Abstract
High-resolution manometry (HRM) has revolutionized esophageal motility testing, and the evolving Chicago Classification has been critical in codifying HRM metrics and definitions of old and new motility disorders. The latest Chicago Classification (version 4.0) is the result of a working group of 52 members (10 women) from 20 countries. Two critical new elements are the expansion of the normal database from 75 to 469 healthy volunteers and the recommendation of ancillary function tests (timed barium esophagram, functional lumen imaging planimetry, and/or impedance) to help with inconclusive HRM metrics, especially in cases of suspected achalasia, esophagogastric junction outflow obstruction (EGJOO), and ineffective esophageal motility (IEM). Important changes relevant to clinical practice include (1) refinement of the diagnosis criteria for EGJOO, which now require elevated integrated relaxation pressure in an upright position along with primary symptoms of dysphagia/noncardiac chest pain and obstruction at the esophago-gastric junction; (2) exclusion of mechanical obstruction in cases of suspected distal esophageal spasm and hypercontractile esophagus; and (3) a shift to a more restrictive metric (>70% ineffective peristalsis) for a diagnosis of IEM. In addition, the working group urged caution in using treatments such as pneumatic dilation or myotomy, which can irreversibly destroy lower esophageal sphincter competency and peristalsis, as the natural history of EGJOO/hypercontractile esophagus is poorly understood and spontaneous symptom resolution is common. Future versions should address the routine use of impedance with HRM, the role of HRM in pharyngeal/upper esophageal sphincter diseases, and the need for better criteria to determine which subsets of spastic disorders warrant aggressive treatment, as is done with achalasia.
Keywords: High-resolution manometry, Chicago Classification, achalasia, esophagogastric junction outflow obstruction, diffuse esophageal spasm, hypercontractile esophagus, ineffective esophageal motility, absent contractility
It has been 30 years since high-resolution manometry (HRM) revolutionized the field of esophageal motility testing,1 vastly improving diagnostic accuracy for certain esophageal motility diseases (achalasia), enabling the identification of new manometric abnormalities (esophagogastric junction outflow obstruction [EGJOO]), and making esophageal motility testing easier to perform and interpret. Along with HRM came the need for a new classification system, coined the Chicago Classification in recognition of the important work led by Drs Peter Kahrilas and John Pandolfino at Northwestern University. This system has been refined over time based on the results of worldwide clinical studies. The first 3 iterations of the Chicago Classification have been cited more than 2000 times,2 and the new version, 4.0, was published online in 2020.3 This article examines the history of HRM and the Chicago Classification, the important diagnostic updates in the latest version, and the clinical areas that still need to be addressed.
Historical Perspective
High-Resolution Manometry
Older manometry techniques recorded esophageal peristalsis using 5-mm to 8-mm widely spaced water- perfusion channels in an esophageal motility catheter. Two significant advances in the 1990s—an increase in pressure sensors along the catheter and the use of spatiotemporal plots for data display—led to what is now recognized as HRM.
HRM was conceived and later developed by Dr Ray Clouse at Washington University in St Louis, Missouri. Dr Clouse decided that there was hidden information in the esophagus between the widely spaced recording ports of the conventional manometry catheter, leading health care providers (HCPs) to possibly miss important information for assessing peristalsis and lower esophageal sphincter (LES) function. Over 10 years of experiments,4 Clouse and colleagues developed a 21-lumen water-perfusion catheter, digitized the information to give a smooth topographic map of esophageal peristalsis, and assigned colors to amplitude levels in spatiotemporal contour plots (an idea from his university training in architecture).1,5,6 As technology progressed toward solid-state pressure sensors, Clouse found a key collaborator in Dr Thomas Park, who formed a new company, Sierra Scientific, Inc, to advance the field. Together, they developed a solid-state catheter with 36 high-fidelity circumferential sensors,7 new software programs, and an electronic sleeve8 that more accurately measured postswallow residual pressures. With Dr Clouse’s untimely death from cancer, HRM technology moved from St Louis to Chicago and beyond. In honor of his contributions, the revolutionizing color pressure topography plots will always be known as Clouse plots.
