory viruses for Johnson & Johnson (Janssen).
Supported by funding from the Government of Canada through the COVID-19 Immunity Task Force.
References
- 1.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.Statistics Canada Population and Dwelling Count Highlight Tables, 2016 Census. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/pd-pl/comprehensive.cfm
- 3.Kalton G., Flores-Cervantes I. Weighting methods. J Off Stat. 2003;19:81–97. [Google Scholar]
- 4.Ainsworth M., Andersson M., Auckland K., et al. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Science M., Bolotin S., Silverman M., et al. SARS-CoV-2 antibodies in Ontario health care workers during and after the first wave of the pandemic: a cohort study. CMAJ Open. 2021;9:E929–E939. doi: 10.9778/cmajo.20210044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 Immunity Task Force Canadian seroprevalence estimates from Canadian Blood Services, May 2020 to January 2021. https://www.covid19immunitytaskforce.ca/canadian-seroprevalence-estimates-from-canadian-blood-services-may-2020-to-january-2021/
- 7.O’Brien S.F., Lieshout-Krikke R.W., Lewin A., et al. Research initiatives of blood services worldwide in response to the covid-19 pandemic. Vox Sang. 2021;116:296–304. doi: 10.1111/vox.12995. [DOI] [PubMed] [Google Scholar]
- 8.Castro Dopico X., Muschiol S., Christian M., et al. Seropositivity in blood donors and pregnant women during the first year of SARS-CoV-2 transmission in Stockholm, Sweden. J Intern Med. 2021;290:666–676. doi: 10.1111/joim.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant R., Dub T., Andrianou X., et al. SARS-CoV-2 population-based seroprevalence studies in Europe: a scoping review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Canada Table 3: provincial or regional SARS-CoV-2 antibody seroprevalence estimates, by antibody seroprevalence type. https://www150.statcan.gc.ca/n1/daily-quotidien/210706/t003a-eng.htm
- 11.COVID-19 Immunity Task Force Latest Canadian Blood Services data show improvements in vaccine uptake and equity in vaccine coverage. https://www.covid19immunitytaskforce.ca/latest-canadian-blood-services-data-show-improvements-in-vaccine-uptake-and-equity-in-vaccine-coverage/
- 12.Pacella-LaBarbara M.L., Park Y.L., Patterson P.D., et al. COVID-19 vaccine uptake and intent among emergency healthcare workers: a cross-sectional. J Occup Environ Med. 2021;63:852–856. doi: 10.1097/JOM.0000000000002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory ME, Powell JR, MacEwan SR, et al. COVID-19 vaccinations in EMS professionals: prevalence and predictors. Prehospital Emerg Care. Published online November 3, 2021. https://doi.org/10.1080/10903127.2021.1993391 [DOI] [PMC free article] [PubMed]
- 14.Nohl A., Afflerbach C., Lurz C., et al. Acceptance of COVID-19 vaccination among front-line health care workers: a nationwide survey of emergency medical services personnel from Germany. Vaccines. 2021;9:424. doi: 10.3390/vaccines9050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown A., Schwarcz L., Counts C.R., et al. Risk for acquiring coronavirus disease illness among emergency medical service personnel exposed to aerosol-generating procedures. Emerg Infect Dis. 2021;27:2340–2348. doi: 10.3201/eid2709.210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caban-Martinez A.J., Schaefer-Solle N., Santiago K., et al. Epidemiology of SARS-CoV-2 antibodies among firefighters/paramedics of a US fire department: a cross-sectional study. Occup Environ Med. 2020;77:857–861. doi: 10.1136/oemed-2020-106676. [DOI] [PubMed] [Google Scholar]
- 17.Mutambudzi M., Niedwiedz C., Macdonald E.B., et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78:307–314. doi: 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnusson K., Nygård K., Methi F., et al. Occupational risk of COVID-19 in the first versus second epidemic wave in Norway, 2020. Euro Surveill. 2021;26:2001875. doi: 10.2807/1560-7917.ES.2021.26.40.2001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]