Dear Editor,
We read with great interest the article in this journal by Luo et al. regarding the use of tocilizumab in patients with COVID-19 infection1. As the global cases of COVID-19 continue to rise and the virus continues to mutate, monoclonal antibodies have emerged as a novel option with potential therapeutic and prophylactic applications.
Sotrovimab is a neutralizing monoclonal antibody targeting the conserved epitope on the spike protein receptor of SARS-CoV-2. It was granted emergency use authorization by the United States Food and Drug Administration in May 2021 with several countries closely following suit2. Given as a single 500 mg IV infusion, sotrovimab inhibits the fusion of viral and cell membranes to decrease viral internalization2. In addition, it was reported that the Omicron spike was resistant against most therapeutic antibodies but remained susceptible to inhibition by sotrovimab2 , 3. However, on March 25, 2022, the FDA revised the authorization for sotrovimab to limit its use for the treatment of COVID-19 in certain U.S. regions with high frequency of the omicron BA.2 subvariant. Currently, there were a few studies that evaluated the effect of sotrovimab on clinical outcomes in patients with mildto-moderate COVID-19. Thus, we aim to perform a meta-analysis to evaluate the effect of sotrovimab in patients with COVID-19.
An electronic search was performed in (PubMed, Embase, Cochrane Library databases, Scopus and medRxiv) from December 1 2019 to March 20th, 2022. No language, year, or publication restrictions were applied. The following medical subject Heading Terms (MeSH) and key words were searched: (“2019-nCoV or coronavirus disease 2019 or COVID-19 or SARS-CoV-2 or novel coronavirus”) AND (sotrovimab or VIR-7831).
The inclusion criteria were defined as follows: (1) patients with confirmed COVID-19; (2) comparison for clinical outcomes between sotrovimab treatment (administered alone) and control groups (standard care, placebo) was reported. Following studies were excluded (1) reviews, letters, editorials, conference abstracts, and case reports; and (2) duplicated publications. We also extracted information on baseline characteristics of the studies and participants, including first author's name, year of publication, study design, country of origin, age, gender, number of participants, usage of sotrovimab, outcomes of interest (mortality and disease severity).
The statistical analysis was performed using Review Manager, version 5.2 (Cochrane Collaboration, Oxford). Dichotomous variables were analyzed using the odds ratio (OR) with a 95% confidence interval (95% CI). We assessed the heterogeneity using Cochran's Q test and the I2 statistic. A P value <0.05 is considered to be statistically significant. This meta-analysis is registered with the PROSPERO international prospective register of systematic reviews (registration number CRD42022322696).
The electronic literature search identified a total of 4 studies4, 5, 6, 7 comprising of 3866 adult patients with COVID-19, including 1040 in the sotrovimab (administered alone) and 2826 in the control group arm, were included in this meta-analysis. The patient demographics and baseline disease characteristics of the study population are shown in Table 1 . Three studies were from USA and one from Singapore. Two studies were RCTs, one study was retrospective and one study was prospective cohort study. Most studies included mild to severe COVID-19 patients. Sotrovimab was intravenously administered in the included studies. The four studies were published between 2021 and 2022 with different sample patient sizes that ranged from 94 to 2357 patients with COVID-19.
Table 1.
Characteristics of included studies.
| Study | Region | Sotrovimab |
No Sotrovimab |
Study design | Sample size | Patients included | Usage of sotrovimab | ||
|---|---|---|---|---|---|---|---|---|---|
| Agea | Male (%) | Agea | Male (%) | ||||||
| Gupta4 2022 | USA | 53 (41.5–62) | 229 (43) | 53 (43–63) | 256 (48) | RCT | 1057 | Non-hospitalized patients with mild to moderate COVID-19 | A single intravenous infusion with 500 mg of sotrovimab |
| Huang5 2021 | USA | NR | NR | 52.8 ± 19.5 | 889 (43.4) | Prospective cohort study | 2357 | Non-hospitalized patients with mild to moderate COVID-19 | Intravenously sotrovimab |
| Ong6 2022 | Singapore | 81 (75–88) | 14(73.7) | 70 (59–80) | 45 (60) | Retrospective cross-sectional study | 94 | Hospitalized patients with mild-to-moderate COVID-19 | A single dose of sotrovimab |
| Self7 2021 | USA | 61 (50–74) | 107 (59) | 60 (49–70) | 103 (58) | RCT | 358 | Adults hospitalized with COVID-19 without organ failure | Intravenously sotrovimab 500 mg |
Age data presented as median (IQR) or mean (SD); RCT: randomized controlled trial; NR: not reported.
