Introduction
As patients with Plasma Cell Disorders (PCDs) live longer due to therapeutic advances, outcomes may be further improved by optimizing nutrition. Additionally, monoclonal gammopathy of undetermined significance (MGUS) and low- to intermediate-risk smoldering multiple myeloma (SMM) present unique opportunities for early intervention, given the standard of care is observation over time [1].
Epidemiologic studies have confirmed that diet is a known risk factor for PCDs [2]. Two large prospective cohort studies support that Western diets, noted for their high inflammatory or insulinemic potential, may be linked to an increased risk of multiple myeloma (MM), while vegetarians and vegans have decreased risk compared to meat-eaters [3, 4]. Further studies based on individual dietary components suggest that increased consumption of fruits, vegetables, whole grains, and seafood is associated with a reduced risk of PCDs [5–7] (https://pubmed.ncbi.nlm.nih.gov/9639389/). In addition, MM-specific mortality is lower in patients with healthful pre-diagnosis dietary patterns, suggesting the potential for diet to affect survival outcomes as well [8]. Although the exact mechanistic basis of diet in plasma cell dyscrasias is unknown, early studies suggest the microbiome may play a significant role (https://www.medrxiv.org/content/10.1101/2022.03.29.22272361v1).
Patients with PCDs are often interested in learning how to optimize their physical health through diet, but oncologists and hematologists commonly do not address these concerns possibly due to the lack of PCD-specific dietary guidelines, although general guidelines by the American Institute for Cancer Research (AICR) and the American Cancer Society (ACS) for cancer prevention and survivorship do exist [9, 10]. Therefore, they are applicable to MGUS and SMM in addition to MM. The aim of this 24-question online survey was to explore patients’ nutrition information needs, perceptions, and practices and to identify areas for further research.
Subjects and methods
We utilized HealthTree® Cure Hub, an online tool created by HealthTree Foundation (a division of the 501(c)3 non-profit organization, CrowdCare Foundation), and invited participants with PCDs to answer questions pertaining to their diet and nutrition and related experience with their hematologists and oncologists [11]. This study was reviewed by the Memorial Sloan Kettering Cancer Center Institutional Review Board and determined to be exempt from further review (IRB X20-091). Over 8000 patients with a known history of PCDs in the United States had access to this survey from January to June 2021. Participants provided written informed consent at survey initiation. Deidentified survey responses and pre-collected health data for each participant were retrieved through the HealthTree platform at study conclusion. Summary statistics were used to estimate the distribution of responses across questions as a function of the number of participants that answered a given question. Differences in question responses between patients diagnosed with malignant (primary plasma cell leukemia (PCL), MM) versus precursor conditions (MGUS, SMM, plasmacytoma) were tested using Fisher’s exact test. McNemar’s Chi-square test was used to assess dietary shifts pre- and post-diagnosis.