Chicago Classification
The move to Chicago was heralded with a pivotal decision by Drs Kahrilas and Pandolfino to develop a new classification system, not limited to their own institution, but through collaboration with motility experts throughout the world via the American Neurogastroenterology and Motility Society and the European Society of Neurogastroenterology and Motility. They evolved the concept of a living document, which would be regularly updated as clinical and scientific data dictated to coincide with the anticipated 3-year cycle of Digestive Disease Week (DDW) being hosted in Chicago. The first major version of the Chicago Classification was published in 2009 after the inaugural meeting of the International HRM Working Group in San Diego in 2008.9 The next major update followed from a meeting of the International HRM Workshop Group in Ascona, Switzerland in 2011 and was endorsed by numerous international motility and gastroenterology societies.10 Later, an expanded International HRM Working Group met in Chicago in conjunction with DDW 2014 to formulate the Chicago Classification version 3.0.11 Chicago Classification version 4.0 (CCv4.0) was delayed by the COVID-19 pandemic but was published online in 2020.3 The working group was composed of 52 members (10 women) selected by 6 international motility societies, representative of 20 countries. The initiative was a 2-year process with 3 international meetings and multiple Web conferences. In addition to expert consensus, one of the group’s main objectives was to use formally validated methodologies to determine both the appropriateness of statements and the level of supportive evidence for each statement. The RAND/University of California Los Angeles Appropriateness Method was used, with 2 rounds of independent electronic voting to determine the appropriateness of each statement.12 Statements with greater than or equal to 85% agreement were considered strong recommendations, whereas statements with 80% to 85% agreement were considered conditional recommendations. Moreover, statements that met criteria for inclusion in the final document underwent further independent evaluation to assess the level of supportive evidence using the Grading of Recommendations Assessment, Development, and Evaluation process.13
What Makes Chicago Classification Version 4.0 Different?
The definition of normality is critical for any classification system after the manometric criteria have been defined. For the first 3 versions of the Chicago Classification, normal values were primarily obtained from 75 healthy controls studied in Chicago.14 This group consisted of 47% women with an age range of 19 to 48 years. Version 4.0 (Table 1) has expanded the normal database to 469 healthy volunteers (55% women, median age of 28 years, age range of 18-79 years) acquired from 15 countries across 4 continents using the 3 available commercial HRM systems.15 Three-quarters had a normal HRM pattern and none had achalasia. Ineffective esophageal motility (IEM) was the most frequent diagnosis (15.1%), followed by EGJOO (5.3%). The supine position reduced the portion with IEM to 7.9% and the upright position reduced the portion with EGJOO to 2 volunteers (1.1%). Expanding the normal database also impacted the diagnosis of hypercontractile esophagus (HE), increasing the distal contractile integral (DCI) from greater than 5000 to greater than 8000 mm Hg-s-cm, as well as requiring the presence of 20% or more hypercontractile supine swallows to define abnormality.16
Table 1.
Key Points About the Chicago Classification Version 4.0 Working Group, Expanded Database, and New Definitions
|
|
|
|
DES, distal esophageal spasm; EGJOO, esophagogastric junction outflow obstruction; IEM, ineffective esophageal motility.
Although not specifically stated, the metrics used in the Chicago Classification hoped to allow HRM to define all features related to esophageal pump vigor of contractions, peristalsis, and esophagogastric junction (EGJ) compliance. It was hoped the new measures of distal latency (DL)17 would replace the old terminology of simultaneous contractions and correlate more closely with bolus clearance by impedance studies, and integrated relaxation pressure (IRP)18 would be an improvement on LES relaxation and better correlate with EGJ compliance. This has not been the case, especially with achalasia and EGJOO, where the median IRP of less than 15 mm Hg misses the diagnosis of achalasia in up to 20% of patients,19 and an IRP of greater than 15 mm Hg over-diagnoses EGJOO in the supine position in more than 50% of patients.20 Therefore, the CCv4.0 now encourages supportive testing with timed barium esophagram (TBE) combined with a 13-mm barium tablet21 and/or endoluminal functional lumen imaging planimetry (FLIP)22 in patients with an inconclusive diagnosis of achalasia/ EGJOO with dysphagia as the presenting symptom.3
Disorders of Esophagogastric Junction Outflow
Achalasia
As shown in Table 2, the basic definitions of the 3 types of achalasia have not changed in CCv4.0. Abnormal median IRP is the first key measurement, with a threshold of 15 mm Hg in the supine position using the Medtronic system and 22 mm Hg for the Laborie/Diversatek system.16 The major improvement in CCv4.0 is the recognition that these absolute cutoff values do not always accurately measure outflow obstruction and the compliance of the EGJ. Rather, high values with aperistalsis give us confidence in a diagnosis of achalasia, but values of less than 15 mm Hg can still be seen in up to 20% to 25% of patients with achalasia and dysphagia, both in the naive or treated state.19 For these inconclusive cases, a TBE with a barium tablet or FLIP is critical for making the diagnosis. Both measure distensibility (compliance) of the EGJ with the TBE column at 5 minutes greater than 2 cm correlating closely with a FLIP distensibility index greater than 2.9 mm2/mm Hg.23 For this reason, many HCPs routinely use HRM first, followed by a TBE in patients with suspected achalasia. A repeat TBE after treatment is then performed to assess improvement in esophageal emptying.24
Table 2.
