Abstract
Chronic multisite musculoskeletal pain (CMP) is common and highly morbid. However, vulnerability factors for CMP are poorly understood. Previous studies have independently shown that both small hippocampal brain volume and genetic risk alleles in a key stress system gene, FKBP5, increase vulnerability for chronic pain. However, little is known regarding the relationship between these factors and CMP. Here we tested the hypothesis that both small hippocampal brain volume and FKBP5 genetic risk, assessed using the tagging risk variant, FKBP5rs3800373, increase vulnerability for CMP. We used participant data from 36,822 individuals with available genetic, neuroimaging, and chronic pain data in the UK Biobank study. Although no main effects were observed, the interaction between FKBP5 genetic risk and right hippocampal volume was associated with CMP severity (β = −0.020, praw = 0.002, padj = 0.01). In secondary analyses, severity of childhood trauma further moderated the relationship between FKBP5 genetic risk, right hippocampal brain volume, and CMP (β = −0.081, p = 0.016). This study provides novel evidence that both FKBP5 genetic risk and childhood trauma moderate the relationship between right hippocampal brain volume and CMP. The data increases our understanding of vulnerability factors for CMP and builds a foundation for further work assessing causal relationships that might drive CMP development.
Subject terms: Genetics of the nervous system, Genetics, Neuroscience, Psychology
Introduction
Up to one-third of individuals globally suffer from chronic pain in their lifetime1–3. Of those individuals experiencing chronic pain, a substantial subset have musculoskeletal pain in multiple sites across the body4–6. Such chronic multisite musculoskeletal pain (CMP) is highly severe7,8, is one of the most common reasons for years lived with disability9–11, contributes to high health care costs12,13, and can lead to opioid addiction/abuse14,15. Despite the substantial burden that CMP has on the individual and society, vulnerability factors for CMP and the interplay between these factors are poorly understood. This lack of understanding precludes the development of effective diagnostic tools and impedes the identification of promising targets for therapeutic intervention.
Increasing evidence indicates that two highly influential vulnerability factors for chronic musculoskeletal pain are smaller hippocampal volumes16 and the presence of one or more genetic risk alleles in a key stress system gene, FKBP517,18. While the data supporting these vulnerability factors originate from independent studies, multiple lines of evidence suggest that these two risk factors are related and might interact to influence CMP development. First, both the hippocampus and FKBP5 are involved in the regulation of the physiological response to stress19–23. Second, these two vulnerability factors have been shown to influence chronic pain states in both human and animal studies24–28. Third, FKBP5 gene expression increases in the hippocampus in response to stress (which can lead to decreased glucocorticoid receptor sensitivity)29,30. Fourth, studies assessing neuropsychiatric disorders that often overlap with CMP, such as depression and anxiety, have shown (albeit in modest sample sizes) that genetic/molecular risk in FKBP5 influences hippocampal brain volume and that these factors together are associated with neuropsychiatric pathology31–34. Altogether this data suggests that small hippocampal volume and risk alleles in FKBP5 might interact to influence CMP vulnerability.
In addition to brain anatomy and FKBP5 genetics, another previously identified vulnerability factor for CMP is early life adversity via childhood trauma35,36. Previous data indicates that individuals with a history of trauma have increased rates of CMP37–39. Further, related to FKBP5 genetic risk and hippocampal volumes as vulnerability factors for CMP, individuals with a history of trauma and adverse sequelae have been shown to have smaller hippocampal volumes40,41 and genetic/molecular risk in the FKBP5 locus42–44. Therefore, it is possible that the combination of all three vulnerability factors — the stress of childhood trauma, FKBP5 genetic risk, and hippocampal volume — increases risk for CMP.
In the current study, we tested the registered hypotheses45 that (1) hippocampal volume is associated with CMP severity, (2) FKBP5 genetic risk via rs3800373 is associated with CMP severity, and (3) that these two vulnerability factors interact to influence CMP, i.e. FKBP5rs3800373 moderates the relationship between hippocampal volume and CMP severity. These hypotheses were tested using neuroimaging, genetic, and musculoskeletal pain data collected from 36,822 participants enrolled in the UK Biobank study. In secondary analyses, we assessed whether childhood trauma further moderated the relationship between hippocampal volume, FKBP5rs3800373, and CMP. Together, this work contributes to our understanding of vulnerability factors for CMP.
Results
Participants
The UK Biobank cohort consisted of 502,487 participants, of which 36,822 participants had genetic, brain imaging, and chronic pain data and were included in the current study. Characteristics of participants (n = 36,822; 54% women) are shown in Table 1. The majority of individuals self-identified as White (97%), were more than sixty years of age, had at least some college education, and were overweight (BMI > 25).
Table 1.
Characteristics of UK Biobank participants with available data for primary study analyses (n = 36,822).
| Characteristic | |
| Age, years, mean (SD) | 63.6 (7.5) |
| Women, n (%) | 19,288 (52.4) |
| Education, n (%) | |
| NVQ, HND, HNC, or equivalent, or other professional qualification | 6281 (17.1) |
| O levels, GCSEs, CSEs, or equivalent | 7938 (21.6) |
| A levels, AS levels, or equivalent | 4517 (12.3) |
| College or university degree | 17,967 (48.8) |
| Self-identified race/ethnicity | |
| White | 35,770 (97.1) |
| Asian or Asian British | 377 (1.0) |
| Black or Black British | 213 (0.6) |
| Multiethnic or other | 462 (1.3) |
| BMI, mean (SD) | 26.5 (4.4) |
| Current smoker, n (%) | 1272 (3.5) |
SD standard deviation, n sample size, NVQ national vocational qualification, HND higher national diploma, HNC higher national certificate, O levels ordinary levels, GCSE general certificate of secondary education, CSE certificate of secondary education, A level advanced levels, AS level advanced subsidiary levels, BMI body mass index.