Results
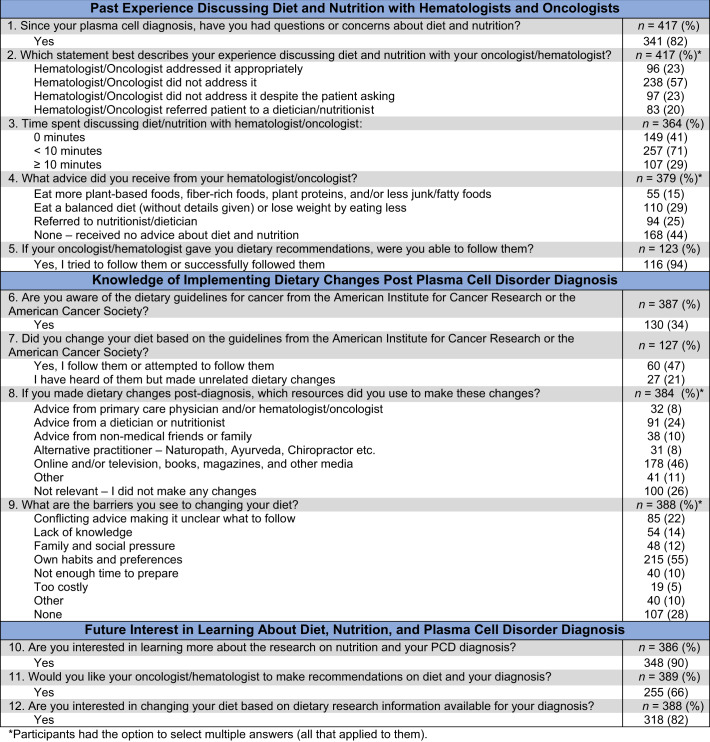
We obtained 421 survey respondents: 205 (49%) ≤65 years, 153 (36%) male, and 282 (67%) white. A range of PCD diagnoses were represented, including 299 (71%) MM, 63 (15%) SMM, 18 (4%) MGUS, 6 (1%) solitary plasmacytoma, 1 (0%) PCL, and 34 (8%) unknown. There was no statistically significant difference in survey responses between those diagnosed with malignant versus precursor conditions. Overall, the majority of respondents (82%) reported having questions or concerns about diet and nutrition (i.e., foods to eat or avoid, portion sizes, and special diets) while fewer than half (43%) indicated that their hematologist or oncologist either appropriately addressed them directly (23%) or referred the patient to a dietician or nutritionist (20%). Moreover, 57% stated that diet and nutrition were not addressed by their hematologists or oncologists at all and 23% stated this topic was not addressed despite asking. Most patients (71%) reported that their hematologist or oncologist spent <10 min discussing nutrition with them; 41% spent 0 min (Table 1).
Table 1.
Perceptions and Experiences with Hematologists and Oncologists Regarding Diet and Nutrition.
About a third of respondents (29%) reported receiving non-specific dietary advice from their hematologist or oncologist, such as to eat a “balanced diet” or to consume less to lose weight, while 15% reported receiving more detailed meaningful guidance (i.e., recommended specific plant-based foods, fiber-rich foods, plant proteins, and/or less junk/fatty foods). Survey results reveal that of the patients that were able to receive dietary recommendations from hematologists or oncologists, the vast majority (94%) stated that they attempted to follow the advice. Additionally, although the ACS and the AICR have published dietary guidelines, 34% of respondents were aware of these guidelines, and of this group 47% attempted to follow them (Table 1).
Lack of knowledge and conflicting advice were barriers to making dietary changes for 14 and 23% of respondents, respectively. Presently, most receive post-diagnosis dietary guidance from non-medical sources, online, television, books, magazines, and other media (46%), advice from non-medical friends or family (10%) and alternative practitioners (naturopath, Ayurvedic doctor, chiropractor, etc.) (8%). Hematologists, oncologists, or primary care providers were a resource in making post-diagnosis dietary changes for 8%, and 24% received advice from dieticians or nutritionists (Table 1).
Most respondents (90%) indicated that they were interested in learning more about nutrition research and their diagnosis, 82% confirmed their interest in changing their diet based on this research, and 66% expressed that they would like their oncologist to make recommendations (Table 1). The most common motivating reasons reported by patients for implementing dietary changes include feeling better physically (68%), taking more control of one’s health (62%), feeling better emotionally (47%), looking better (42%), and losing weight (37%).