Disorders of Esophagogastric Junction Outflow
| Achalasia |
|
|
|
|
|
|
| EGJOO |
|
|
|
|
Bold text indicates important new criteria in Chicago Classification version 4.0.
EGJOO, esophagogastric junction outflow obstruction; FLIP, functional lumen imaging planimetry; IRP, integrated relaxation pressure; TBE, timed barium esophagram.
A relatively new observation is that Type III achalasia and EGJOO are significantly more likely to be associated with the use of opioids compared with achalasia Types I and II.25,26 Studies suggest that 11% to 13% of Type III achalasia and 13% to 37% of EGJOO may be due to activation of μ and κ receptors by opiates, which impair LES relaxation. The most common narcotics are oxycodone, hydrocodone, and tramadol. It is suggested, but not known, that studying patients off their opioids for variable times based on drug half-life will reduce this potential confusion. Although an interesting observation, there are no good data that patients on opioids with Type III achalasia/EGJOO and dysphagia and abnormal TBE/ FLIP will not do well with traditional therapies if the opioids cannot be discontinued.
Esophagogastric Junction Outflow Obstruction
A critical update in CCv4.0 is the clarification and rigorous definition of EGJOO (Table 2).3 Following the introduction of EGJOO as a motility disorder, nearly 10% of patients undergoing HRM were identified to have this pattern (Figure).27 An unknown subset of these patients present with a variant of achalasia, but the vast majority of presentations are related to benign conditions (eg, peptic strictures, large hiatal hernia, eosinophilic esophagitis, tight Nissen fundoplication), opioid use, subtle cancers, or artifacts of the pressure measurements. All these presentations usually have dysphagia for solid food and, less frequently, noncardiac chest pain as the predominant symptoms.
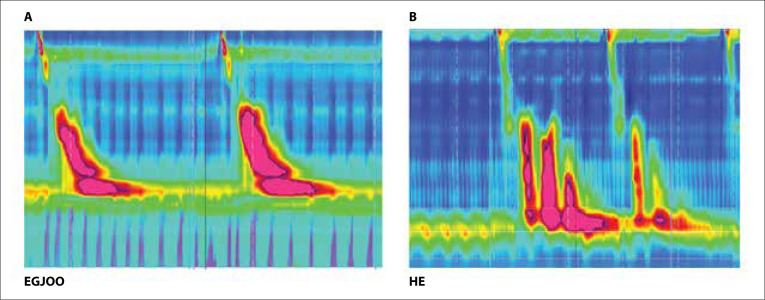
Figure.
High-resolution manometry images (A: EGJOO; B: HE) from the University of South Florida’s Swallowing Center. The vertical axes are esophageal length (cm), horizontal axes are time (s), and colors increasing from blue to red represent pressures (intrathoracic chest pressure) in mm Hg.
EGJOO, esophagogastric junction outflow obstruction; HE, hypercontractile (jackhammer) esophagus.