FKBP5rs3800373 genetic risk and hippocampal brain volume were not directly associated with CMP in UKBB participants
Our three primary hypotheses, as submitted in our UK Biobank proposal45, were that (a) hippocampal brain volume, (b) FKBP5rs3800373 genetic risk, and c) the interaction between these two biological factors are associated with CMP. To test these hypotheses, we first examined the relationship between FKBP5rs3800373 genetic risk and CMP severity, defined by the number body sites with musculoskeletal pain for ≥ 3 months. In the UK Biobank cohort used here, 32.7% (n = 12,038) of individuals reported one or more sites of CMP, with the average number of sites of CMP equal to 0.48 (SD = 0.81) (Supplementary Table 1). Using linear regression modeling, we found that FKBP5rs3800373 genetic risk was not statistically significantly associated with CMP (β = −0.003, p = 0.701; Supplementary Table 2). We next examined the relationship between hippocampal volume and CMP severity. Because previous literature has indicated that the left and right hippocampi play different roles in the development of pain24,26,46, and because these two brain regions had different mean volumes (t = 44.095, p = 2.2 × 10–16), we assessed for associations between these brain regions and CMP severity independently. As shown in Supplementary Table 3, we found that neither left (β = −0.005, p = 0.238) nor right (β = −0.007, p = 0.130) hippocampal volumes were directly associated with CMP.
A statistically significant interaction between FKBP5rs3800373 genetic risk and right hippocampal brain volume was associated with CMP severity in UKBB study participants
We next assessed our third hypothesis, that the interaction between FKBP5rs3800373 and hippocampal brain volume is associated with CMP severity. Here, as shown in Table 2, we found that the interaction between FKBP5rs3800373 genetic risk and right hippocampal brain volume (β = −0.020, praw = 0.002, padj = 0.01), but not left hippocampal brain volume (β = −0.015, praw = 0.023, padj = 0.12) was statistically significantly associated with CMP. The interaction explained 0.01% of the variance in CMP. Further, as shown in Fig. 1, we identified a dose-dependent inverse relationship between the number of FKBP5rs3800373 risk alleles and the relationship between right hippocampal brain volume on CMP severity such that individuals with two FKBP5rs3800373 risk alleles showed the strongest inverse relationship between right hippocampal volume and CMP (i.e. smaller right hippocampal volume was associated with increased CMP severity; β = −0.031, p = 0.045; Supplementary Table 4). In those individuals with one FKBP5rs3800373 risk allele, there was a less strong but statistically significant inverse relationship between right hippocampal volume and CMP (β = −0.016, p = 0.022). Finally in individuals with zero FKBP5rs3800373 risk alleles, we detected no statistically significant relationship between right hippocampal volume and CMP (β = 0.004, p = 0.639).
Table 2.
Linear regression analyses assessing the interaction between FKBP5 genetic risk, using the tagging allele rs3800373, and left and right hippocampal volumes on chronic multisite musculoskeletal pain in the UK Biobank (n = 36,822).
| Left hippocampus | Right hippocampus | ||||||
|---|---|---|---|---|---|---|---|
| β | S.E. | p value | β | S.E. | p value | ||
| Intercept | −0.302 | 0.047 | < 0.001 | −0.299 | 0.047 | < 0.001 | |
| FKBP5rs3800373 | −0.003 | 0.007 | 0.692 | −0.003 | 0.007 | 0.694 | |
| Hippocampal volume | 0.003 | 0.006 | 0.638 | 0.004 | 0.006 | 0.476 | |
| Age | 0.001 | 0.0006 | 0.057 | 0.001 | 0.0006 | 0.065 | |
| Sex | −0.116 | 0.009 | < 0.001 | −0.117 | 0.009 | < 0.001 | |
| BMI | 0.029 | 0.001 | < 0.001 | 0.029 | 0.001 | < 0.001 | |
| Genotyping array | 0.011 | 0.014 | 0.461 | 0.011 | 0.014 | 0.444 | |
| Imaging center (Reading) | −0.020 | 0.013 | 0.137 | −0.020 | 0.013 | 0.133 | |
| Imaging center (Newcastle) | 0.026 | 0.010 | 0.012 | 0.026 | 0.010 | 0.013 | |
|
FKBP5rs3800373* Hippocampal volume |
−0.015 | 0.007 | 0.023 | −0.020 | 0.007 | 0.002 | |
Chronic multisite musculoskeletal pain was defined as the number of musculoskeletal sites with pain that persisted for more than 3 months, ranging from 0 to 4. An additive genetic model was used to assess the effect of rs3800373. Genetic principal components 1–10 and ethnic background were included as covariates but omitted from the table for brevity.
Figure 1.
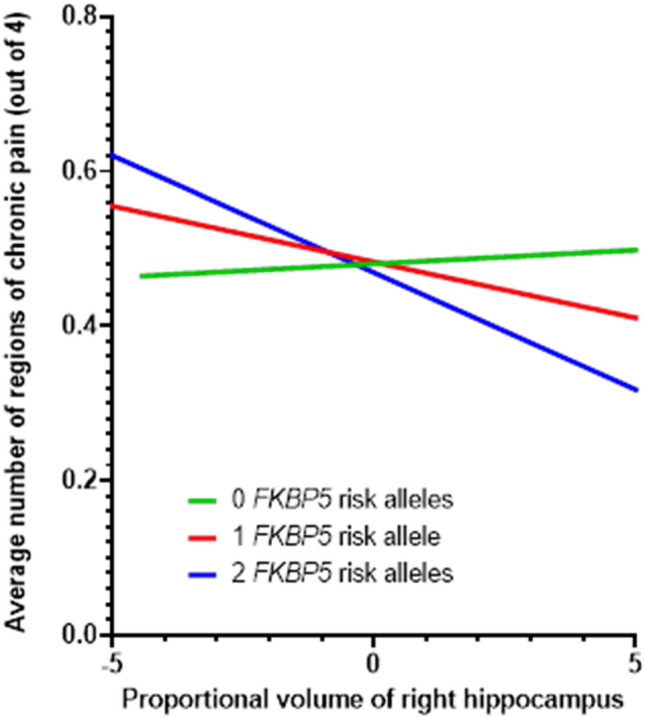
The number of FKBP5rs3800373 risk alleles moderates the relationship between the right hippocampus and CMP severity. Shown is the relationship between right hippocampal brain volume and chronic multisite musculoskeletal pain (CMP) severity in individuals from the UKBB study cohort (n = 36,822). Right hippocampal brain volume was adjusted for head size and standardized (mean = 0, SD = 1), see “Methods”. Individuals with two copies of the FKBP5rs3800373 risk allele (n = 3005) are represented by the blue line, individuals with one copy of the FKBP5rs3800373 risk allele (n = 15,327) are represented by the red line, and individuals with no risk alleles are represented by the green line (n = 18,490). As shown in Supplementary Table 4, relationships represented by the blue and red lines were statistically significant (p < 0.05).
