Abstract
The burden of cancer diseases is increasing every year, therefore, the demands to figure out novel drugs that can retain antitumor properties have been raised. This study aimed to investigate the anti-tumor properties of amygdalin (Amy) against Ehrlich ascites carcinoma (EAC) bearing mice and its protective properties against liver damage. Amy and the standard anticancer drug Sorafenib (Sor) were given alone or in combination to Swiss albino female mice that had been injected with EAC cells. Biochemical parameters of liver function (AST, ALT, GGT, total protein, albumin), tumor volume, oxidative stress [malondialdehyde, (MDA)] and antioxidative [superoxide dismutase (SOD), and reduced glutathione (GSH)] markers were measured. The hepatic expression of the antioxidant-related gene [nuclear factor erythroid-2-related factor 2 (Nrf2)], the migration-related gene [matrix metalloprotease 9 (MMP9)], and the angiogenesis-related gene [vascular endothelial growth factor (VEGF)] were evaluated by qPCR. The results revealed that EAC-bearing mice treated with Amy and/or Sor showed a decrease in the tumor burden and hepatic damage as evidenced by (1) decreased tumor volume, number of viable tumor cells; (2) increased number of dead tumor cells; (3) restored the liver function parameters; (4) reduced hepatic MDA levels; (5) enhanced hepatic GSH and SOD levels; (6) upregulated expression of Nrf2; (7) downregulated expression of MMP9 and VEGF, and (8) improved hepatic structure. Among all treatments, mice co-treated with Amy (orally) and Sor (intraperitoneally) showed the best effect. With these results, we concluded that the Amy improved the antitumor effect of Sor and had a protective role on liver damage induced by EAC in mice.
Subject terms: Biochemistry, Drug discovery
Introduction
Cancer is characterized by an uncontrolled division of cells which can spread by direct growth into the tissues through invasion or by metastasis1. Ehrlich ascites carcinoma (EAC) induces a local inflammatory reaction with cumulative vascular permeability which results in strong edema, progressive ascitic fluid formation, and cellular migration, which are essential for tumor growth2–4. Cancer therapy involves surgery, chemotherapy, radiation, hormonal, and biological approaches. Struggles have been made to recognize natural and synthetic anticancer with antioxidant properties5–7. Alternatively, both anticancer and herbal extract with antioxidant properties could be given together during cancer treatment. Indeed, some trials have been conducted and the obtained results revealed the presence of a dual synergistic effect between synthetic antitumor and herbal agent1,8–10.
Amygdalin (Amy, Vit B12) has been recently accepted as one of the main sources of cancer chemoprevention drugs due to their diverse pharmacological properties11,12. Amygdalin is rich in anti-cancer compounds such as hydrocyanic acid in addition to benzaldehyde, which can induce an analgesic action13. Co-administration of a synthetic anti-tumor agent with amygdalin relieves hepatotoxicity and liver fibrosis14. Sorafenib (Sor) is a drug that has been shown to prolong overall survival in patients with advanced liver cancer. Sorafenib is an oral multi-kinase inhibitor exerting its effects via RAF/MEK/ERK pathway15, vascular endothelial growth factor receptor (VEGFR)16, and platelet-derived growth factor receptor beta (PDGFR-β) tyrosine kinases17. Little data are available in the literature regarding the combined effect of Amy and Sor against tumor burden and the liver damage induced by EAC. Therefore, this study was conducted to investigate this effect.
Results
Effect of amygdalin and/or sorafenib on body weight
Among all groups, only Cnt, Amy, EAC, Amy + SorIP, and EAC + Amy showed significantly (P ≤ 0.05) higher body weight as revealed by positive values of the body weight change with highest value in EAC followed by Cnt, then EAC + Amy, and finally Amy and Amy + SorIP group (Table 1). Moreover, EAC mice treated with Sor either IP (EAC + SorIP) or orally (EAC + SorOS) showed a significant decrease in body weight compared to EAC and control groups. The treatment with Amy alone showed either a significant decrease or insignificant increase in body weight when compared with EAC or control (Cnt) group, respectively. Dual treatment with Amy and Sor (OS or IP) resulted in a significant decrease in the body weight as compared with EAC-treated and control mice (Table 1).
Table 1.
Mice initial body weights and change in body weight in different experimental groups.
| Groups | Initial body weight (g) | Body weight change (g) |
|---|---|---|
| Cnt | 20.25 ± 0.75 | 4.26 ± 0.33b |
| Amy | 21.47 ± 0.67 | 2.75 ± 0.12c |
| SorIP | 21.75 ± 0.85 | − 7.23 ± 0.41f |
| SorOS | 20.75 ± 0.65 | − 8.28 ± 0.60f |
| Amy + SorIP | 21.31 ± 0.71 | 2.25 ± 0.12c |
| Amy + SorOS | 21.00 ± 0.40 | − 4.22 ± 0.45e |
| EAC | 20.48 ± 0.63 | 7.53 ± 0.92a |
| EAC + Amy | 21.63 ± 0.55 | 4.62 ± 0.46b |
| EAC + SorIP | 21.26 ± 0.40 | − 3.75 ± 0.30e |
| EAC + SorOS | 20.60 ± 0.85 | − 3.52 ± 0.31e |
| EAC + Amy + SorIP | 21.50 ± 0.69 | − 0.24 ± 0.10d |
| EAC + Amy + SorOS | 21.06 ± 0.41 | − 2.87 ± 0.12e |
Data were presented as means ± SEM. Small (a–f) letters showed the marked change at P ≤ 0.05. The significant were expressed by dissimilar letters in the same column. Cnt Control, Amy Amygdalin, Sor Sorafenib, EAC Ehrlich ascetic carcinoma, IP Intraperitoneal, OS Per os (oral).