When a diagnosis of EGJOO is suspected after 10 swallows in the supine position, the patient should be positioned upright and asked to provide at least 5 swallows. An upright median IRP of greater than 12 mm Hg (Medtronic) or greater than 15 mm Hg (Laborie/Diver-satek) is defined as abnormal20 and rarely seen in healthy patients.15 The reason for the decrease in IRP from supine to an upright position may be a catheter impingement artifact.27 In patients with hiatal hernia, catheter angulation of 1 to 2 sensors located in the EGJ segment by the hernia sac can result in erroneous elevation of the IRP. Sitting the patient upright is the easiest way to resolve this issue. Other provocative tests to assess for outflow obstruction during HRM include rapid drink challenge with subsequent esophageal pressurization, solid test meal with replication of symptoms, or pharmacologic provocation with amyl nitrate.27
As my colleague and I have previously suggested,28 an elevated IRP should be evaluated for other mechanical causes of obstruction using upper endoscopy with biopsies, barium esophagram, or endoscopic ultrasound (especially in patients with significant weight loss), while also reviewing history of opioid use. If no etiologies are found, then a functional obstruction (sometimes called Type IV achalasia) must be confirmed with an abnormal TBE with barium pill or FLIP. In a retrospective study of TBE by Blonski and colleagues,21 only 27% of patients had an abnormal test, which was similar to the 30% reported by Triggs and colleagues.20
Based on the published data, the key question is whether idiopathic EGJOO is a manometric curiosity or an obstructive disease that warrants treatment. Proposed treatments in case reports or series include muscle relaxants, bougie dilation, botulinum toxin (BTX), pneumatic dilation, or surgical myotomy, with improvements (usually determined only by symptom assessment) ranging from 35% to 100%.28 Importantly, no treatment with observation alone may result in spontaneous resolution over 6 months to 2 years in 15% to 72% of patients.28 One study, however, did find that symptoms were likely to persist if dysphagia was the predominant symptom and the IRP was very high (>32 mm Hg).29 Thus, members of the CCv4.0 Working Group suggest avoiding invasive, irreversible treatments on the LES, especially pneumatic dilation or myotomy. If symptoms are severe, then first consideration should be given to BTX injection, as it is safe and reversible.27 Porter and Gyawali30 describe the beneficial results of a single injection lasting up to 1.5 years in 55% of patients. However, my colleagues and I did this early in our experience with EGJOO and saw 2 patients evolving to Type III achalasia.31 We currently treat healthy patients with EGJOO, severe dysphagia, and an abnormal TBE with pneumatic dilation and recently reported a symptomatic response rate of 67% and marked improvement in esophageal emptying.32 Patients have been doing well for up to 7 years, and none have returned with achalasia, raising the possibility that more aggressive improvement of EGJ compliance may prevent the loss of peristalsis over time.
Disorders of Peristalsis
Distal Esophageal Spasm
Distal esophageal spasm (DES) is a rare and elusive motility disorder due to partial loss of inhibitory innervation via nitric oxide, producing premature, rapid, or simultaneous contractions.33,34 As shown in Table 3, CCv4.0 has not changed the primary manometric criteria of (1) greater than or equal to 20% premature contractions and (2) DCI of greater than 450 mm Hg-s-cm.3 For an unexplained reason, some normal peristalsis being present is no longer required.11 This omission could cause some confusion with Type III achalasia with a normal IRP unless careful assessment of EGJ compliance is performed. The presence of premature contractions with a DCI of less than 450 mm Hg-s-cm is inconclusive for the manometric diagnosis of DES. In this scenario, these manometric changes are often part of the gastroesophageal reflux disease (GERD) spectrum. The additional observation of abnormal inhibition defined by the persistence of peristalsis in the distal esophagus during multiple rapid swallows supports a diagnosis of DES.3 Importantly, to prevent confusion with normal variations, GERD, or neuropathic entities such as diabetes mellitus, CCv4.0 now requires the clinically relevant symptoms of dysphagia and/or noncardiac chest pain to make a definitive diagnosis of DES.
Table 3.
Disorders of Peristalsis
| DES |
|
|
|
|
|
|
| IEM |
|
|
|
| Absent Contractility |
|
Bold text indicates important new criteria in Chicago Classification version 4.0.
DCI, distal contractile integral; DES, distal esophageal spasm; HE, hyper-contractile (jackhammer) esophagus; IEM, ineffective esophageal motility; IRP, integrated relaxation pressure.
Hypercontractile Esophagus
HE is the HRM version of the old nutcracker esophagus first described by Benjamin and colleagues.35 The mano-metric criteria (Table 3) are identical for both versions 3.0 and 4.0 of the Chicago Classification. That is, a manometric diagnosis of HE is defined as 20% or more hypercontractile swallows in the supine position (DCI >8000 mm Hg-s-cm). Esophageal hypercontractility can either be limited to the esophageal body or include the LES. A variant form with prominent, high-amplitude, repetitive contractions has been given the colorful name of jackhammer esophagus (Figure).