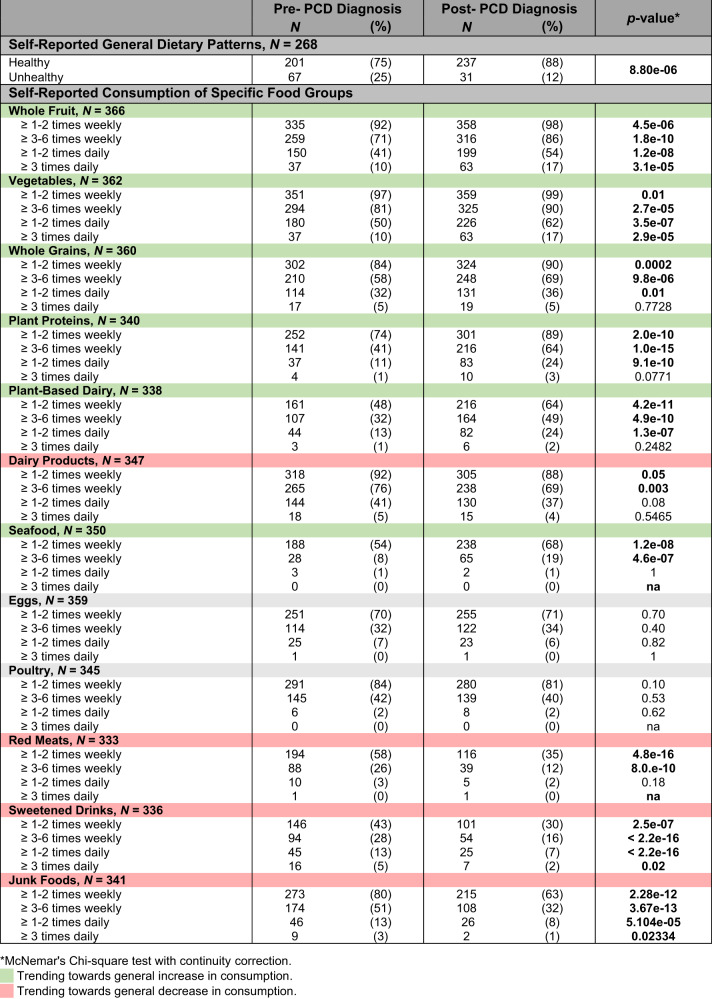
A significant number of patients self-reported that they consumed a healthier diet after diagnosis (75% pre versus 88% post, p < 0.0001). Furthermore, among patients with a self-reported unhealthy diet pre-diagnosis, 73% improved their diet post-diagnosis. In contrast, 6% with a healthy diet pre-diagnosis worsened their diet post-diagnosis (Table 2). Patients reported consuming food groups such as whole fruits, vegetables, whole grains, plant proteins, plant-based dairy, and seafood at significantly higher rates post-diagnosis (p < 0.0001). There was a concurrent decrease in the consumption of red meats, dairy products, sweetened drinks, and junk foods (Table 2).
Table 2.
Self-reported dietary patterns in patients pre-PCD diagnosis versus post-PCD diagnosis.
Discussion
Survey responses indicated that patients often change their diets post-diagnosis, suggesting that they may be amenable to dietary interventions. Cancer patients have been well-documented to make dietary changes following a diagnosis, and this trend extends to PCD patients [12] (https://pubmed.ncbi.nlm.nih.gov/12616253/). A cancer diagnosis can induce psychological stress which can motivate individuals to reduce known risk factors and promote general health [13]. Our results confirm that besides patients with active plasma cell malignancies, patients with precursor conditions such as MGUS may be similarly empowered to make dietary and lifestyle changes as they are apprehensive about their cancer progression risk. The lack of difference in survey responses between patients with active cancer and precursor disorders maybe due to the small sample size. The role of diet is possibly different across the plasma cell disorder spectrum and may be dependent on disease stage, nutritional status, comorbidities, and patient preference.
Additionally, a meta-analysis evaluating the effectiveness of primary care-based dietary interventions showed that personalized guidance from healthcare professionals can usher sustainable healthy diets in patients (https://doi.org/10.1002/1099-1611(200009/10)9:5%3C418::AID-PON474%3E3.0.CO;2-E). This suggests that patients who get professional guidance make healthier shifts. Of the 123 patients that reported receiving dietary advice directly from hematologists and oncologists, an overwhelming 94% stated that they attempted to follow the advice. This highlights the positive influence physicians may have in propelling healthful dietary changes.
Our results also highlight the important role that dieticians and non-medical sources (internet, books, magazines, social media) play, given that despite 90% of respondents desired dietary information, only 66% expressed interest in receiving guidance from their oncologist or hematologist. This study indicates that though patients with PCDs are inclined to eat more healthfully post-diagnosis, the majority currently do not receive this information from physicians and may benefit from professional input from dietitians or physicians to alleviate any uncertainties regarding diet and nutrition.