Effect of amygdalin and/or sorafenib on tumor volume and EAC cell count
Tumor volume (volume of the ascitic fluid) in the EAC group was significantly (P < 0.05) higher than that in all other treated groups (Fig. 1). In the treated groups, EAC + Amy + SorIP group showed the lowest ascitic fluid volume followed by EAC + SorIP, then EAC + Amy + SorIP, EAC + SorOS, and finally EAC + Amy group. Treatment with Amy and Sor (IP or OS) alone or in combination significantly decreased the total and live tumor cells count, with lowest count in EAC + SorIP, and EAC + Amy + SorIP groups, followed by EAC + Amy + SorOS, EAC + SorOS, and finally EAC + Amy as compared to the EAC group (Fig. 1). However, dead cells were significantly higher in EAC + Amy and EAC + Amy + SorOS than other groups.
Figure 1.
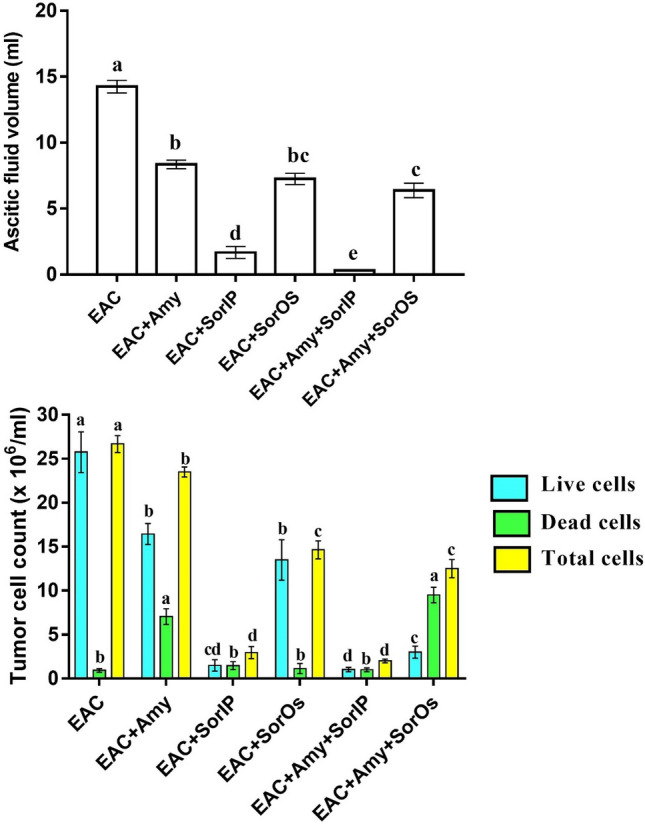
Tumor ascitic fluid volume and count of live and/or dead EAC cells in EAC-bearing mice after treatment with amygdalin and/or sorafenib. Data were presented as means ± SEM (n = 7). Small (a–e) letters showed the marked change at P ≤ 0.05. The significant were expressed by dissimilar letters above columns with the same color.
Effect of amygdalin and/or sorafenib on liver functions
EAC mice treated with Amy and/or Sor showed significantly reduced serum levels of AST, ALT, and GGT, total protein, and a significantly higher level of albumin as compared to the untreated EAC mice (Table 2). The best improvement in these serum biochemical parameters was noticed in EAC + SorIP, and EAC + Amy + SorIP groups, followed by EAC + Amy + SorOS, EAC + SorOS, and finally EAC + Amy group. Additionally, serum ALT, AST, and GGT levels in SorIP, SorOS, Amy + SorIP, and Amy + SorOS groups were significantly increased compared to the control group. However, the Amy group showed insignificant change relative to the control group (Table 2).
Table 2.
Liver function parameters in EAC-bearing mice after treatment with amygdalin and/or sorafenib.
| Group | ALT (U/L) | AST (U/L) | Albumin (g/dL) | Total protein (g/dL) | GGT (U/L) |
|---|---|---|---|---|---|
| Cnt | 44.75 ± 1.26e,f | 59.50 ± 3.87f | 4.34 ± 0.15a | 6.12 ± 0.18a | 19.75 ± 2.01e |
| Amy | 48.25 ± 1.93e | 62.75 ± 2.21f | 3.67 ± 0.11b | 5.15 ± 0.20d | 22.00 ± 2.41e |
| SorIP | 56.08 ± 1.58d | 67.00 ± 2.73e,f | 3.62 ± 0.16b | 6.23 ± 0.22a | 35.50 ± 2.75c,d |
| SorOS | 54.21 ± 1.35d | 69.21 ± 2.13e | 3.40 ± 0.09b | 5.37 ± 0.17b,d | 33.75 ± 2.75d |
| Amy + SorIP | 54.03 ± 1.84d | 64.73 ± 2.28a | 4.14 ± 0.13a | 6.05 ± 0.19a | 31.25 ± 3.25d |
| Amy + SorOS | 57.50 ± 2.21d | 77.50 ± 3.21d | 3.62 ± 0.12b | 5.50 ± 0.11b | 38.00 ± 3.13c |
| EAC | 86.47 ± 3.51a | 165.0 ± 6.72a | 2.35 ± 0.09d | 4.17 ± 0.23e | 154.37 ± 9.21a |
| EAC + Amy | 75.61 ± 3.15b | 103.8 ± 5.40b | 3.13 ± 0.24b | 5.65 ± 0.14b | 99.50 ± 7.46b |
| EAC + SorIP | 50.00 ± 2.38d,e | 74.00 ± 3.80d,e | 3.25 ± 0.11b | 5.70 ± 0.16b | 33.00 ± 2.13d |
| EAC + SorOS | 66.50 ± 2.49c | 88.00 ± 4.41c | 3.07 ± 0.15b | 5.97 ± 0.24a,b | 46.25 ± 2.80c |
| EAC + Amy + SorIP | 39.37 ± 1.48f | 48.75 ± 2.15g | 3.45 ± 0.13b | 6.47 ± 0.17a | 39.75 ± 2.13c |
| EAC + Amy + SorOS | 50.25 ± 2.38d,e | 75.75 ± 3.59d | 3.20 ± 0.11b | 5.73 ± 0.17b | 34.30 ± 2.34c,d |
Data were presented as means ± SEM. Small (a–e) letters showed the marked change at P ≤ 0.05. The significant were expressed by dissimilar letters in the same column.