HE is a rare condition that ranges from 1.5% to 3% of manometric diagnoses in motility centers.36 The pathophysiology is the result of excessive cholinergic drive with temporary asynchrony of circular and longitudinal muscle contractions.37,38 HE can sometimes be associated with EGJOO as well as other causes of mechanical obstruction, including GERD, obstructing hiatal hernia, eosinophilic esophagitis, and gastric laparoscopic bands.39 Opioid use also has been associated with HE.25 CCv4.0 recognizes this potential causality and emphasizes the importance of appropriately addressing these diseases before treatment directed only at the hypercontracting esophagus, such as smooth muscle relaxants, BTX, or long surgical myotomy.
As with DES, diagnosis of HE requires that patients have symptoms of dysphagia and/or noncardiac chest pain. Noncardiac chest pain is not associated with any specific manometric criteria, but several studies have reported dysphagia associated with DCI of the hyper-contractile swallows and with intrabolus pressure.39,40 The jackhammer subgroup is typically associated with higher DCI values and greater symptom severity.39
Given the heterogenicity of hypercontractile patterns, the CCv4.0 Working Group advocates for a cautious approach in treating contractile vigor as an endpoint3 because its relationship to symptoms, especially chest pain, and natural history are poorly understood. Relevant to these points are 2 important observations. A French randomized study of BTX in patients with HE demonstrated that symptom improvement after BTX was not superior to sham and that symptoms and manometric patterns may improve spontaneously over time.41 Specifically, 3 of 10 patients who did not receive any treatment had resolution of their HE, including 1 with jackhammer esophagus, over 3 to 12 months. This could be due to a placebo effect or to spontaneous resolution. Similarly, in a Mayo Clinic retrospective study29 of HRM scans, which included 40 patients with HE, 72% of patients had resolution of their symptoms over 2.8 years of follow-up. Interestingly, predictors of continued symptoms included dysphagia as the main symptom and a DCI of greater than 32,132 mm Hg-s-cm. These studies need to be appropriately weighed when considering treating refractory EGJOO and HE with peroral endoscopic myotomy surgery.42
Ineffective Esophageal Motility
A major improvement in CCv4.0 is the change in the diagnostic criteria for IEM, which was driven by the finding that 71 healthy volunteers (15.1%) in the expanded database met this criteria.16 Therefore, a definitive diagnosis of IEM was made more restrictive, now requiring greater than 70% ineffective swallows (DCI >100 mm Hg-s-cm but <450 mm Hg-s-cm) or greater than or equal to 50% failed swallows (DCI <100 mm Hg-s-cm).3 With that, the presence of 50% to 70% ineffective swallows will be inconclusive for a definitive diagnosis of IEM and will require confirmatory testing to strengthen confidence in these cases. Supportive testing for the diagnosis of IEM may include poor bolus transit on impedance or barium esophagram or lack of contraction reserve on multiple rapid swallows. Fragmented peristalsis (rarely seen in practice) was eliminated as a distinct esophageal motility disorder and absorbed into the definition of IEM.3
On the surface, these changes may seem like minor fine-tuning, but in clinical practice, they serve as important new guidance given that IEM is the most commonly reported esophageal motility disorder in patients (20%-58%).43 GERD is observed in nearly half of patients with IEM, and patients with Barrett esophagus have a strong predilection for IEM. In addition, IEM has been noted as a frequent abnormality in patients with diabetes mellitus with autonomic dysfunction, alcoholism with neuropathy, and collagen vascular diseases. However, it can be seen in healthy patients, even up to 10% with the more rigid diagnostic criteria, and many patients have minimal or no symptoms of dysphagia.44 The finding of IEM becomes clinically important, especially in patients with GERD, when dysphagia is the dominant symptom and antireflux surgery is planned. Patients with IEM have an increased risk of postfundoplication dysphagia if a 360-degree Nissen fundoplication is performed,45 and IEM is a contraindication for magnetic sphincter augmentation.46 Reserving the diagnosis of IEM for the more severe form with additional evidence of poor bolus clearance and impaired peristaltic reserve will expand the potential for antireflux surgery without increasing the chance for troubling dysphagia.
Absent Contractility
As shown in Table 3, the criteria for the diagnosis of absent contractility (previously known as absent peristalsis) were not revised in CCv4.0.3 A definitive diagnosis will require a normal median IRP in the supine and upright positions and 100% failed contractions. In this context, a borderline high IRP should prompt consideration of Type I achalasia, and supportive testing with TBE or FLIP should be performed if dysphagia is the dominant symptom.