Strengths of this study include the large sample size. A limitation includes the flexible branching logic of the survey instrument which allowed patients to selectively answer certain questions. Thus, we captured differing response rates across some sections (i.e., Table 1 versus Table 2 questions) as participants were less likely to complete questions further along the survey. Alternatively, this scheme allowed for a larger clinical sample size. The retrospective nature of surveys may have led to recall bias in patients, producing an overestimation of effect size when comparing pre-diagnosis habits with those post-diagnosis. Although the selection of HealthTree Cure Hub as the platform to disseminate the survey lent itself to greater outreach amongst patients, this may have led to a self-selection bias from patients who are interested in this topic and may already have made dietary changes. Beyond selection bias, the generalizability of these results may be constrained by the low response rate (5.3%) given 421 responses were captured despite 8000 site visitors. However, the exact number of patients active on the site during the survey period is unknown and is likely under 8000.
Conclusions
To summarize, our survey reveals a missed opportunity between patients’ need for dietary advice and the potential for hematologists and oncologists to provide helpful counsel. Patients with PCDs are interested in dietary advice from hematologists and oncologists to make healthful dietary switches. Most patients currently make dietary changes post-diagnosis. However, they receive advice pertaining to diet and nutrition from non-medical sources and report barriers related to lack of consistent information. Our findings highlight a need for additional research into standardized guideline (AICR and ACS) implementation as well as for the development of PCD-specific guidelines by hematologists and oncologists. Further disease focused dietary studies among patients with PCDs, especially those aiming to assess the impact of defined dietary interventions on biomarkers of disease prognosis and survival outcomes (e.g., NCT04920084), are essential to fill this gap.
Acknowledgements
This research was made possible through the support of HealthTree Foundation (Lehi, Utah, USA) and the NIH/NCI Cancer Center Support Grant P30 CA008748. M.M. received research funding through the National Cancer Institute Summer Medical Student Research Fellowship program at Memorial Sloan Kettering Cancer Center. U.A.S. received research grant support from International Myeloma Society Career Development Award, Paula and Rodger Riney Foundation, the Allen Foundation Inc, the HealthTree Foundation, and the NCI MSK Paul Calabresi Career Development Award for Clinical Oncology 2K12CA184746. Her research is also supported by the American Society of Hematology Clinical Research Training Institute Award (U.A.S) and the TREC Training Workshop R25CA203650 (PI: Melinda Irwin).
Author contributions
UAS, MJ, SC, NS, JA, CC, AML, JMA, NI conceived and designed the study. UAS, MM, AD, NWS, curated, analyzed, accessed, verified, and interpretated data. UAS, MM, AD, NWS, JH, SM, SC, ADS, AML, SZU, SAG, MVDB, NI, SEM, SM, NK, CRT, HH, MH, PAA drafted and edited the manuscript. UAS, MM, AD, NWS, had full access to the data and share final responsibility for submission of the publication. All authors wrote and approved the article and are accountable for publication.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
UAS has received grants and research support from Celgene/Bristol Myers Squibb, Janssen paid to the institution, personal fees from Janssen, MJH Life Sciences, ACCC, MashUpMD all outside of the submitted work. SG reports personal fees and advisory role (scientific advisory board) from Actinnum, Celgene, Bristol Myers Squibb, Sanofi, Amgen, Pfizer, GlaxoSmithKline, JAZZ, Janssen, Omeros, Takeda, and Kite, outside the submitted work. HH reports grants from Celgene, during the conduct of the study; and grants from Celgene, Takeda, and Janssen, outside the submitted work. NK reports research funding through Amgen and participates in advisory board with Medimmune. OLan reports grants from Amgen, Janssen, and Takeda; Data Monitoring Committee from Janssen, Merck, and Takeda; and personal fees from Amgen, Janssen, GlaxoSmithKline, AstraZeneca, and The Binding Site, outside the submitted work. AML reports grants