Effect of amygdalin and/or sorafenib on oxidative and antioxidative markers
Untreated EAC-bearing mice have significantly higher hepatic levels of lipid peroxidation marker MDA and lower hepatic levels of the antioxidant markers (GSH and SOD) than all control groups (Table 3). Treatment with Amy and/or Sor (IP and OS) restored these markers to levels near that of the control groups with best improvement (lowest MDA and highest GSH and SOD) observed in EAC + SorIP, and EAC + Amy + SorIP groups, followed by EAC + Amy + SorOS, EAC + SorOS, and finally EAC + Amy group.
Table 3.
Hepatic oxidative (MDA) and antioxidative (GSH and SOD) parameters in EAC-bearing mice after treatment with amygdalin and/or sorafenib.
| Groups | MDA (nmol/g tissue) | GSH (nmol/g tissue) | SOD (U/g tissue) |
|---|---|---|---|
| Cnt | 3.98 ± 0.11f | 6.31 ± 0.16b | 58.63 ± 1.23a |
| Amy | 4.01 ± 0.14f | 7.22 ± 0.23a | 54.09 ± 1.99a |
| SorIP | 4.08 ± 0.09f | 6.06 ± 0.19b | 55.28 ± 1.76a |
| SorOS | 4.17 ± 0.17f | 6.13 ± 0.18b | 56.21 ± 2.38a |
| Amy + SorIP | 4.12 ± 0.15f | 5.98 ± 0.14b | 54.14 ± 2.14a |
| Amy + SorOS | 4.23 ± 0.16f | 6.04 ± 0.15b | 56.82 ± 1.77a |
| EAC | 23.58 ± 0.76a | 3.23 ± 0.11e | 25.26 ± 0.54g |
| EAC + Amy | 15.37 ± 0.43b | 4.43 ± 0.13d | 32.12 ± 0.77f |
| EAC + SorIP | 10.50 ± 0.35d | 5.14 ± 0.14c | 39.32 ± 0.54d |
| EAC + SorOS | 13.51 ± 0.32c | 5.03 ± 0.15c | 36.39 ± 0.42e |
| EAC + Amy + SorIP | 7.30 ± 0.26e | 6.35 ± 0.22b | 47.05 ± 1.01b |
| EAC + Amy + SorOS | 9.76 ± 0.36d | 5.33 ± 0.16c | 42.31 ± 0.63c |
Data were presented as means ± SEM. Small (a–g) letters showed the marked change at P ≤ 0.05. The significant were expressed by dissimilar letters in the same column.
Effect of amygdalin and/or sorafenib on the expression of Nrf2, MMP9, and VEGF genes
The obtained qPCR results revealed significantly downregulated hepatic expression of Nrf2 and upregulated hepatic expression of MMP9 and VEGF in the EAC group as compared to all control groups (Fig. 2). Administration of Amy and/or Sor (IP and OS) restored the expression of these genes to levels comparable to the control groups with best improvement (highest Nrf2 and lowest MMP9 and VEGF) noticed in EAC + Amy + SorIP, followed by EAC + Amy + SorOS, then EAC + SorIP, EAC + SorOS, and finally EAC + Amy group (Fig. 2).
Figure 2.
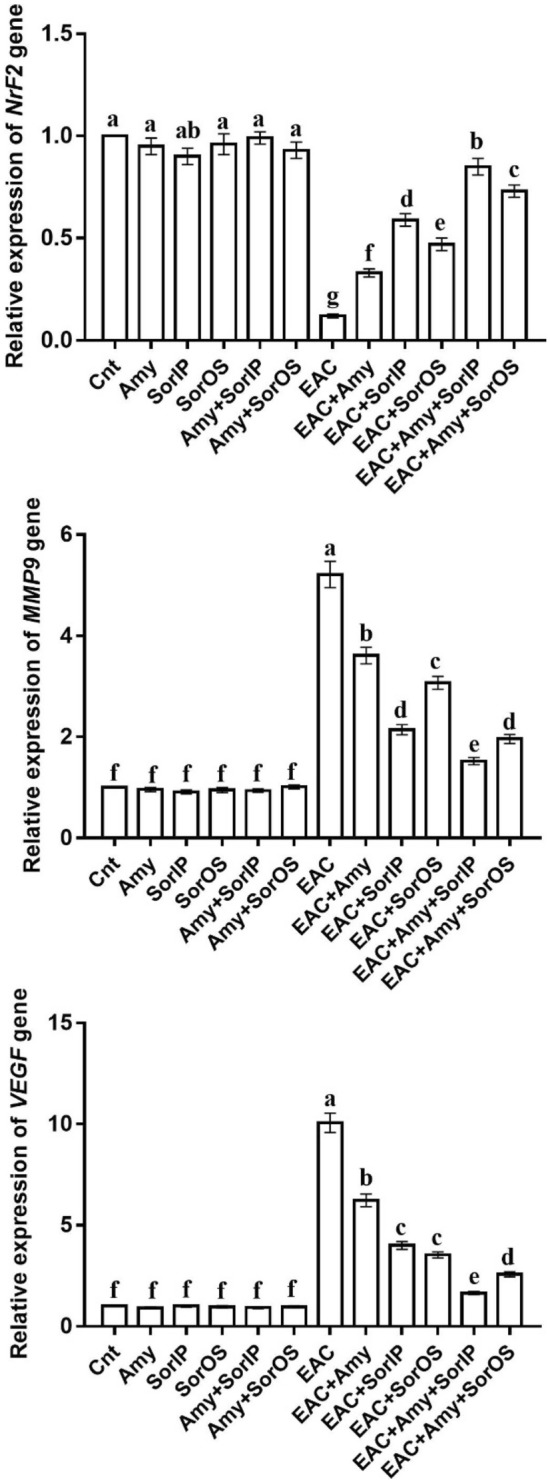
Changes in Nrf2, MMP9, and VEGF gene expression in liver of different groups as detected by real-time PCR. β-actin was used as internal control. The expression was expressed as fold changes mean ± SEM (n = 5/group). Columns with various letters [a (the highest fold change)–f (the lowest fold change)] showed significance at P < 0.05.