Similar to IEM, absent contractility is not a specific diagnosis and has been reported in many diseases with a neuropathic or myopathic etiology. However, in clinical practice, this motility pattern is most suggestive of a collagen vascular disease, and although sometimes termed scleroderma esophagus, this pattern has been reported in all the collagen vascular diseases, including healthy patients with isolated Raynaud’s phenomenon.47 Esophageal disease is observed in up to 90% of patients with either limited or diffuse forms of scleroderma.47 In a recent prospective study of 200 patients with scleroderma, absent contractility was reported in 56% of patients, followed by normal motility in 26%, and IEM in 10%. Interestingly, the classic scleroderma esophagus motility pattern with low LES pressure and absent contractility was only observed in 33% of patients.48
Future Directions
CCv4.0 highlights areas ripe for future research. Although many issues need to be addressed, there are several areas that may be most relevant to clinical practice. First, future iterations of the Chicago Classification must incorporate impedance topography to better assess intrabolus pressure and bolus flow.49 This may be most relevant to the perplexing diagnosis of DES, where at least 20% premature contractions (DL <4.5 s) makes the diagnosis. However, catheters combining HRM and impedance routinely find these abnormal contractions associated with normal bolus clearance. Thus, like the HRM metric of IRP, a DL of less than 4.5 seconds needs to be supported by impaired bolus clearance before abnormality can be assured. Another area needing attention is the use of HRM for pharyngeal and upper esophageal sphincter (UES) function in health and disease.50 This may be more challenging because the striated muscle can be markedly affected by psychological factors. Here, the modified barium swallow will need to be included to ensure the relevance of HRM metrics with bolus flow and UES relaxation.
Although CCv4.0 has refined the diagnosis of EGJOO and HE, it leaves HCPs with the conundrum of who to treat and how to treat. Telling patients that their symptoms of dysphagia and chest pain are likely to improve spontaneously will be met with resistance and added confusion like the old nutcracker esophagus dilemma. Natural history and treatment outcome studies, preferably with a randomized control design, are greatly needed. Furthermore, the roles of TBE and FLIP in defining who to treat or just follow need refinement. Future iterations may propose manometric criteria for therapy selection, such as the role of peroral endoscopic myotomy for spastic disorders and risk stratification and tailoring of fundoplication to prevent postfundoplication dysphagia.
Lastly, there remains a vast unknown between achalasia (and its 3 phenotypes) and normal, with 5 manometric patterns representing heterogenous diseases whose natural histories are unknown and treatments remain uncertain. It is hoped that future versions of the Chicago Classification will provide guidance on these areas as well.
Summary
The Chicago Classification has been critical in codifying HRM metrics and definitions of old and new motility disorders. The latest version (4.0) has 2 critical new elements: (1) expansion of the normal database from 75 to 469 healthy volunteers and (2) the recommendation of ancillary function tests (TBE, FLIP, and/or impedance) to help with inconclusive HRM metrics, especially in cases of suspected achalasia, EGJOO, and IEM. Important changes for the HCP include (1) refinement of the diagnosis of EGJOO, (2) exclusion of mechanical obstruction in cases of suspected DES and HE, and (3) a shift to a more restrictive metric (>70% ineffective peristalsis) for a diagnosis of IEM. Additionally, the working group urged caution in using treatments such as pneumatic dilation or surgical myotomy, which can irreversibly destroy LES competency and peristalsis, as the natural history of EGJOO/HE, unlike that of classic achalasia Types I to III, is poorly understood and spontaneous symptom resolution is common.