Secondary analyses identify childhood trauma as a moderator of the interaction between FKBP5rs3800373 genetic risk and right hippocampal brain volume on CMP
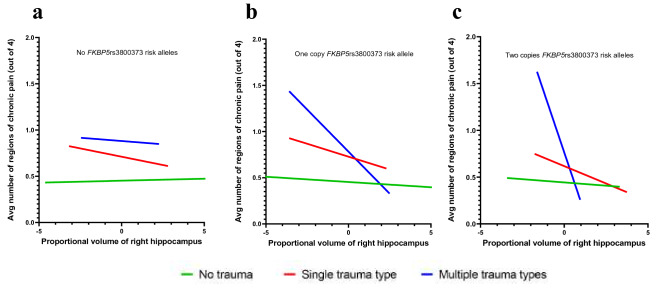
Numerous previous studies have identified significant gene-by-environment interactions between FKBP5 risk alleles and significant stressors47,48. Therefore, in extension to the above findings, via secondary analyses, we assessed whether distress in the form of early childhood trauma moderates the relationship between FKBP5rs3800373/right hippocampal volume and CMP (Supplementary Fig. 2). We performed this analysis in a sub-cohort of UKBB participants with available childhood trauma data (n = 25,280). Because this subset only included a portion of the full cohort used for genetic and brain volume analyses, we first assessed whether the above identified relationships remained significant in the sub-cohort. Consistent with the above findings, in this sub-cohort we found no direct association between FKBP5rs3800373 genetic risk and CMP (β = −0.001, p = 0.853; Supplementary Table 5), between left hippocampal brain volume and CMP (β = −0.005, p = 0.401; Supplementary Table 6), or between right hippocampal brain volume and CMP (β = −0.006, p = 0.285; Supplementary Table 6). Additionally, consistent with above results, we identified a statistically significant interaction between FKBP5rs3800373 genetic risk and right hippocampal volume on CMP (β = −0.017, p = 0.032; Supplementary Table 7). We then examined the interaction between childhood trauma severity and right hippocampal volume on CMP in participants with 0, 1, or 2 FKBP5rs3800373 risk alleles. The interaction was statistically significant in participants with 1 (β = −0.084, p = 0.006; Table 3) or 2 (β = −0.168, p = 0.042; Table 3) FKBP5rs3800373 risk alleles such that in individuals with higher levels of childhood trauma and FKBP5rs3800373 genetic risk, decreased hippocampal volume was associated with increased CMP (Fig. 2). For individuals with 1 or 2 risk alleles, the interaction explained 0.58% and 0.30% of the variance in CMP, respectively. No interaction between the level of childhood trauma and right hippocampal volume was observed in those without an FKBP5rs3800373 risk allele (β = −0.020, p > 0.05; Table 3). Further, when we assessed an interactive relationship between these three factors, right hippocampal volume, FKBP5rs3800373 genetic risk, and childhood trauma severity on CMP, we identified a statistically significant relationship between the interaction of these three factors and CMP (β = −0.064, p = 0.047; Supplementary Table 8) that persisted when accounting for symptoms of anxiety and depression (β = −0.081, p = 0.016; Supplementary Table 9). Of note, because a second functional SNP, FKBP5rs1360780 (linkage disequilibrium with FKBP5rs3800373, D′ = 0.89, r2 = 0.78) has been shown to be associated with childhood trauma and is functional, we also tested the triple interaction using FKBP5rs1360780 (β = −0.063, p = 0.048; Supplementary Table 10).
Table 3.
Linear regression analyses assessing the interaction between childhood trauma and right hippocampal volume on chronic multisite musculoskeletal pain in individuals with 0, 1, or 2 FKBP5rs3800373 risk alleles (n = 25,280).
| 0 risk alleles (n = 12,777) | 1 risk allele (n = 10,464) | 2 risk alleles (n = 2,039) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Intercept | −0.311 | 0.078 | < 0.001 | −0.260 | 0.084 | 0.002 | −0.301 | 0.188 | 0.110 |
| Right hippocampal volume | 0.004 | 0.008 | 0.577 | −0.012 | 0.008 | 0.158 | −0.014 | 0.019 | 0.452 |
| Childhood trauma severity | 0.192 | 0.028 | < 0.001 | 0.189 | 0.032 | < 0.001 | 0.178 | 0.067 | 0.008 |
| Age | 0.0008 | 0.001 | 0.399 | 0.0007 | 0.001 | 0.520 | 0.002 | 0.002 | 0.331 |
| Sex | −0.103 | 0.014 | < 0.001 | −0.105 | 0.016 | < 0.001 | −0.078 | 0.035 | 0.027 |
| BMI | 0.029 | 0.002 | < 0.001 | 0.027 | 0.002 | < 0.001 | 0.022 | 0.004 | < 0.001 |
| Genotyping array | 0.020 | 0.024 | 0.408 | 0.001 | 0.026 | 0.972 | 0.0347 | 0.059 | 0.427 |
| Imaging center (Reading) | −0.029 | 0.022 | 0.177 | 0.017 | 0.024 | 0.489 | 0.034 | 0.054 | 0.528 |
| Imaging center (Newcastle) | 0.004 | 0.018 | 0.803 | 0.035 | 0.019 | 0.074 | 0.152 | 0.043 | < 0.001 |
| Right hippocampal volume*childhood trauma severity | −0.020 | 0.028 | 0.470 | −0.084 | 0.031 | 0.006 | −0.168 | 0.083 | 0.042 |
Chronic multisite musculoskeletal pain was defined as the number of musculoskeletal sites with pain that persisted for 3 months or more, ranging from 0 to 4 sites. Genetic principal components 1–10 and ethnic background were included as covariates but omitted from the table for brevity.