Histopathological examination
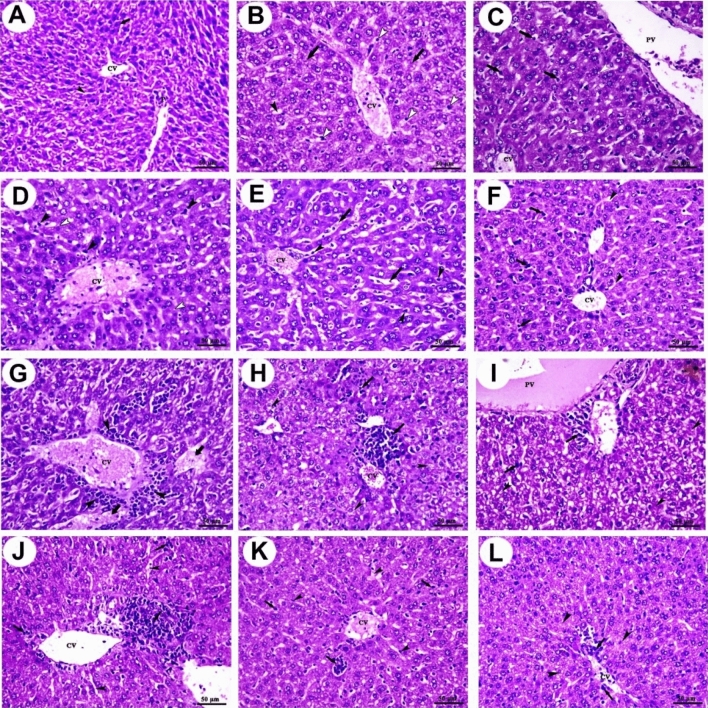
As shown in Fig. 3A, the liver of the control group showed normal hepatic architecture, central vein, portal vein area, polyhedral-shaped hepatocytes, and blood sinusoids. The liver of the Amy group showed congested central vein, mild dilation of blood sinusoids, mild degeneration of hepatocytes, and mild Kupffer cells activity (Fig. 3B). The liver of the SorIP group showed congested central vein, portal vein, mild vacuolation of hepatocytes, and moderate Kupffer cells activity (Fig. 3C). The liver of the SorOS group showed congested central vein, mild vacuolation of hepatocytes, and moderate Kupffer cells activity (Fig. 3D). The liver of the Amy + SorIP group showed congested central vein, congestion, and dilation of blood sinusoids in addition to Kupffer cells activity (Fig. 3E). The liver of the Amy + SorOS group showed mild congestion of the central vein, mild dilation of blood sinusoids, and a moderate increase of Kupffer cells activity (Fig. 3F).
Figure 3.
Photomicrographs of mice liver sections stained with H&E. (A) Control group shows central vein (CV), portal vein area (P), polyhedral-shaped hepatocytes (arrow), and blood sinusoids (arrowhead). (B) Amy group shows congested central vein (CV), mild dilation of blood sinusoids (black arrowhead), mild degeneration of hepatocytes (arrows), and mild Kupffer cells activity. (C) SorIP group shows congested central vein (CV), portal vein (PV), mild vacuolation of hepatocytes (white arrowheads), and moderate Kupffer cells activity (arrows). (D) SorOS group shows congested central vein (CV), mild vacuolation of hepatocytes (white arrowheads), and moderate Kupffer cells activity (black arrowheads). (E) Amy + SorIP group shows congested central vein (CV), congestion, and dilation of blood sinusoids (arrows) in addition to Kupffer cells activity (arrowheads). (F) Amy + SorOS group shows mild congestion of central vein (CV), mild dilation of blood sinusoids (arrowheads), and a moderate increase of Kupffer cells activity (arrows). (G) EAC group shows aggregations of pleomorphic, hyperchromatic, and darkly basophilic cells (arrowheads) around central vein (CV) and hepatocellular necrosis (arrows). (H) EAC + Amy group shows a moderate number of pleomorphic cells (arrows) with moderate degeneration (arrowheads). (I) EAC + SorIP group shows a small focal area of pleomorphic cells (arrows) with moderate degenerative changes (arrowheads). (J) EAC + SorOS group shows a moderate focal area of pleomorphic cells (arrows) and mild dilation of some blood sinusoids (arrowheads). (K) EAC + SorOS group shows the smallest focal area of the pleomorphic cells (arrows) with the mildest congestion in some blood sinusoids (arrowheads). (L) EAC + Amy + SorOS group shows small diffuse pleomorphic cells (arrows) with mild vacuolar degeneration of hepatocytes (arrowheads). Scale bars = 50 µm.
On the other hand, livers of untreated EAC-bearing mice (EAC group) showed aggregations of pleomorphic, hyperchromatic, and darkly basophilic cells (these cells were assumed to be EAC cells) in the perivascular area around the dilated and congested central vein as well as a notable area of hepatocellular necrosis (Fig. 3G). The five treated groups showed notable improvement in liver structure with less damage as compared to the EAC group. In the EAC + Amy group, the liver showed a moderate number of pleomorphic cells with moderate degeneration (Fig. 3H). The liver of the EAC + SorIP group showed a small focal area of pleomorphic cells with moderate degenerative changes (Fig. 3I). The liver of the EAC + SorOS group showed a moderate focal area of pleomorphic cells and mild dilation of some blood sinusoids (Fig. 3J). The liver of the EAC + SorOS group showed the smallest focal area of the pleomorphic cells with the mildest congestion in some blood sinusoids (Fig. 3K). The liver of the EAC + SorOS group showed small diffuse pleomorphic cells with mild vacuolar degeneration of hepatocytes (Fig. 3L).
Discussion
Mice injected with EAC cells and treated with Amy and/or Sor (IP or OS) showed a significant reduction in the ascitic fluid (tumor volume), the body weight, and viable tumor cell as compared to mice in the untreated EAC group. Among the five treated groups, the best antitumor effect (as revealed by less ascitic fluid, body weight, and viable EAC cells) was noticed in animals treated with both Amy and Sor with better effect for those injected IP by Sor. This antitumor effect could be attributed to enhanced phagocytes and cytokines production by Amy and/or Sor on EAC cells18. Our results also revealed that treatment with Amy and/or Sor not only significantly reduced the viable EAC cell count but also increased the count of non-viable cells. This infers that the antitumor action of Amy and Sor could be mediated through a direct inhibitory potential on the proliferation of tumor cells.
