Abstract
Malnutrition has been one of the most common complications of older COVID-19 survivors. COVID-19 associated symptoms like loss of appetite as well as changes in taste and smell may trigger the deterioration of nutritional status, while other complications of the disease may contribute to it, like respiratory failure that necessitates admission to the ICU. Especially in nursing home residents reduced food intake may be related to preexisting and also to incident geriatric syndromes like delirium. Sarcopenia has also been highly prevalent in older COVID-19 survivors. It is caused and exacerbated by COVID-19-associated inflammatory processes, total or partial immobilization, and malnutrition. COVID-19 survivors may be at high risk of developing the vicious circle that results from the interaction of deteriorating nutritional status and declining functionality. Regular monitoring of nutritional and functional status is, therefore, indicated in all older COVID-19 survivors. If malnutrition and/or functional decline have been identified in this patient population, low-threshold provision of individualized nutritional and exercise interventions should be installed. In those that are most seriously affected by malnutrition and sarcopenia ambulatory or inpatient rehabilitation has to be considered. Geriatric rehabilitation programs should be specifically adapted to the needs of older patients with COVID-19.
Keywords: Sarcopenia, Malnutrition, COVID-19 survivors, Long COVID, Nursing home
Key points
-
•
Malnutrition and sarcopenia are both highly prevalent among older COVID-19 survivors.
-
•
Admission to intensive care in older hospital patients and delirium in nursing home residents are negative predictors for both syndromes.
-
•
Routine monitoring of nutritional and functional status is indicated in all older COVID-19 survivors.
-
•
Individually adapted geriatric rehabilitation should be provided especially for those that are at risk for loss of independence.
The observation that nutritional status and functionality are deeply intertwined in older persons holds especially true in the context of the COVID-19 pandemic. One of the key symptoms of infections with the SARS-CoV-2 virus has been its negative impact on taste and smell thereby significantly decreasing the attractiveness of adequate food intake in infected older individuals.1 As this sensory deficit may persist for several months the nutritional status of older patients with post–COVID-19 may be severely impaired. In acute hospital patients, the risk of malnutrition has been linked to the severity of the infection, while in the subacute and chronic stage of the disease inadequate food intake may also be seen in patients that are not affected by a serious course of the disease. Inadequate intake of calories and protein has been established as one of the main etiologic factors of sarcopenia, that in patients with COVID-19 often combines with the deleterious effects of immobilization and inflammation. For these reasons, functional status and overall prognosis may be negatively affected in older COVID-19 survivors. With this article, we will provide some additional insight into the specifics of this patient population.
Malnutrition in different health care settings
Hospital and Ambulatory Setting During Acute Disease
During the acute stage of the disease each of 407 Dutch patients with COVID-19 hospital (mean age 64y) had at least one nutritional complaint (Box 1 ).2 Among those were decreased appetite in 58% and the feeling of being full in 49%. One in 3 patients experienced a change in taste, loss of taste, and/or loss of smell. In this study, 35% of the patients were diagnosed as being malnourished.
Box 1. Nutrition-related complaints among hospital patients with COVID-19.
Decreased appetite and loss of appetite
Feeling of being full
Change in taste
Loss of taste
Loss of smell
Tiredness (assistance with eating required)
Swallowing difficulties and/or dysphagia
Nausea
Vomiting
Chewing difficulties
Pain in the oral cavity
Adapted from Wierdsma NJ, Kruizenga HM, Konings LA, et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021;43:369–376. https://doi.org/10.1016/j.clnesp.2021.03.021; with permission
With regard to the subacute stage of the disease, it was shown in a French study that 30 days after hospital discharge (D30) 33.3% of respondents (mean age 60 years) were malnourished with 13.2% fulfilling the criteria of severe malnutrition and 20.1% fulfilling those of moderate malnutrition3 , 4. In this study, severe malnutrition was defined as weight loss > 10% compared to the weight before COVID-19 or a BMI less than 17 kg/m2 (<18.5 for patients >70 year old). Moderate malnutrition was defined as weight loss > 5% compared to the weight before COVID-19 or BMI less than 18.5 kg/m2 (<21 for patients >70 year old). 23.2% had been malnourished at discharge, but were no longer malnourished at D30. A higher risk for persistent malnutrition was associated with admission to ICU, subjective functional loss at discharge, and male sex. In this study, it was also shown that subjective functional loss was present in 76.8% of patients at discharge and in 26.2% still at D30.