Figure 2.
Childhood trauma and FKBP5rs3800373 risk alleles moderate the relationship between the right hippocampus and CMP severity. Shown is the relationship between right hippocampal brain volume and chronic multisite musculoskeletal pain (CMP) severity in individuals from the UK Biobank study with (a) 0, (b) 1, and (c) 2 FKBP5rs3800373 risk alleles and varying degrees of childhood trauma (n = 25,280). Right hippocampal brain volume was adjusted for head size and standardized (mean = 0, SD = 1), see “Methods”. Individuals who did not often experience childhood trauma are represented by the green line (“no trauma”), individuals who often experienced one type of childhood trauma are represented by the red line (“single trauma type”), and individuals who often experienced multiple (> 2) types of childhood trauma are represented by the blue line (“multiple trauma types”). As shown in Supplementary Table 8, the interaction between FKBP5rs3800373, right hippocampal volume, and childhood trauma was statistically significant (p < 0.05).
Discussion
Here we show for the first time and using the largest sample size to date that includes genetic, neuroimaging, and chronic pain data, that vulnerability to CMP is highest in individuals with a combination of FKBP5rs3800373 genetic risk alleles and smaller right hippocampal brain volumes. Further, we show that increased exposure to childhood trauma further extends this biological vulnerability to CMP. Importantly, these associations were not accounted for by differences in age, sex, and BMI, nor were they accounted for by symptoms of depression or anxiety. Together, these findings add to the growing body of literature indicating that multiple factors, including important biological × environmental interactions, compound risk for CMP. In addition, this work provides an important foundational framework for subsequent mechanistic work that aims to identify causal relationships between the identified factors.
While the main effect relationships that were hypothesized between FKBP5 genetic risk and CMP and between hippocampal volume and CMP did not hold up in the current set of analyses (as they had in previous studies of chronic pain16,49–51) the interaction between these two factors did show association with CMP. Since both FKBP5 and the hippocampus are central to stress system functioning19–23,52,53, and the combination of these stress system mediators substantially increases vulnerability to CMP (especially in individuals with a history of substantial childhood stressors), it suggests that the physiological stress-response system is critical to the development of CMP. Such findings are important because they provide further support to existing literature indicating that therapeutic strategies aimed at reducing CMP might be more effective if targeted to stress system mediators vs tissue injury generators54,55.
The HPA axis stress system and its role in the development of neuropsychiatric disorders has been studied extensively previously48. Data from this field, in addition to nascent literature from the pain field, can be used to help generate further hypotheses to later test how the identified stress-associated vulnerability factors might be contributing to the pathogenesis of CMP. For instance, because the protein encoded by FKBP5, FKBP51, regulates the affinity of the glucocorticoid receptor (GR) to the glucocorticoid cortisol53, and the FKBP5 risk allele causes an increase in FKBP51 synthesis (thus decreasing glucocorticoid sensitivity30), one could hypothesize that in tissues with a high density of glucocorticoid receptors (e.g., hippocampus23), that increased glucocorticoid resistance (e.g., in individuals with FKBP5 genetic risk and in individuals that have been exposed to substantial stressors) could cause maladaptive changes to tissue morphology (such as tissue atrophy). Such glucocorticoid-induced tissue atrophy has been observed previously via studies assessing cortisol inhibition of cellular formation and proliferation in the hippocampus56, likely via apoptosis in the subgranular zone of the dentate gyrus57. One might then hypothesize that lower glucocorticoid sensitivity and atrophied hippocampal tissue (due to glucocorticoids) lead to smaller anatomical hippocampal sizes that predispose an individual to CMP via the reduced negative feedback inhibition control of the hippocampus on the HPA axis 58–61. Chronic pain poses an allostatic load on the system62, and elevated basal glucocorticoid cortisol in chronic pain patients with smaller hippocampal volumes have been previously observed63. Therefore, it is possible that glucocorticoid resistance in the hippocampus due to FKBP5 genetic risk, reduces this negative feedback inhibition control, thus sustaining a maladaptive endocrine stress response, and exacerbating CMP. While substantial data, including the data presented here, supports these mechanistic hypotheses, the direct causal relationships between the identified vulnerability factors and CMP has yet to be studied.
In addition to hippocampal volume and FKBP5 genetic risk being important to the development of CMP, we also showed in this study that childhood stress via emotional, physical, and/or sexual abuse increases an individual’s vulnerability to CMP. In extension to the above mechanistic hypotheses, one might further expand upon the hypotheses to include the role that childhood trauma plays in the pathogenic mechanisms driving CMP. For instance, evidence from multiple species indicates that traumatic events in childhood alters normal development of brain structure, function and behaviour64, most notably in the hippocampus65–70. Additionally, the severity of childhood trauma and the developmental timing of this trauma influence stress responses in adulthood such that children who suffered from physical or sexual abuse in the first five years of life were more likely to have HPA axis dysregulation71. These HPA axis changes due to childhood abuse are thought to be mediated by epigenetic changes such as DNA de-methylation of FKBP572, which occurs more commonly in individuals with at least one copy of the genetic risk allele in this gene42,47. Finally, as might be relevant to individuals in the UK Biobank cohort (i.e. with an average age of ~ 64 in the current sub-cohort), previous studies indicate that the effect that childhood trauma has on FKBP51, the hippocampus, and HPA axis persists throughout the lifespan72–75.