Hepatocytes are the main target for hepatic enzyme metabolism. Injury of these cells results in leakage of liver enzymes such as AST, ALT, and GGT from the liver into the circulation with an ultimate elevation of enzyme serum levels19–21. Hepatocytes are rigorously injured in animals with EAC cells22. This hepatic damage is accompanied by an increase in the levels of ALT and AST in the serum of EAC-bearing mice22–24. Consistent with these findings, we also found a significant elevation in ALT, AST, and GGT in the EAC group as compared to the control group. Reduced level of these hepatic damage enzymes in serum is associated with the antitumor potential of synthetic and natural anticancer compounds19,25,26. In agreement, we also found a significant reduction in the serum levels of these enzymes in EAC mice treated with Amy and/or Sor with best effect for animals treated with both Amy and SorIP. In line with our findings, another study reported that administration of Amy decreased the elevated ALT, AST, ALP, and GGT levels in N-nitrosodiethylamine-intoxicated rats27. Albumin and total proteins are other hepatic markers that are very important to follow up the progression of liver damage. We found a significant decrease in these two markers in EAC-bearing mice when compared to the control mice. Similarly, decreased levels in serum total protein and albumin were also observed in hepatic dysfunction cases2,22. Again, administration of Amy and/or Sor elevated these two markers in EAC-bearing mice with superior effect in co-treated mice, especially with Amy and SorIP. Similarly, Amy pretreatment increased albumin, total proteins in intoxicated rats27.
In the present study, treatment with Amy and/or Sor neutralized the effects induced by oxidative stress in EAC-bearing mice. This overall conclusion is based on our data which revealed that administration of Amy and/or Sor inhibited the lipid peroxides (MDA) and increased the antioxidant markers (GSH and SOD). In support, Amy increases the free radical scavenging activity through its stimulatory effect on GSH level and SOD activity in the liver of intoxicated rats27. The therapeutic potential of Amy is mainly attributed to its components that exhibit a wide range of biological effects including free radical scavenging28. Amy also improved the level of GSH in dimethylnitrosamine-induced liver fibrosis27. At a molecular level, mice treated with Amy and/or Sor showed a significant upregulation of the antioxidant-related Nrf2 gene, with best effect in mice co-treated with Amy and SorIP, as compared to the untreated EAC-bearing mice. Nrf2 is mandatory for defending the body against cancer29.
It was found that tumor-induced angiogenesis is initiated by angiogenic cytokines such as basic fibroblast growth factor (bFGF) and VEGF that are expressed in the tumor itself30. The expression of VEGF is increased by the treatment of TGFβ, which is activated by MMP931. Our data revealed a significantly upregulated expression of the migration-related MMP9 gene and the angiogenesis-related VEGF gene in the liver of the untreated EAC-bearing mice. Our results are consistent with El Bakary, et al. (32) who reported a significant elevation in the expressions of MMP9 and VEGF in EAC mice. This expression was downregulated following treatment with Amy and/or Sor, with best effect for Amy + SorIP.
Histopathological examination showed aggregations of pleomorphic, hyperchromatic, and darkly basophilic cells assumed to be EAC cells in the perivascular area around the dilated and congested central vein and hepatocellular necrosis in the liver of the EAC group. Additionally, degenerative changes such as loss of histo-architecture, due to microvascular fatty changes in addition to mild Kupffer cells activity were also observed. It was also reported that EAC cells can migrate from the peritoneal cavity and reach the liver causing liver injury32. EAC-bearing mice treated with Amy and/or Sor showed notable improvement in liver structure, especially in Amy + SorIP co-treated mice.
Conclusions
Treatment of EAC-bearing mice with Amy and/or Sor reduced tumor burden and lipid peroxidation, improved antioxidant status, and restored the damaged hepatocytes. Amy and SorIP are more effective in ameliorating the effect of liver damage in EAC- bearing mice. Amy could improve the antitumor effect of Sor on EAC. Hence, it could be utilized as an adjuvant for Sor during cancer therapy. Therefore, further studies are encouraged to create a novel strategy targeting cancer cells using Amy and Sor co-therapy.
Materials and methods
Animals
Research ethical approval was obtained from the research ethical committee, Faculty of Science, Tanta University, Egypt, as established by the institutional animal care and use committee (IACUC). All methods were completed in accordance with ARRIVE guidelines. The experiment was carried out on female Swiss albino mice weighing 20–25 g and of 10–12 weeks ages. Mice were maintained under standardized conditions. Mice were kept in a controlled temperature environment with a 24 h cycle. All mice were adapted to the place for two weeks before the start of the experiment. The animals were provided with a normal diet and water ad libitum. Four EAC-bearing mice at day 14 of EAC intraperitoneal (IP) injection were obtained from the Cancer Biology Unit, Cairo, Al-Kaser Al-Eini, Egypt. In our lab, the ascitic fluid containing EAC cells was maintained and propagated by serial aseptic IP transplantation in mice. Each mouse was injected with 200 mL of 1 × 106 EAC cells33. EAC cells filled the peritoneal cavity by fast division of cells, resulting in accumulation of ascitic fluid, and the animal could be died 17–18 days after EAC injection if did not receive appropriate treatment3.