Additional follow-up data for the above patient population were published by Gérard and colleagues.5 Six months after discharge 36% (43/119) of respondents fulfilled the criteria for persistent malnutrition, 14.3% (17/119) described persistent subjective functional loss, and 14.9% (18/119) a reduced performance status. Obesity and admission to intensive care were both identified as risk factors for persistent functional impairments.
In the above-cited study by Wierdsma and colleagues, appetite reduction was still evident in 30% of patients 3 to 5 months after discharge. In this population, severity of illness and admission to intensive care were associated with the persistence of complaints.2
Taking into account the persistent nature of malnutrition in patients with COVID-19 that were admitted to hospital during the acute course of disease, it will be indicated to routinely assess their nutritional status before discharge and to monitor it regularly during the first post–COVID-19 year. Nutritional interventions should be started early after diagnosis of malnutrition and preventive measures should be initiated in those that are at high risk for developing it during the course of the disease, especially in those patients that are admitted to intensive care. There have been first indications that this approach may allow for good results in younger patient populations.6 At the moment it remains to be proven to what extent these positive results can be reproduced in older multimorbid patients with COVID-19.
The Nursing Home Setting
In a US study that focused on nursing home residents, it was shown that early during an outbreak of COVID-19 17.9% showed symptoms of anorexia and that this percentage increased to 70.8% after 21 days.7 This increase of anorexia symptoms was paralleled by a significant increase of delirium in patients with COVID-19, which increased from 18.6% to 56% during the same period. Therefore, it may be reasoned that the presence of delirium aggravated anorectic symptoms in this patient population. However, it should also be considered that insufficient nutritional intake may have triggered delirium. This two-way relationship has already been addressed in two recent articles.8 , 9 Considering the above the presence of delirium warrants special consideration in the context of malnutrition in older nursing home patients with COVID-19.
Focusing on the subacute course of COVID-19 in older nursing home residents, Martinchek et all demonstrated that there was a mean weight loss of 4.6% (3.5 kg) in patients with COVID-19 2 months after the onset of COVID-19-related symptoms.10
It also seems important to mention that preventive restrictions during a local outbreak10 and general contact restrictions for nursing homes during the pandemic11 can have a negative impact on the nutritional status including weight loss of COVID-19 negative residents. However, the respective consequences seem to be less severe than in those that have been infected by the coronavirus.10 In this context psychological factors and a lack of mealtime assistance may be of special relevance.
Summarizing the above, nursing home residents are in general at a high risk of deteriorating nutritional status during the pandemic. Food and fluid intake deserves special attention in positive residents with signs of delirium.
Sarcopenia
From a subacute and chronic perspective, an inadequate intake of calories and protein will lead to a decrease in muscle mass and subsequently to a loss of strength and function. This process may finally cause overt sarcopenia in patients with post–COVID-19. However, in those that have been affected by a more serious course of disease, sarcopenia may be present already before hospital discharge. Systemic inflammation, immobilization, hypoxemia, and malnutrition may all cause loss of muscle mass and function, while the relative contribution of the etiologic factors may vary to the relevant degree.12 In this context it also has to be appreciated that the presence of sarcopenia in patients with COVID-19 already early after admission to hospital has been identified as an independent prognostic parameter that was associated with length of stay.13
A study from Spain which was based on data from the first COVID-19 wave focused on the nutritional and functional status of COVID-19 survivors, average age of 60 years, who had been treated in intensive care units.14 The study participants had suffered an average weight loss of 16.6% during their hospital stay. While 83.5% were at risk of malnutrition at discharge, 86.9% were at risk of sarcopenia based on the SARC-F questionnaire. Those latter groups had a longer length of hospital and ICU stay than those patients that were not at risk. Furthermore, they had received tracheostomy and invasive ventilation more often. 86.3% of those at risk of sarcopenia showed moderate or total dependency according to the Barthel-Index at discharge, while the respective percentage was only 34.8% in those that were not at risk. Although nutritional therapy would have been warranted in a high percentage of those patients it was prescribed only in 38%, mainly in form of oral nutritional supplements.