Several limitations should be considered when interpreting these study results. First, the cross-sectional study design implemented here limited our ability to assess for direct causation (e.g. between an inciting traumatic event and subsequent transition from acute to chronic pain). Second, the UK Biobank cohort is comprised of mainly European/White individuals, thus the generalizability of our results to other racial/ethnic groups is not known. Third, we did not control for potential confounding by medication use (e.g. opioids) or by comorbid disorders other than frequently co-occurring neuropsychiatric conditions. Fourth, the extent of both CMP and childhood trauma in this cohort was relatively low, limiting the sample size in some stratified analyses. Fifth, since our dataset did not include severity or duration of pain, our CMP variable has a more limited characterization of pain than other studies; however, we created our CMP variable using methodology that is consistent with other research that studies pain in the UK Biobank dataset76,77. Finally, the presented results remain to be validated in an independent dataset.
In conclusion, we presented novel evidence that both FKBP5rs3800373 genetic risk and childhood trauma moderate the relationship between hippocampal volume and CMP. While previous evidence highlighted these factors as independent predictors of chronic musculoskeletal pain, we found here that these factors interact such that individuals with a combination of these related risk factors have the highest risk for CMP. Future studies should investigate whether these factors not only indicate vulnerability for CMP but whether they also contribute to the underlying pathogenesis of disease. Altogether, these findings increase our understanding of risk factors for CMP and identify potential targets for therapeutic intervention within the stress axis pathway.
Methods
Study design and population
The UK Biobank is a population-based cohort of over 500,000 participants that was designed to improve the prevention, diagnosis, and treatment of chronic conditions. Between 2006 and 2010, all UK residents registered with the National Health Service, aged 40–69 years, and living within 25 miles of one of the 22 assessment centers across the country, were invited to participate. At baseline assessment, participants completed a detailed touch-screen questionnaire which collected demographic, lifestyle, and medical information. Biological samples (such as blood and saliva) and physical measurements (such as height and weight) were also collected. Between 2014 and 2020, enrolled participants were invited to participate in a follow-up assessment in which they repeated the initial questionnaire and underwent brain imaging protocols. Participants also regularly responded to web-based questionnaires including a mental health questionnaire between 2016 and 2017. The current study includes 36,822 participants with genetic, brain imaging, and chronic pain data. A sub-cohort of 25,280 participants with childhood trauma data was used for secondary analyses.
The present analyses were conducted under UK Biobank application number 4816845. All participants provided informed consent and all methods described in the current manuscript were performed in accordance with the relevant guidelines and regulations. Further information on the consent procedure can be found elsewhere (http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=200).
Assessments
Chronic multisite musculoskeletal pain (CMP)
At the follow-up visit between 2014 and 2020 (at the same time that brain imaging data was acquired), participants were asked “In the last month have you experienced any of the following that interfered with your usual activities?” The response options were seven specific sites of pain (headache, facial pain, neck or shoulder pain, back pain, stomach or abdominal pain, hip pain, and knee pain) as well as “pain all over the body”, “none of the above”, or “prefer not to say.” Participants were permitted to select multiple painful sites unless they reported having “pain all over the body,” “none of the above”, or “prefer not to say.” Participants who indicated that they had pain at specific sites were then asked whether the pain at each site had been present for more than 3 months78. Consistent with previous studies76,77, only the neck or shoulder, back, hip, and knee sites were used to investigate musculoskeletal pain. Participants who reported having pain all over the body were excluded since chronic widespread pain is distinct from chronic musculoskeletal pain79,80. Chronic multisite musculoskeletal pain (CMP) was defined as the number of musculoskeletal sites with pain that persisted for more than 3 months, ranging from 0 to 4.
Hippocampal brain volume
Brain images were acquired using Siemens Skyra 3 T scanners in UK Biobank’s imaging centers in Cheadle (n = 23,495), Reading (n = 4491), and Newcastle (n = 8836) using identical acquisition protocols across sites81. T1-weighted brain images were processed using FreeSurfer v6.0 to automatically estimate right and left hippocampal volumes and total intracranial volumes82–84. Hippocampal volumes were divided by total intracranial volume in order to adjust for head size85,86. Right and left hippocampal volumes were normally distributed both before and after adjustment (Supplementary Fig. 1). We used a t-test to compare the difference in means (mean volume of right hippocampus = 3795 mm3, mean volume of left hippocampus = 3677 mm3; adjusted mean volume of right hippocampus = 0.00246 and the adjusted mean volume of left hippocampus = 0.00238). Standardized adjusted hippocampal volumes (mean = 0, SD = 1) were used in statistical models.
FKBP5 genetic risk
Genotyping was performed by the UK Biobank on the full cohort. In the current study, we utilized genotyping data from only those participants with brain imaging data. Genotyping was performed using the Affymetrix UK BiLEVE Axiom array (n = 3469) and the Affymetrix UK Biobank Axiom array (n = 33,353). Quality control procedures, as described previously87, were performed centrally by UK Biobank personnel prior to distribution to individual investigators. In the current set of analyses we focused on a single genetic variant in the FKBP5 gene, rs3800373, based on its ability to tag the main risk haplotype88 and because it has been shown previously to be associated with persistent musculoskeletal pain via its allele-specific influence on microRNA regulation of FKBP5 expression18,49. This allele was in Hardy–Weinberg equilibrium (p > 0.05) and had an excellent call rate (> 99%). Consistent with the reported minor allele frequency (MAF) of rs38003873 for the ‘British in England and Scotland’ (GBR) sub-population of European (EUR) ancestry (MAF = 32%, 1000 Genomes), the MAF for participants included in the current study was 29%. An additive genetic model (homozygous for the major allele (AA) = 0, heterozygous (CA) = 1, and homozygous for the minor allele (CC) = 2) was used to assess the relationship between FKBP5rs3800373, hippocampal brain volume, childhood trauma and CMP. Of note, rs1360780 is in high linkage disequilibrium with rs3800373 (D′ = 0.89, r2 = 0.78), is associated with childhood trauma and has been shown to influence FKBP5 transcription via DNA methylation42. Due to high LD between this allele and FKBP5rs3800373, we used this allele to confirm key findings demonstrated with FKBP5rs3800373.