Experimental design
Seventy-two mice were divided into 12 groups (n = 6/group). Normal (control) group: mice were injected IP with normal saline (0.9% w/v, 300 µl/mouse). Amy group: mice were administrated amygdalin. SorIP group: mice were IP treated with sorafenib. SorOS group: mice were treated orally with sorafenib. Amy + SorIP group: mice were administered amygdalin and injected IP with sorafenib. Amy + SorOS group: mice were orally given amygdalin and sorafenib. EAC group: mice were injected IP by 200 mL of 1 × 106 EAC cells and left for 14 days without treatment. EAC + Amy group: animals were injected once with EAC cells and 24 h later they were treated with amygdalin. EAC + SorIP: mice were inoculated with EAC cells and 24 h later they were IP injected with sorafenib. EAC + SorOS: mice were inoculated once with EAC cells and 24 h later they were orally administered sorafenib. EAC + Amy + SorIP: mice were inoculated once with EAC and 24 h later they were co-treated with amygdalin and sorafenib (IP). EAC + Amy + SorOS group: mice were inoculated with EAC and 24 h later they were co-treated with amygdalin and sorafenib. All treatments were given daily for 14 days and the doses of amygdalin (300 mg/kg mouse) and sorafenib (30 mg/kg mouse for both IP and OS) were chosen based on a pilot study with aid of previous studies34,35. Amygdalin (Vitamin B17) and sorafenib were purchased from Sigma-Aldrich and BAYER companies, respectively. The animals were weighed at the beginning of the experiment (initial body weight, g), at the end of the experiment (final weight, g) and the mean body weight change (g) was then calculated by subtracting the initial weight from the final weight. It is well-known that changes in body weight of EAC-bearing mice is an additional indirect measure of changes in tumor mass in these animals.
Sampling
At the end of the experimental period (2 weeks), overnight fasted rats have sacrificed for 24 h. After the last treatment, blood samples were centrifuged in clean glass tubes for 15 min at 3000×g to get clear, non-hemolyzed sera. Eppendorf tubes with labels were immediately shipped to − 20 °C; the sera were frozen for biochemical analysis. After euthanization by exsanguination, livers were immediately removed and some specimens were fixed in 10% formalin (pathological investigation), and the others were either homogenized (biochemical assay) or frozen in − 70 °C (RNA extraction).
Tumor (ascitic fluid) volume and EAC count
At the end of the experiment (day 14), ascitic fluid containing EAC cells was withdrawn from the peritoneal cavity of each mouse before they were dissected. A graduated centrifuge tube was used to measure how much ascitic fluid was collected. Following the suspension of EAC cells in sterile isotonic saline, a Neubauer hemocytometer was used to count the total, viable and non-viable EAC cells33.
Assessment of biochemical parameters
The serum level of liver damage enzymes [aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT)], albumin, and total proteins were measured using commercially available kits (Biomed Diagnostics, Cairo, Egypt). Liver homogenates were prepared as previously described36. The hepatic levels of lipid peroxidation marker malondialdehyde (MDA) and the antioxidant markers reduced glutathione (GSH) and superoxide dismutase (SOD) were measured colorimetrically using kits purchased from Biodiagnostics and as previously described37,38.
Real-time PCR
Real-time PCR was used to determine changes in the relative expression of Nrf2, MMP9, and VEGF genes in liver specimens of all groups. Total RNA was first isolated and then reverse transcribed into cDNA by kits purchased from Thermo Scientific, USA (# K0731 and #EP0451, respectively). The sequences of primers were as following: F: 5′ CACATCCAGACAGACACCAGT 3′ and R: 5′ CTACAAATGGGAATGTCTCTG C 3′ for Nrf2; F: 5′ TCGAAGGCGACCTCAAGTG 3′ and R: 5′ TTCGGTGTAGCTTTG GATCCA 3′ for MMP9; F: 5′ GATCATGCGGATCAAACCTCACC 3′ and R: 5′ CCTCCGGACCCAAAGTGCTC 3′ for VEGF; F: 5′ CATGGATGACGATATCGCT 3′ and R: 5′ CATGAGGTAGTCTGTCAGGT 3′ for β actin (internal control). Thermal and melting curve conditions were done as previously detailed39–41. The fold change in gene expression was determined using the 2−∆∆Ct method.
Histopathological investigation
Liver tissues were dehydrated in ascending series of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 5 µm thickness by a microtome, stained with eosin and hematoxylin, examined, and photographed under a light microscope to detect histopathological changes.
Statistical analysis
GraphPad Prism 5.0 was used to analyze the data. The experimental results were expressed as mean ± standard error mean (SEM). Data were assessed by one-way analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons test. Values for which P < 0.05 were considered statistically significant.
Institutional review board statement
The study was conducted according to the guidelines of ARRIVE and approved by Ethical committee at the Faculty of Science, Tanta University.