The high probability of being at risk of sarcopenia among hospital patients admitted with COVID-19 has been confirmed in a recent study from the Netherlands, that also included non-ICU patients. In this study, the SARC-F indicated a risk of sarcopenia in 73% of all included patients (mean age of 64.8 years).2
Reflecting on the presented data some critical comments seem to be justified. In both trials, the SARC-F was applied to hospital patients during the acute course of disease before discharge. Under these circumstances, there might have been a relevant overlap with conditions that affect functionality but that are not closely related to sarcopenia, like partial respiratory insufficiency or acute and subacute cognitive impairment. In addition, the above studies do not provide an estimate of muscle mass in their patient populations. The follow-up of the study by Wierdsma showed that the percentage of patients at risk of sarcopenia fell from 72% at discharge to 21% between 4 and 7 weeks after discharge. This decrease in prevalence would not be expected in truly sarcopenic patients. Correctly the authors only referred to a risk of sarcopenia and not to the diagnosis of sarcopenia. However, it is absolutely obvious that COVID-19 infections in older hospital patients may trigger the development of sarcopenia or worsen already existing sarcopenia, primarily as a consequence of immobilization, malnutrition, and inflammation. Unfortunately, we may not have sufficient information to calculate the precise prevalence of sarcopenia in older COVID-19 survivors at this moment. Therefore, it may be more adequate to describe the decline of functionality and resulting dependence in activities of daily living in this population without applying the diagnosis of sarcopenia.
In an Italian study reporting on 34 patients with post–acute COVID-19, mean age 70 years, that were admitted to rehabilitation 20 patients (58%) were diagnosed as being sarcopenic based on the EWGSOP criteria.15 When compared with the nonsarcopenic patients they showed slightly higher age (71.5 vs 68 years), lower BMI values (21 vs 27.3. kg/m2) and significantly higher ferritin and lower vitamin D values.
Additional factors may contribute to the development of sarcopenia in the subacute and chronic course of the disease. While it is still unclear whether viral infiltration of the muscle affects its function directly, for example, by the impairment of its mitochondria, indirect mechanisms have been shown to be of relevance. In this context, the contribution of prolonged disuse should be regarded as a key factor. The postviral chronic fatigue syndrome may be diagnosed in a relevant percentage of patients with COVID-19. It has been characterized by profound fatigue, sleep disturbances, neurocognitive changes, and postexertional malaise. In a recent systematic review fatigue was present in 32% of patients with COVID-19 12 weeks and beyond after diagnosis of the infection.16 In addition, the proportion of individuals exhibiting cognitive impairment was 22% in the same analysis. Fatigue and cognitive impairment may both significantly contribute to the disuse of the muscle in patients with post–COVID-19.
Summary
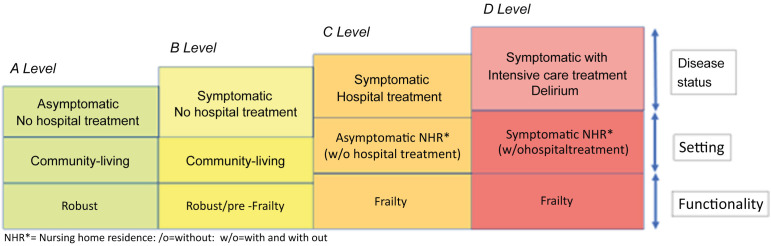
Malnutrition has been one of the most common complications of older COVID-19 survivors. COVID-19 associated symptoms like loss of appetite as well as changes in taste and smell may trigger the deterioration of nutritional status, while other complications of the disease may contribute to it, like respiratory failure that necessitates admission to the ICU. Especially in nursing home residents reduced food intake may be related to preexisting and also to incident geriatric syndromes like delirium. Sarcopenia has also been highly prevalent in older COVID-19 survivors. It is caused and exacerbated by COVID-19-associated inflammatory processes, total or partial immobilization, and malnutrition. COVID-19 survivors may be at high risk of developing the vicious circle that results from the interaction of deteriorating nutritional status and declining functionality. A model for general risk assessment in older COVID-19 survivors with regard to the persistence of malnutrition and sarcopenia is proposed in Fig. 1 . Regular monitoring of nutritional and functional status isindicated in all older COVID-19 survivors. When malnutrition and/or functional decline have been identified in this patient population, low-threshold provision of individualized nutritional and exercise interventions should be installed. In those that are most seriously affected by malnutrition and sarcopenia ambulatory or inpatient rehabilitation has to be considered. Geriatric rehabilitation programs should be specifically adapted to the needs of older patients with COVID-19.