Childhood trauma
A sample of the original cohort (n = 25,280) completed a subsequent online mental health questionnaire between 2016 and 2017 that contained questions pertaining to childhood trauma. Three questions from the Childhood Trauma Questionnaire89 were used to identify participants who experienced physical abuse (“When I was growing up…People in my family hit me so hard that it left me with bruises or marks”), emotional abuse (“When I was growing up…I felt that someone in my family hated me”), and sexual abuse (‘When I was growing up…someone molested me (sexually)”). Possible responses were never true, rarely true, sometimes true, often true, very often true, or prefer not to say. For each question, similarly to previous definitions of frequency90, participants who responded “often true” or “very often true” were said to frequently experience physical, emotional, or sexual abuse. Of those individuals frequently experiencing these childhood traumas, the count of childhood trauma types, ranging from 0 to 3, was calculated and used to capture the frequency and dimensions of childhood trauma that participants experienced.
Depression and anxiety symptoms
To control for neuropsychiatric conditions related to CMP, two additional items from the online mental health questionnaire were used to assess core symptoms of depression and anxiety. The question “Have you ever had a time in your life when you felt sad, blue, or depressed for two weeks or more in a row?” was used to assess lifetime depressive symptoms and the question “Have you ever had a period lasting one month or longer when most of the time you felt worried, tense, or anxious?” was used to assess anxiety symptoms. If participants had an affirmative response to the item, we considered the participant affected by the neuropsychiatric condition.
Statistical analyses
Sociodemographic characteristics were summarized using standard descriptive statistics. The relationship between FKBP5rs3800373, hippocampal brain volume, and chronic multisite musculoskeletal pain in the UK Biobank cohort was assessed using general linear models. Interactions between FKBP5rs3800373 and hippocampal volume were assessed by including corresponding product terms in the model. Based on previous analyses, age, sex, BMI, and self-reported ethnic background were included in models as covariates77,91,92. Models assessing the effect of FKBP5rs3800373 genetic risk also included genotyping array and top 10 genetic principal components as covariates. Models assessing the effect of hippocampal volume also included imaging site as a covariate, similarly to previous studies86,92,93. The coefficient of determination (R2, i.e. the proportion of the variation in the dependent variable that is predictable from the independent variable) of the interaction term was calculated as the difference between R2 of the full model that contains the interaction term along with covariates and the R2 of the null model that only includes covariates. We adjusted for multiple testing using Bonferroni correction and reported both raw and adjusted p-values. Statistical significance was determined based on adjusted p values. The sample (n = 36,822) was sufficient to test for gene-environment interactions. The minimum sample size to achieve 80% power at 5% significance level to detect a small effect gene-environment interaction for a genetically dominant SNP with p = 0.3 frequency is > 20,000 participants94.
Secondary analyses tested moderation via the interaction between childhood trauma and hippocampal volume on CMP in individuals with 0, 1, and 2 FKBP5rs3800373 risk alleles. All analyses were performed using RStudio server (1.4.1106-5) and all graphs were made for publication using GraphPad Prism v 9.1.2.
Supplementary Information
Acknowledgements
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under Award Number R01NS118563 (Linnstaedt), by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under K01AR071504 (Linnstaedt) and by the Rita Allen Foundation (Linnstaedt). L.J. Ayoub was supported by Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Doctoral Research Award. M. Moayedi acknowledges support from the Bertha Rosenstadt Endowment Fund from the University of Toronto’s Faculty of Dentistry. The content is solely the responsibility of the authors and does not necessarily represent the views of these funding agencies. This research has been conducted using data from UK Biobank, a major biomedical database: https://www.ukbiobank.ac.uk. This study was approved by the UK Biobank under project #48168. We would like to thank the study participants and researchers in the UK Biobank study. UK Biobank is generously supported by its founding funders, the Wellcome Trust and UK Medical Research Council, as well as the British Heart Foundation, Cancer Research UK, Department of Health, Northwest Regional Development Agency and Scottish Government.
Author contributions
M.M. and S.D.L. developed the hypothesis. M.M. applied for UKBiobank data. J.J.L. and S.D.L. designed the analysis and J.J.L performed statistical models and data visualization. All authors wrote, reviewed, and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Massieh Moayedi and Sarah D. Linnstaedt.
Change history
7/10/2022
The original online version of this Article was revised: In the original version of this Article the homozygous for the major allele “(AA)” = 0 was incorrectly given as “(CC)”. The correct sentence now reads: “An additive genetic model (homozygous for the major allele (AA) = 0, heterozygous (CA) = 1, and homozygous for the minor allele (CC) = 2) was used to assess the relationship between FKBP5rs3800373, hippocampal brain volume, childhood trauma and CMP.”
Contributor Information
Massieh Moayedi, Email: m.moayedi@utoronto.ca.
Sarah D. Linnstaedt, Email: sarah_linnstaedt@med.unc.edu
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10411-9.