Author contributions
Participated in research design: A.E., A.F.S., M.A.M., S.A.E., and M.A.E. Conducted experiments: A.F.S., A.E., J.G.E., and M.A.E. Performed data analysis: A.A.A., A.E., H.M.N. and M.A.E. Wrote or contributed to writing of the manuscript: all authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data presented in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Badawy AA, Othman RQA, El-Magd MA. Effect of combined therapy with camel milk-derived exosomes, tamoxifen, and hesperidin on breast cancer. Mol. Cell. Toxicol. 2021 [Google Scholar]
- 2.Mutar TF, Tousson E, Hafez E, Abo Gazia M, Salem SB. Ameliorative effects of vitamin b17 on the kidney against ehrlich ascites carcinoma induced renal toxicity in mice. Environ. Toxicol. 2020;35:528–537. doi: 10.1002/tox.22888. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya S, Haldar PK. Trichosanthesdioica root extract induces tumor proliferation and attenuation of antioxidant system in albino mice bearing ehrlich ascites carcinoma. Interdiscip. Toxicol. 2011;4:184–190. doi: 10.2478/v10102-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magdy A, Sadaka E, Hanafy N, El-Magd MA, Allahloubi N, El Kemary M. Green tea ameliorates the side effects of the silver nanoparticles treatment of ehrlich ascites tumor in mice. Mol. Cell. Toxicol. 2020;16:271–282. [Google Scholar]
- 5.Zedan AMG, Sakran MI, Bahattab O, Hawsawi YM, Al-Amer O, Oyouni AAA, Nasr Eldeen SK, El-Magd MA. Oriental hornet (Vespaorientalis) larval extracts induce antiproliferative, antioxidant, anti-inflammatory, and anti-migratory effects on mcf7 cells. Molecules. 2021;26:3303. doi: 10.3390/molecules26113303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansour GH, El-Magd MA, Mahfouz DH, Abdelhamid IA, Mohamed MF, Ibrahim NS, Hady A, Abdel Wahab A, Elzayat EM. Bee venom and its active component melittin synergistically potentiate the anticancer effect of sorafenib against hepg2 cells. Bioorg. Chem. 2021 doi: 10.1016/j.bioorg.2021.105329. [DOI] [PubMed] [Google Scholar]
- 7.Mahfouz DH, El-Magd MA, Mansour GH, Abdel Wahab AH, Abdelhamid IA, Elzayat E. Therapeutic potential of snake venom, l-amino oxidase and sorafenib in hepatocellular carcinoma. Mol. Cell. Toxicol. 2021 [Google Scholar]
- 8.Awad MG, Ali RA, Abd El-Monem DD, El-Magd MA. Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol. Cell. Toxicol. 2020;16:385–399. [Google Scholar]
- 9.El-Magd MA, Mohamed Y, El-Shetry ES, Elsayed SA, Abo Gazia M, Abdel-Aleem GA, Shafik NM, Abdo WS, El-Desouki NI, Basyony MA. Melatonin maximizes the therapeutic potential of non-preconditioned mscs in a den-induced rat model of hcc. Biomed. Pharmacother. 2019;114:108732. doi: 10.1016/j.biopha.2019.108732. [DOI] [PubMed] [Google Scholar]
- 10.Khamis AAA, Ali EMM, El-Moneim MAA, Abd-Alhaseeb MM, El-Magd MA, Salim EI. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018;105:1335–1343. doi: 10.1016/j.biopha.2018.06.105. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Zhang D, Tang D, Sun K, Peng J, Zhu W, Yin S, Wu Y. Amygdalin inhibits tgfβ1-induced activation of hepatic stellate cells (hscs) in vitro and ccl(4)-induced hepatic fibrosis in rats in vivo. Int. Immunopharmacol. 2021;90:107151. doi: 10.1016/j.intimp.2020.107151. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer T, Oluwaseun Adetunji C, El Omari N, Balahbib A, Taheri Y, Bouyahya A, Martorell M, Martins N, Cho WC. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules. 2019;9:679. doi: 10.3390/biom9110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Desouky MA, Fahmi AA, Abdelkader IY, Nasraldin KM. Anticancer effect of amygdalin (vitamin b-17) on hepatocellular carcinoma cell line (hepg2) in the presence and absence of zinc. Anticancer Agents Med. Chem. 2020;20:486–494. doi: 10.2174/1871520620666200120095525. [DOI] [PubMed] [Google Scholar]
- 14.Moslehi A, Komeili-movahed T, Moslehi M. Antioxidant effects of amygdalin on tunicamycin-induced endoplasmic reticulum stress in the mice liver: Cross talk between endoplasmic reticulum stress and oxidative stress. J. Rep. Pharm. Sci. 2019;8:298–302. [Google Scholar]
- 15.Ma Y, Xu R, Liu X, Zhang Y, Song L, Cai S, Zhou S, Xie Y, Li A, Cao W, Tang X. Ly3214996 relieves acquired resistance to sorafenib in hepatocellular carcinoma cells. Int. J. Med. Sci. 2021;18:1456–1464. doi: 10.7150/ijms.51256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niklander S, Bordagaray MJ, Fernández A, Hernández M. Vascular endothelial growth factor: A translational view in oral non-communicable diseases. Biomolecules. 2021;11:85. doi: 10.3390/biom11010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berk V, Kaplan MA, Tonyali O, Buyukberber S, Balakan O, Ozkan M, Demirci U, Ozturk T, Bilici A, Tastekin D, Ozdemir N, Unal OU, Oflazoglu U, Turkmen E, Erdogan B, Uyeturk U, Oksuzoglu B, Cinkir HY, Yasar N, Gumus M. Efficiency and side effects of sorafenib therapy for advanced hepatocellular carcinoma: A retrospective study by the anatolian society of medical oncology. Asian Pac. J. Cancer Prev. APJCP. 2013;14:7367–7369. doi: 10.7314/apjcp.2013.14.12.7367. [DOI] [PubMed] [Google Scholar]
- 18.Samudrala PK, Augustine BB, Kasala ER, Bodduluru LN, Barua C, Lahkar M. Evaluation of antitumor activity and antioxidant status of Alternantherabrasiliana against ehrlich ascites carcinoma in swiss albino mice. Pharmacogn. Res. 2015;7:66–73. doi: 10.4103/0974-8490.147211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkeiy M, Khamis A, El-Gamal M, Abo Gazia M, Zalat Z, El-Magd M. Chitosan nanoparticles from artemia salina inhibit progression of hepatocellular carcinoma in vitro and in vivo. Environ. Sci. Pollut. Res. Int. 2018;27:19016–19028. doi: 10.1007/s11356-018-3339-6. [DOI] [PubMed] [Google Scholar]
- 20.Abdelhady DH, El-Magd MA, Elbialy ZI, Saleh AA. Bromuconazole-induced hepatotoxicity is accompanied by upregulation of pxr/cyp3a1 and downregulation of car/cyp2b1 gene expression. Toxicol. Mech. Methods. 2017;27:544–550. doi: 10.1080/15376516.2017.1333555. [DOI] [PubMed] [Google Scholar]