References
- Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261(4pt 1):G677–G684. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- Sweis R, Fox M. High-resolution manometry—observations after 15 years of personal use—has advancement reached a plateau? Curr Gastroenterol Rep. 2020;22(10):49. doi: 10.1007/s11894-020-00787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadlapati R, Kahrilas PJ, Fox MR. et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0. Neurogastroenterol Motil. 2021. 33 133e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali CP. High resolution manometry: the Ray Clouse legacy. Neurogastroenterol Motil. 2012;24(suppl 1):2–4. doi: 10.1111/j.1365-2982.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- Clouse RE, Staiano A, Alrakawi A. Development of a topographic analysis system for manometric studies in the gastrointestinal tract. Gastrointest Endosc. 1998;48(4):395–401. doi: 10.1016/s0016-5107(98)70010-0. [DOI] [PubMed] [Google Scholar]
- Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95(10):2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- Clouse RE, Parks T, Haroian LR, Zakko SF. Development and clinical validation of a solid-state high resolution pressure measurement system for simplified and consistent esophageal manometry. Am J Gastroenterol. 2003;98(suppl):532–533. [Google Scholar]
- Clouse RE, Parks TR, Staiano A, Haroian LR. Creation of an electronic sleeve emulation (eSleeve) for use with high solid-state high resolution manometry. Gastroenterology. 2004;126(suppl 2):A111. [Google Scholar]
- Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21(8):796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrilas PJ, Bredenoord AJ, Fox M et al. International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadlapati R, Gawron AJ, Keswani RN et al. Identification of quality measures for performance of and interpretation of data from esophageal manometry. Clin Gastroenterol Hepatol. 2016;14(4):526–534.e1.. doi: 10.1016/j.cgh.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G988–G997. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- Rengarajan A, Rogers BD, Wong Z High-resolution manometry thresholds and motor patterns among asymptomatic individuals [published online November 2, 2020]. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2020.10.052. [DOI] [PubMed]
- Herregods TVR, Roman S, Kahrilas PJ, Smout AJ, Bredenoord AJ. Normative values in esophageal high-resolution manometry. Neurogastroenterol Motil. 2015;27(2):175–187. doi: 10.1111/nmo.12500. [DOI] [PubMed] [Google Scholar]
- Roman S, Lin Z, Pandolfino JE, Kahrilas PJ. Distal contraction latency: a measure of propagation velocity optimized for esophageal pressure topography studies. Am J Gastroenterol. 2011;106(3):443–451. doi: 10.1038/ajg.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1033–G1040. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- Nicodème F, de Ruigh A, Xiao Y et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol. 2013;11(2):131–137. doi: 10.1016/j.cgh.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs JR, Carlson DA, Beveridge C et al. Upright integrated relaxation pressure facilitates characterization of esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol. 2019;17(11):2218–2226.e2.. doi: 10.1016/j.cgh.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol. 2018;113(2):196–203. doi: 10.1038/ajg.2017.370. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Kahrilas PJ, Lin Z et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111(12):1726–1735. doi: 10.1038/ajg.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficiency of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50(6):765–770. doi: 10.1136/gut.50.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratuapli SK, Crowell MD, DiBaise JK et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol. 2015;110(7):979–984. doi: 10.1038/ajg.2015.154. [DOI] [PubMed] [Google Scholar]
- Babaei A, Szabo A, Shad S, Massey BT. Chronic daily opioid exposure is associated with dysphagia, esophageal outflow obstruction, and disordered peristalsis. Neurogastroenterol Motil. 2019;31(7):e13601. doi: 10.1111/nmo.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenoord AJ, Babaei A, Carlson D Esophagogastric junction outflow obstruction [published online June 12, 2021]. Neurogastroenterol Motil. doi:10.1111/nmo.14193. [DOI] [PubMed]
- Richter JE, Clayton SB. Diagnosis and management of esophagogastric junction outflow obstruction. Am J Gastroenterol. 2019;114(4):544–547. doi: 10.14309/ajg.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Schupack D, Katzka DA, Geno DM, Ravi K. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil. 2017;29(10):1–9. doi: 10.1111/nmo.13105. [DOI] [PubMed] [Google Scholar]