References
- 1.Kuehn B. Chronic pain prevalence. JAMA. 2018;320:1632–1632. doi: 10.1001/jama.2018.16009. [DOI] [PubMed] [Google Scholar]
- 2.Rice AS, Smith BH, Blyth FM. Pain and the global burden of disease. Pain. 2016;157:791–796. doi: 10.1097/j.pain.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 3.Fayaz A, Croft P, Langford R, Donaldson L, Jones G. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open. 2016;6:e010364. doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2007;21:403–425. doi: 10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2003;17:547–561. doi: 10.1016/S1521-6942(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 6.Carnes D, et al. Chronic musculoskeletal pain rarely presents in a single body site: Results from a UK population study. Rheumatology (Oxford) 2007;46:1168–1170. doi: 10.1093/rheumatology/kem118. [DOI] [PubMed] [Google Scholar]
- 7.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain–A gender perspective. Eur. J. Pain. 2004;8:435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Silva AG, Alvarelhão J, Queirós A, Rocha NP. Pain intensity is associated with self-reported disability for several domains of life in a sample of patients with musculoskeletal pain aged 50 or more. Disabil. Health J. 2013;6:369–376. doi: 10.1016/j.dhjo.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Cieza A, et al. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396:2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith E, et al. The global burden of other musculoskeletal disorders: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014;73:1462–1469. doi: 10.1136/annrheumdis-2013-204680. [DOI] [PubMed] [Google Scholar]
- 11.Butera KA, Roff SR, Buford TW, Cruz-Almeida Y. The impact of multisite pain on functional outcomes in older adults: Biopsychosocial considerations. J. Pain Res. 2019;12:1115–1125. doi: 10.2147/JPR.S192755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mäntyselkä PT, Kumpusalo EA, Ahonen RS, Takala JK. Direct and indirect costs of managing patients with musculoskeletal pain—Challenge for health care. Eur. J. Pain. 2002;6:141–148. doi: 10.1053/eujp.2001.0311. [DOI] [PubMed] [Google Scholar]
- 13.Phillips CJ. The cost and burden of chronic pain. Rev. Pain. 2009;3:2–5. doi: 10.1177/204946370900300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat. Rev. Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 15.Beaudoin FL, et al. Persistent pain after motor vehicle collision: comparative effectiveness of opioids versus non-steroidal anti-inflammatory drugs prescribed from the emergency department–A propensity matched analysis. Pain. 2017;158:289. doi: 10.1097/j.pain.0000000000000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vachon-Presseau E, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139:1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linnstaedt SD, et al. A functional riboSNitch in the 3' untranslated region of FKBP5 alters microRNA-320a binding efficiency and mediates vulnerability to chronic post-traumatic pain. J. Neurosci. 2018;38:8407–8420. doi: 10.1523/JNEUROSCI.3458-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortsov AV, et al. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. PAIN®. 2013;154:1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levone BR, Cryan JF, O'Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress. 2015;1:147–155. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 21.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 22.Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: Time-course analysis. Brain Res. Mol. Brain Res. 2000;80:142–152. doi: 10.1016/S0169-328X(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 23.Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol. Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci. Bull. 2009;25:237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutso AA, et al. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayoub LJ, et al. The medial temporal lobe in nociception: A meta-analytic and functional connectivity study. Pain. 2019;160:1245–1260. doi: 10.1097/j.pain.0000000000001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiarù, M. et al. The stress regulator Fkbp51: A novel and promising druggable target for the treatment of persistent pain states across sexes. Pain. (2018). [DOI] [PMC free article] [PubMed]
- 28.Maiarù M, et al. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci. Translat. Med. 2016;8:325ra319–325ra319. doi: 10.1126/scitranslmed.aab3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner KV, et al. Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: Influence of paroxetine treatment. Neuropsychopharmacology. 2012;37:2797–2808. doi: 10.1038/npp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharf SH, Liebl C, Binder EB, Schmidt MV, Muller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE. 2011;6:e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikolas P, et al. Effects of early life adversity and FKBP5 genotype on hippocampal subfields volume in major depression. J. Affect. Disord. 2019;252:152–159. doi: 10.1016/j.jad.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Levy-Gigi E, Szabó C, Kelemen O, Kéri S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiat. 2013;74:793–800. doi: 10.1016/j.biopsych.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Fani N, et al. FKBP5 and attention bias for threat: Associations with hippocampal function and shape. JAMA Psychiat. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tozzi L, et al. Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology. 2018;43:1138–1145. doi: 10.1038/npp.2017.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Generaal E, et al. Biological stress systems, adverse life events and the onset of chronic multisite musculoskeletal pain: a 6-year cohort study. Ann. Rheum. Dis. 2016;75:847–854. doi: 10.1136/annrheumdis-2014-206741. [DOI] [PubMed] [Google Scholar]
- 36.Kopec JA, Sayre EC. Stressful experiences in childhood and chronic back pain in the general population. Clin. J. Pain. 2005;21:478–483. doi: 10.1097/01.ajp.0000139909.97211.e1. [DOI] [PubMed] [Google Scholar]
- 37.Van Houdenhove, B. & Patrick, L. The role of childhood trauma in chronic pain and fatigue. in Trauma and Physical Health. 51–78. (Routledge, 2008).
- 38.Sansone, R.A., Watts, D.A. & Wiederman, M.W. Childhood trauma and pain and pain catastrophizing in adulthood: A cross-sectional survey study. Prim. Care Companion CNS Disord.15 (2013). [DOI] [PMC free article] [PubMed]
- 39.Walsh CA, Jamieson E, MacMillan H, Boyle M. Child abuse and chronic pain in a community survey of women. J. Interpers. Violence. 2007;22:1536–1554. doi: 10.1177/0886260507306484. [DOI] [PubMed] [Google Scholar]
- 40.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U S A. 2012;109:E563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riem MM, Alink LR, Out D, Van Ijzendoorn MH, Bakermans-Kranenburg MJ. Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 2015;27:507–520. doi: 10.1017/S0954579415000127. [DOI] [PubMed] [Google Scholar]
- 42.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenen KC, Uddin M. FKBP5 polymorphisms modify the effects of childhood trauma. Neuropsychopharmacology. 2010;35:1623–1624. doi: 10.1038/npp.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collip D, et al. FKBP5 as a possible moderator of the psychosis-inducing effects of childhood trauma. Br. J. Psychiatry. 2013;202:261–268. doi: 10.1192/bjp.bp.112.115972. [DOI] [PubMed] [Google Scholar]
- 45.Moayedi, M. Identifying risk factors for chronic musculoskeletal pain: A multifactorial approach combining brain imaging and genetics. in Approved Research. Vol. 2021. (UK Biobank Limited, 2019).