- 21.Abdelhady D, El-Abasy M, Abou-Asa S, Elbialy Z, Shukry M, Hussein A, Saleh A, El-Magd M. The ameliorative effect of aspergillus awamori on aflatoxin b1-induced hepatic damage in rabbits. World Mycotoxin J. 2017;10:363–373. [Google Scholar]
- 22.Tousson E, Hafez E, Abo Gazia MM, Salem SB, Mutar TF. Hepatic ameliorative role of vitamin b17 against ehrlich ascites carcinoma-induced liver toxicity. Environ. Sci. Pollut. Res. Int. 2020;27:9236–9246. doi: 10.1007/s11356-019-06528-6. [DOI] [PubMed] [Google Scholar]
- 23.Alyan M, Shalaby M, El-Sanousi A, Mohamed A, Shebl R. Antiviral and anticancer potentials of snake and scorpion venom derivatives. Invent. Impact Mol. Pharmacol. 2014;2:15–22. [Google Scholar]
- 24.Aldubayan MA, Elgharabawy RM, Ahmed AS, Tousson E. Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxid. Med. Cell. Longev. 2019;2019:5162687. doi: 10.1155/2019/5162687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badawy A, Hassanean H, Ibrahim AK, Habib ES, El- Magd MA, Ahmed SA. Isolates from Thymelaeahirsuta inhibit progression of hepatocellular carcinoma in vitro and in vivo. Nat. Prod. Res. 2019;35:1799–1807. doi: 10.1080/14786419.2019.1643859. [DOI] [PubMed] [Google Scholar]
- 26.Abu Khudir R, El-Magd MA, Salama AF, Tousson EM, El-Dsoki SM. Curcumin attenuated oxidative stress and inflammation on hepatitis induced by fluvastatin in female albino rats. Alex. J. Vet. Sci. 2019;62:102–115. [Google Scholar]
- 27.Ramadan A, Kamel G, Awad NE, Shokry AA, Fayed HM. The pharmacological effect of apricot seeds extracts and amygdalin in experimentally induced liver damage and hepatocellular carcinoma. J. Herbmed. Pharmacol. 2020;9:400–407. [Google Scholar]
- 28.Abboud MM, Al Awaida W, Alkhateeb HH, Abu-Ayyad AN. Antitumor action of amygdalin on human breast cancer cells by selective sensitization to oxidative stress. Nutr. Cancer. 2019;71:483–490. doi: 10.1080/01635581.2018.1508731. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the keap1–nrf2 pathway in stress response and cancer evolution. Genes Cells Devoted Mol. Cell. Mech. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang SF, Yang WE, Kuo WH, Chang HR, Chu SC, Hsieh YS. Antimetastatic potentials of flavones on oral cancer cell via an inhibition of matrix-degrading proteases. Arch. Oral Biol. 2008;53:287–294. doi: 10.1016/j.archoralbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Westergren-Thorsson G, Bagher M, Andersson-Sjöland A, Thiman L, Löfdahl CG, Hallgren O, Bjermer L, Larsson-Callerfelt AK. Vegf synthesis is induced by prostacyclin and tgf-β in distal lung fibroblasts from copd patients and control subjects: Implications for pulmonary vascular remodelling. Respirology (Carlton, Vic) 2018;23:68–75. doi: 10.1111/resp.13142. [DOI] [PubMed] [Google Scholar]
- 32.El Bakary NM, Alsharkawy AZ, Shouaib ZA, Barakat EMS. Role of bee venom and melittin on restraining angiogenesis and metastasis in γ-irradiated solid ehrlich carcinoma-bearing mice. Integr. Cancer Ther. 2020;19:1534735420944476. doi: 10.1177/1534735420944476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Magd MA, Khamis A, Nasr Eldeen SK, Ibrahim WM, Salama AF. Trehalose enhances the antitumor potential of methotrexate against mice bearing ehrlich ascites carcinoma. Biomed. Pharmacother. 2017;92:870–878. doi: 10.1016/j.biopha.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Fendrich V, Maschuw K, Rehm J, Buchholz M, Holler JP, Slater EP, Bartsch DK, Waldmann J. Sorafenib inhibits tumor growth and improves survival in a transgenic mouse model of pancreatic islet cell tumors. Sci. World J. 2012;2012:529151. doi: 10.1100/2012/529151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Masry TA, Al-Shaalan NH, Tousson E, Buabeid M, Alyousef AM. The therapeutic and antineoplastic effects of vitamin b17 against the growth of solid-form ehrlich tumours and the associated changes in oxidative stress, DNA damage, apoptosis and proliferation in mice. Pak. J. Pharm. Sci. 2019;32:2801–2810. [PubMed] [Google Scholar]
- 36.Mohamed AE, El-Magd MA, El-Said KS, El-Sharnouby M, Tousson EM, Salama AF. Potential therapeutic effect of thymoquinone and/or bee pollen on fluvastatin-induced hepatitis in rats. Sci. Rep. 2021;11:15688. doi: 10.1038/s41598-021-95342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Bayomi KM, Saleh AA, Awad A, El-Tarabany MS, El-Qaliouby HS, Afifi M, El-Komy S, Essawi WM, Almadaly EA, El-Magd MA. Association of cyp19a1 gene polymorphisms with anoestrus in water buffaloes. Reprod. Fertil. Dev. 2018;30:487–497. doi: 10.1071/RD16528. [DOI] [PubMed] [Google Scholar]
- 38.Saleh AA, El-Magd MA. Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. Environ. Sci. Pollut. Res. 2018;25:27031–27038. doi: 10.1007/s11356-018-2730-7. [DOI] [PubMed] [Google Scholar]
- 39.Selim NM, Elgazar AA, Abdel-Hamid NM, El-Magd MRA, Yasri A, Hefnawy HME, Sobeh M. Chrysophanol, physcion, hesperidin and curcumin modulate the gene expression of pro-inflammatory mediators induced by lps in hepg2: In silico and molecular studies. Antioxidants. 2019;8:371. doi: 10.3390/antiox8090371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elgazar AA, Selim NM, Abdel-Hamid NM, El-Magd MA, El Hefnawy HM. Isolates from alpinia officinarum hance attenuate lps induced inflammation in hepg2: Evidence from in silico and in vitro studies. Phytother. Res. 2018;32:1273–1288. doi: 10.1002/ptr.6056. [DOI] [PubMed] [Google Scholar]
- 41.Saleh AA, Amber K, El-Magd MA, Atta MS, Mohammed AA, Ragab MM, Abd El-Kader H. Integrative effects of feeding Aspergillusawamori and fructooligosaccharide on growth performance and digestibility in broilers: Promotion muscle protein metabolism. Biomed. Res. Int. 2014;2014:946859. doi: 10.1155/2014/946859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.