from Novartis, during the conduct of the study; grants from Bristol Myers Squibb; personal fees from Trillium Therapeutics; grants, personal fees and nonfinancial support from Pfizer; and grants and personal fees from Janssen, outside the submitted work. AML also has a patent US20150037346A1 with royalties paid. SM reports research funding from Allogene Therapeutics, Juno/Bristol Myers Squibb, Takeda Oncology, and Janssen Oncology; personal fees from Plexus communication, and Physician Education Resource, outside the submitted work. CT reports other from Janssen Research and Development outside the submitted work. JH has received personal fees and scientific advisory board fees from Amgen, Janssen Biotech, Glaxo Smith Kline, Bristol Myers Scribbs, Skyline, Oncopeptides and Sanofi.SZU reports grant support from Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX and Takeda; personal fees from Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda and TeneoBio; outside the submitted work.MVDB has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, WindMIL Therapeutics, Rheos Medicines, Merck & Co, Inc., Magenta Therapeutics, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Forty Seven Inc., Ceramedix, Lygenesis, Pluto Therapeutics, GlaskoSmithKline, Da Volterra, Novartis (Spouse), Synthekine (Spouse), and Beigene (Spouse); he has IP Licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kyle RA, Baudi FI, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncol (Williston Park) [Internet]. 2011;25:578. [cited 2021 Aug 9]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3923465/.. [PMC free article] [PubMed]
- 2.Parikh R, Tariq SM, Marinac CR, Shah UA A comprehensive review of the impact of obesity on plasma cell disorders. Leuk 2021 [Internet]. 2021 Oct [cited 2021 Nov 5];1–14. Available from: https://www.nature.com/articles/s41375-021-01443-7. [DOI] [PMC free article] [PubMed]
- 3.Lee DH, Fung TT, Tabung FK, Colditz GA, Ghobrial IM, Rosner BA, et al. Dietary pattern and risk of multiple myeloma in two large prospective US cohort studies. JNCI Cancer Spectr [Internet]. 2019 Jun [cited 2021 Jul 27];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6532330/. [DOI] [PMC free article] [PubMed]
- 4.Key T, Appleby P, Crowe F, Bradbury K, Schmidt J, Travis R Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr [Internet]. 2014 Jul [cited 2021 Jul 27];100 Suppl. Available from: https://pubmed.ncbi.nlm.nih.gov/24898235/. [DOI] [PMC free article] [PubMed]
- 5.Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Steingrimsdottir L, et al. Dietary intake is associated with risk of multiple myeloma and its precursor disease. PLoS One [Internet]. 2018 Nov [cited 2021 Jul 27];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6211667/. [DOI] [PMC free article] [PubMed]
- 6.Vlajinac H, Pekmezović T, Adanja B, Marinković J, Kanazir M, Suvajdzić N, et al. Case-control study of multiple myeloma with special reference to diet as risk factor. Neoplasm [Internet] 2003;50:79–83. [PubMed] [Google Scholar]
- 7.Hosgood H, Baris D, Zahm S, Zheng T, Cross A. Diet and risk of multiple myeloma in Connecticut women. Cancer Causes Control [Internet] 2007;18:1065–76. doi: 10.1007/s10552-007-9047-z. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Fung TT, Tabung FK, Marinac CR, Devore EE, Rosner BA, et al. Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int J Cancer [Internet] 2020;147:1823–30. doi: 10.1002/ijc.32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin [Internet] 2020;70:245–71. doi: 10.3322/caac.21591. [DOI] [PubMed] [Google Scholar]
- 10.American Institute for Cancer Research. How to Prevent Cancer: 10 Recommendations [Internet]. 2021 [cited 2022 Jan 29]. Available from: https://www.aicr.org/cancer-prevention/.
- 11.MyelomaCrowd. HealthTree - Improving Myeloma Patient Outcomes & Accelerating a Cure [Internet]. CrowdCare Foundation. 2021 [cited 2021 Aug 10]. Available from: https://www.myelomacrowd.org/healthtree/.
- 12.Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. Eur J Cancer Care (Engl) [Internet] 2001;10:12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Mcbride CM, Clipp E, Peterson L, Lipkus IM, Demark-Wahnefried W. Psychological impact of diagnosis and risk reduction among cancer survivors. Psychooncology. 2000;9:418–27. doi: 10.1002/1099-1611(200009/10)9:5<418::AID-PON474>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.