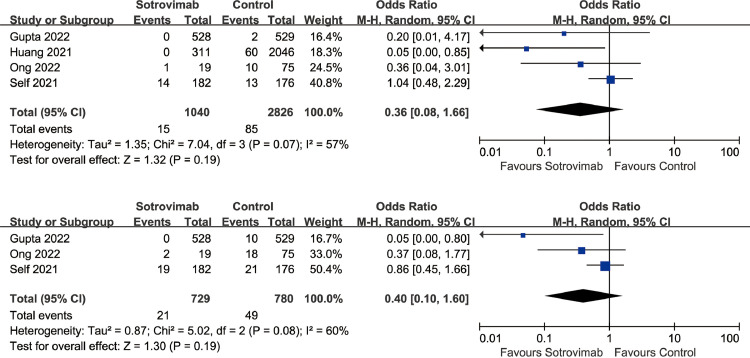
The meta-analysis showed the overall mortality was not statistically different between the sotrovimab group and control group (OR=0.36, 95%CI: 0.08 to 1.66, P = 0.19; I2=57%) (Fig.1 A). In addition, sotrovimab treatment was not associated with developing severe COVID-19 disease (OR=0.40, 95%CI: 0.10 to 1.60, P = 0.19; I2=60%) (Fig.1B) compared with control group.
Fig. 1.
A Association between sotrovimab treatment and mortality. Figure 1B Association between sotrovimab treatment and developing severe COVID-19.
In this study, we find that sotrovimab administration in patients with COVID-19 infection does not have a significant benefit with either mortality rate or severity of disease. Since most of the patients in our study were mild to moderate COVID-19, an alternative view of this result is that sotrovimab may not significantly improve the mortality or severity among non-severe COVID-19 patients because the risk of death is very low regardless of the interventions used8.
The level of effectiveness of sotrovimab in patient with COVID-19 is most likely multifactorial. While the studies by Gupta et al. and Huang et al. investigated the use of sotrovimab in non-hospitalized patients, the other studies had hospitalized patients in their inclusion criteria. These populations have differing baseline risks for severity and mortality following COVID-19 infection9. As such, further investigation is needed to probe the effectiveness separately between inpatient and outpatient settings. Emerging evidence has also suggested that the use of neutralizing monoclonal antibody therapy may not as effective in patients with endogenous anti-SARS-CoV-2 antibodies as compared to those without10. While one included study attempted to correct through randomization, the others did not test for presence of anti-SARS-CoV-2 antibodies. As such, it is possible that monoclonal antibody therapy may have a higher indication for use in patients without a past COVID-19 infection history or with lower levels of endogenous anti-SARS-CoV2 antibodies.
SARS-CoV-2 continues to develop mutations creating new variant of concerns (VOC) with differing susceptibilities to medication treatment and differing immune escape properties. While Huang et al. only included the delta variant, the other studies did not use the VOC as exclusion criteria. In addition, Ong et al. excluded fully vaccinated patients, while the other studies did not consider vaccination status in their inclusion criteria. These results thus may not be completely applicable to new and emerging VOCs, including the Omicron subvariant BA.2 which has recently been documented as displaying considerable resistance to sotrovimab11. Thus, sotrovimab is no longer authorized by the FDA to treat COVID-19 in eight states and two territories.
Our study has several limitations that should be noted. There was a small sample size of included articles for use in meta-analysis of four studies. Both the mortality and severity analysis results also had significant heterogeneity (I2>50%). Furthermore, the studies had differing patient populations, including healthcare setting, type of VOC, and vaccination status. Despite these limitations, our study offers considerable value as the first meta-analysis to investigate the impact of sotrovimab treatment in patients with COVID-19 infection.
More studies are needed to explore the association between treatment with sotrovimab and outcomes of patients with COVID-19 to better understand the factors involved in the effectiveness of medication, including inpatient versus outpatient settings, the severity of COVID-19 infection, the time of medication administration, and the presence or absence of endogenous anti-SARS-CoV-2 antibodies.
In conclusion, the use of sotrovimab to treat patients with COVID-19 infection does not have a significant benefit in either the mortality rate or severity of illness. Additional research is needed to corroborate these findings.
Funding information
None declared.
Declaration of Competing Interest
The authors declare that they have no competing interest.
Acknowledgements
None.
References
- 1.Luo L., Luo T., Du M., Mei H., Hu Y. Efficacy and safety of tocilizumab in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Infect. 2022;84(3):418–467. doi: 10.1016/j.jinf.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo Y.A. Sotrovimab: First Approval. Drugs. 2022;82(4):477–484. doi: 10.1007/s40265-022-01690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., Krüger N., Schulz S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3) doi: 10.1016/j.cell.2021.12.032. 447-456.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang D.T., McCreary E.K., Bariola J.R., et al. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial. medRxiv 2021:2021.12.23.21268244. doi: 10.1101/2021.12.23.21268244. [DOI] [PMC free article] [PubMed]
- 6.Ong S.W.X., Ren D., Lee P.H., et al. Real-world use of sotrovimab for pre-emptive treatment in high-risk hospitalized COVID-19 patients: an observational cross-sectional study. Antibiotics (Basel) 2022;11(3):345. doi: 10.3390/antibiotics11030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00751-9. S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemieniuk R.A., Bartoszko J.J., Díaz Martinez J.P., et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergquist S.H., Partin C., Roberts D.L., et al. Non-hospitalized adults with COVID-19 differ noticeably from hospitalized adults in their demographic, clinical, and social characteristics. SN Compr Clin Med. 2020;2(9):1349–1357. doi: 10.1007/s42399-020-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022 doi: 10.1038/s41586-022-04594-4. 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]