- Porter RF, Gyawali CP. Botulinum toxin injection in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol Motil. 2011;23(2):139–144, e27-e28. doi: 10.1111/j.1365-2982.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- Clayton SB, Patel R, Richter JE. Functional and anatomic esophagogastic junction outflow obstruction: manometry, timed barium esophagram findings, and treatment outcomes. Clin Gastroenterol Hepatol. 2016;14(6):907–911. doi: 10.1016/j.cgh.2015.12.041. [DOI] [PubMed] [Google Scholar]
- Clayton SB, Shin CM, Ewing A, Blonski W, Richter J. Pneumatic dilation improves esophageal emptying and symptoms in patients with idiopathic esophago-gastric junction outflow obstruction. Neurogastroenterol Motil. 2019;31(3):e13522. doi: 10.1111/nmo.13522. [DOI] [PubMed] [Google Scholar]
- Konturek JW, Thor P, Lukaszyk A, Gabryelewicz A, Konturek SJ, Domschke W. Endogenous nitric oxide in the control of esophageal motility in humans. J Physiol Pharmacol. 1997;48(2):201–209. [PubMed] [Google Scholar]
- Murray JA, Ledlow A, Launspach J, Evans D, Loveday M, Conklin JL. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology. 1995;109(4):1241–1248. doi: 10.1016/0016-5085(95)90584-7. [DOI] [PubMed] [Google Scholar]
- Benjamin SB, Gerhardt DC, Castell DO. High amplitude, peristaltic esophageal contractions associated with chest pain and/or dysphagia. Gastroenterology. 1979;77(3):478–483. [PubMed] [Google Scholar]
- de Bortoli N, Gyawali PC, Roman S et al. Hypercontractile esophagus from pathophysiology to management: proceedings of the Pisa symposium. Am J Gastroenterol. 2021;116(2):263–273. doi: 10.14309/ajg.0000000000001061. [DOI] [PubMed] [Google Scholar]
- Quader F, Mauro A, Savarino E et al. Jackhammer esophagus with and without esophagogastric junction outflow obstruction demonstrates altered neural control resembling type 3 achalasia. Neurogastroenterol Motil. 2019;31(9):e13678. doi: 10.1111/nmo.13678. [DOI] [PubMed] [Google Scholar]
- Jung HY, Puckett JL, Bhalla V et al. Asynchrony between the circular and the longitudinal muscle contraction in patients with nutcracker esophagus. Gastroenterology. 2005;128(5):1179–1186. doi: 10.1053/j.gastro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Herregods TVK, Smout AJPM, Ooi JLS, Sifrim D, Bredenoord AJ. Jackhammer esophagus: observations on a European cohort. Neurogastroenterol Motil. 2017;29(4):e12975. doi: 10.1111/nmo.12975. [DOI] [PubMed] [Google Scholar]
- Philonenko S, Roman S, Zerbib F et al. Jackhammer esophagus: clinical presentation, manometric diagnosis, and therapeutic results—results from a multicenter French cohort. Neurogastroenterol Motil. 2020;32(11):e13918. doi: 10.1111/nmo.13918. [DOI] [PubMed] [Google Scholar]
- Mion F, Marjoux S, Subtil F et al. Botulinum toxin for the treatment of hypercontractile esophagus: results of a double-blind randomized sham-controlled study. Neurogastroenterol Motil. 2019;31(5):e13587. doi: 10.1111/nmo.13587. [DOI] [PubMed] [Google Scholar]
- Khashab MA, Familiari P, Draganov PV et al. Peroral endoscopic myotomy is effective and safe in non-achalasia esophageal motility disorders: an international multicenter study. Endosc Int Open. 2018;6(8):E1031–E1036. doi: 10.1055/a-0625-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Jalil AA, Castell DO. Ineffective esophageal motility (IEM): the old-new frontier in esophagology. Curr Gastroenterol Rep. 2016;18(1):1. doi: 10.1007/s11894-015-0472-y. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Kahrilas PJ, Nicodème F, Lin Z, Roman S, Pandolfino JE. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol. 2014;109(4):521–526. doi: 10.1038/ajg.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello M, Gyawali CP. Esophageal manometry in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2014;43(1):69–87. doi: 10.1016/j.gtc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Gyawali CP, Sifrim D, Carlson DA et al. Ineffective esophageal motility: concepts, future directions, and conclusions from the Stanford 2018 symposium. Neurogastroenterol Motil. 2019;31(9):e13584. doi: 10.1111/nmo.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JO. Esophageal involvement in systematic diseases. In: Richter JE, Castell DO, eds. The Esophagus. 6th ed. London, UK: Wiley Blackwell. 2021. pp. 312–332.
- Crowell MD, Umar SB, Griffing WL, DiBaise JK, Lacy BE, Vela MF. Esophageal motor abnormalities in patients with scleroderma: heterogeneity, risk factors, and effects on quality of life. Clin Gastroenterol Hepatol. 2017;15(2):207–213.e1. doi: 10.1016/j.cgh.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: studies using combined impedance-manometry. Clin Gastroenterol Hepatol. 2004;2(3):230–236. doi: 10.1016/s1542-3565(04)00010-2. [DOI] [PubMed] [Google Scholar]
- Omari TI, Ciucci M, Gozdzikowska K et al. High-resolution pharyngeal manometry and impedance: protocols and metrics—recommendations of a high-resolution pharyngeal manometry international working group. Dysphagia. 2020;35(2):281–295. doi: 10.1007/s00455-019-10023-y. [DOI] [PubMed] [Google Scholar]