Fig. 1.
Level of risk for the persistence of malnutrition and sarcopenia in older COVID-19 survivors–A proposed model.
Clinics care points
-
•
All older COVID-19 survivors should be screened repeatedly for malnutrition and sarcopenia after the acute disease.
-
•
Delirium in COVID-19 survivors warrants special awareness in this regard.
-
•
Nutritional intervention should be started early during the disease, if indicated.
-
•
The repeated assessment of functionality should trigger the allocation of rehabilitative care in older COVID-19 survivors.
References
- 1.Agyeman A.A., Chin K.L., Landersdorfer C.B., et al. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierdsma N.J., Kruizenga H.M., Konings L.A., et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021;43:369–376. doi: 10.1016/j.clnesp.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quilliot D., Gérard M., Bonsack O., et al. Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open. 2021;11(7):e048948. doi: 10.1136/bmjopen-2021-048948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saniasiaya J., Islam M.A., Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope. 2021;131(4):865–878. doi: 10.1002/lary.29286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gérard M., Mahmutovic M., Malgras A., et al. Long-term evolution of malnutrition and loss of muscle strength after COVID-19: a major and neglected component of long COVID-19. Nutrients. 2021;13(11):3964. doi: 10.3390/nu13113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedock D., Couffignal J., Bel Lassen P., et al. Evolution of nutritional status after early nutritional management in COVID-19 hospitalized patients. Nutrients. 2021;13(7):2276. doi: 10.3390/nu13072276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S.M., Bakaev I., Chen H., et al. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21(10):1378–1383. doi: 10.1016/j.jamda.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morandi A., Pozzi C., Milisen K., et al. An interdisciplinary statement of scientific societies for the advancement of delirium care across Europe (EDA, EANS, EUGMS, COTEC, IPTOP/WCPT) BMC Geriatr. 2019;19(1):253. doi: 10.1186/s12877-019-1264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudge A., Young A., Cahill M., et al. In: Interdisciplinary nutritional management and care for older adults. Perspectives in nursing management and care for older adults. Geirsdóttir Ó.G., Bell J.J., editors. Springer; Cham: 2021. Nutrition and delirium. [DOI] [Google Scholar]
- 10.Martinchek M., Beiting K.J., Walker J., et al. Weight loss in COVID-19-positive nursing home residents. J Am Med Dir Assoc. 2021;22(2):257–258. doi: 10.1016/j.jamda.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danilovich M.K., Norrick C.R., Hill K.C., et al. Nursing home resident weight loss during coronavirus disease 2019 restrictions. J Am Med Dir Assoc. 2020;21(11):1568–1569. doi: 10.1016/j.jamda.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares M.N., Eggelbusch M., Naddaf E., et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J Cachexia Sarcopenia Muscle. 2022 doi: 10.1002/jcsm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.W., Yoon J.S., Kim E.J., et al. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus Disease 2019. J Gerontol A Biol Sci Med Sci. 2021;76(8):e110–e116. doi: 10.1093/gerona/glab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuerda C., Sánchez López I., Gil Martinez C., et al. NUTRICOVID study research group of SENDIMAD. impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobbi M., Bezzoli E., Ismelli F., et al. Skeletal muscle mass, sarcopenia and rehabilitation outcomes in post-acute COVID-19 patients. J Clin Med. 2021;10(23):5623. doi: 10.3390/jcm10235623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceban F., Ling S., Lui L.M.W., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]