- 46.Mutso AA, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014;111:1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zannas A, Binder E. Gene–environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- 48.Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene–stress–epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linnstaedt SD, et al. A functional riboSNitch in the 3′ untranslated region of FKBP5 alters microRNA-320a binding efficiency and mediates vulnerability to chronic post-traumatic pain. J. Neurosci. 2018;38:8407–8420. doi: 10.1523/JNEUROSCI.3458-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bortsov AV, et al. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154:1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maiaru M, et al. The stress regulator FKBP51: A novel and promising druggable target for the treatment of persistent pain states across sexes. Pain. 2018;159:1224–1234. doi: 10.1097/j.pain.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J. Steroid Biochem. Mol. Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Hausl, A.S., et al. The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol. Psychiatry (2021). [DOI] [PMC free article] [PubMed]
- 54.Haukka E, Ojajarvi A, Kaila-Kangas L, Leino-Arjas P. Protective determinants of sickness absence among employees with multisite pain-a 7-year follow-up. Pain. 2017;158:220–229. doi: 10.1097/j.pain.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 55.Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 57.Yu S, et al. Depletion of the neural precursor cell pool by glucocorticoids. Ann. Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]
- 58.Grilli M. Chronic pain and adult hippocampal neurogenesis: Translational implications from preclinical studies. J. Pain Res. 2017;10:2281–2286. doi: 10.2147/JPR.S146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: Implications to mood disorders. Front. Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn. Mem. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vachon-Presseau E. Effects of stress on the corticolimbic system: Implications for chronic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;87:216–223. doi: 10.1016/j.pnpbp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Vachon-Presseau E, et al. The stress model of chronic pain: Evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 64.Agorastos A, Pervanidou P, Chrousos GP, Baker DG. Developmental trajectories of early life stress and trauma: A narrative review on neurobiological aspects beyond stress system dysregulation. Front. Psychiatry. 2019;10:118. doi: 10.3389/fpsyt.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremner JD, Krystal JH, Charney DS, Southwick SM. Neural mechanisms in dissociative amnesia for childhood abuse: Relevance to the current controversy surrounding the "false memory syndrome". Am. J. Psychiatry. 1996;153:71–82. doi: 10.1176/ajp.153.12.1658. [DOI] [PubMed] [Google Scholar]
- 66.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 67.McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 68.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychiatry. 1999;46:1472–1479. doi: 10.1016/S0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 69.Arabadzisz D, et al. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol. Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Cinini SM, et al. Social isolation disrupts hippocampal neurogenesis in young non-human primates. Front. Neurosci. 2014;8:45. doi: 10.3389/fnins.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev. Psychopathol. 2001;13:677–693. doi: 10.1017/S0954579401003145. [DOI] [PubMed] [Google Scholar]
- 72.Klengel T, Binder EB. Allele-specific epigenetic modification: A molecular mechanism for gene-environment interactions in stress-related psychiatric disorders? Epigenomics. 2013;5:109–112. doi: 10.2217/epi.13.11. [DOI] [PubMed] [Google Scholar]
- 73.Kerker BD, et al. Adverse childhood experiences and mental health, chronic medical conditions, and development in young children. Acad. Pediatr. 2015;15:510–517. doi: 10.1016/j.acap.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Provencal N, et al. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc. Natl. Acad. Sci. U S A. 2020;117:23280–23285. doi: 10.1073/pnas.1820842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Dev. Psychopathol. 1998;10:871–885. doi: 10.1017/S0954579498001916. [DOI] [PubMed] [Google Scholar]
- 76.Macfarlane GJ, Beasley M, Smith BH, Jones GT, Macfarlane TV. Can large surveys conducted on highly selected populations provide valid information on the epidemiology of common health conditions? An analysis of UK Biobank data on musculoskeletal pain. Br. J. Pain. 2015;9:203–212. doi: 10.1177/2049463715569806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan F, et al. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: results from UK Biobank study. J. Sci. Med. Sport. 2019;22:444–449. doi: 10.1016/j.jsams.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Merskey, H.E. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. Pain (1986). [PubMed]
- 79.Nicholl BI, et al. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry. 2014;14:1–11. doi: 10.1186/s12888-014-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnston KJ, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15:e1008164. doi: 10.1371/journal.pgen.1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller KL, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016;19:1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Littlejohns TJ, et al. The UK Biobank imaging enhancement of 100,000 participants: Rationale, data collection, management and future directions. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alfaro-Almagro F, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischl B, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 85.O'Brien LM, et al. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatr. Res. Neuroimaging. 2011;193:113–122. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grama S, et al. Polygenic risk for schizophrenia and subcortical brain anatomy in the UK Biobank cohort. Transl. Psychiatry. 2020;10:1–10. doi: 10.1038/s41398-020-00940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 89.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Hanlon P, et al. Association between childhood maltreatment and the prevalence and complexity of multimorbidity: A cross-sectional analysis of 157,357 UK Biobank participants. J. Comorbid. 2020;10:2235042X10944344. doi: 10.1177/2235042X10944344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng W, et al. A genome-wide association study finds genetic variants associated with neck or shoulder pain in UK Biobank. Hum. Mol. Genet. 2020;29:1396–1404. doi: 10.1093/hmg/ddaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alfaro-Almagro F, et al. Confound modelling in UK Biobank brain imaging. Neuroimage. 2021;224:117002. doi: 10.1016/j.neuroimage.2020.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gheorghe DA, Li C, Gallacher J, Bauermeister S. Associations of perceived adverse lifetime experiences with brain structure in UK Biobank participants. J. Child Psychol. Psychiatry. 2021;62:822–830. doi: 10.1111/jcpp.13298. [DOI] [PubMed] [Google Scholar]
- 94.Luan J, Wong M, Day N, Wareham N. Sample size determination for studies of gene-environment interaction. Int. J. Epidemiol. 2001;30:1035–1040. doi: 10.1093/ije/30.5.1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.