Abstract
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by hair loss. Patients with AA experience a range of social and emotional impacts, and the lack of effective treatments and multiple affected locations can deepen the burden of illness. The objective of the current study was to assess health-related quality of life (HRQL) among patients with AA, and to evaluate the relationship between patient-reported AA severity, HRQL and treatment patterns.
Methods
A web survey was completed by participants recruited through the National Alopecia Areata Foundation. The survey included questions on disease characteristics, burden and impact (evaluated by the Skindex-16 for AA and items on work/school and sexual relationships), healthcare utilization and treatment experience. Analyses were conducted for the overall sample and by key subgroups, including AA severity and disease duration.
Results
A total of 1327 participants with AA completed the survey. The mean age was 39.7 [standard deviation (SD) 12.3] years and 58.4% were female. On average, participants had experienced signs and symptoms of AA for 11.5 years (SD 12.5) and were diagnosed by a healthcare provider (HCP) 10.5 (SD 12.2) years ago. Participants reported a range of severity of current scalp hair loss, including 0% (2.6%), 1–20% (39.8%), 21–49% (26.2%), 50–94% (10.2%) and 95–100% (21.3%). Participants reporting 95–100% of scalp hair missing were less likely to be currently seeing an HCP and to currently be on treatments for AA. There was a non-linear relationship between HRQL and current AA severity. Participants with 1–20% to 50–94% of current scalp hair missing reported higher symptom, functioning and emotional impacts due to AA than participants with 0% missing scalp hair and/or 95–100% missing scalp hair. Similar findings were observed for current eyebrow and eyelash severity, except for emotional impacts.
Conclusion
Severity of AA plays an important role in understanding the burden of illness and healthcare patterns of people living with AA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00702-4.
Keywords: Alopecia areata, Healthcare, Health-related quality of life, Survey, Symptom severity, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Alopecia areata is an autoimmune disease with a limited number of effective treatment options that results in a substantial and ongoing burden of illness for patients. |
| Severity of AA plays an important role in the burden of illness, with people living with AA having a wide variety of experiences in terms of severity of signs and symptoms, health-related quality of life (HRQL) and treatment. |
| What was learned from the study? |
| Participants with 95-100% of scalp hair missing were less likely to be seeing a healthcare provider for treatment of their AA and were also less likely to be receiving treatment. |
| There was a non-linear relationship between severity of current hair loss and HRQL, with participants with 21-49% or 50-94% scalp hair loss reporting higher impacts due to AA than participants with 95-100% of scalp hair missing. |
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by hair loss that impacts approximately 50,000 individuals in the USA [1]. The location and amount of hair loss can be unpredictable and may occur on the face, scalp and/or other areas of the body. The severity of hair loss also varies significantly among individuals, ranging from one or multiple hairless coin-shaped patches on the scalp to complete hair loss on all parts of the body.
There are presently no medications approved by the US Food and Drug Administration (FDA) for the indication of AA [2, 3]. Depending on the amount of hair loss, physicians may prescribe (off-label) topical corticosteroids, intralesional corticosteroids, topical immunotherapy, Janus kinase (JAK) inhibitors or other treatments, or recommend the use of a wig or scalp prosthesis for coverage. The absence of effective treatments for AA and its unpredictable disease course can greatly deepen the burden of illness. Patients with AA can experience a wide range of social and emotional impacts, including decreased self-confidence/self-worth, social stigma, bullying, social isolation, depression and anxiety [2]. Additionally, patients with AA report feeling like they have minimal control over their condition and treatment [4].
Patients with AA have reported feeling that healthcare providers (HCPs) do not fully understand or empathize with the emotional devastation of hair loss, and also that they had little support to help address the burden of AA [5]. While previous studies have explored the psychosocial impacts of AA, little is known about how the severity of AA relates to health-related quality of life (HRQL) and the healthcare and treatment experience. The objective of this study was to evaluate HRQL among patients with AA and explore the relationship between AA severity, HRQL, as well as the healthcare and treatment experience.
Methods
This was a cross-sectional, web-based survey involving 1327 patients with AA in the USA, fielded from 18 November to 4 December 2020. The study was approved by an institutional review board (IRB) Ethical & Independent Review Services (E&I study number: 20154-01; 15 September 2020) prior to the initiation of any participant recruitment. Participants were recruited through the National Alopecia Areata Foundation (NAAF), a nonprofit organization that supports AA research and educates the public about AA. NAAF identified and recruited all participants by sending out emails to their membership database and posting advertisements on their Facebook account. One email reminder was sent approximately 3 weeks after the initial outreach.
Interested participants clicked on the survey link to access the screener, consent form and subsequent survey. Participants who completed the survey in its entirety were remunerated for their time. To reduce the likelihood of duplicate responses, the survey restricted IP addresses from being able to access the survey after full completion of the survey or after assessment of screening ineligibility. Duplicates were also detected by checking email addresses provided for remuneration.
Participants were considered eligible for the study if they were at least 18 years of age, had a self-reported diagnosis of AA and were a resident of the USA. Participants were ineligible if they had androgenetic alopecia or alopecia barbae involvement only. Recruitment targets were developed to ensure a diverse sample.
Measures
Participants completed the web-based survey after providing electronic informed consent; the survey took most participants approximately 20 min to complete. The survey was divided into four key areas of interest, including disease characteristics, patient burden and social impacts, healthcare and treatment experience and sociodemographic and clinical characteristics.
For the disease characteristics section, participants completed single-item patient-reported outcomes (PROs) to evaluate their current severity and severity at its worst for key AA signs and symptoms, including hair loss on the scalp, eyebrows, eyelashes, nail appearance and eye irritation. The PROs were developed with input from clinical experts and patients with AA [6, 7]. Scalp hair loss was defined as “no missing scalp hair (0%),” “a limited area of scalp hair loss (1–20%),” “a moderate area of scalp hair loss (21–49%),” “a large area of scalp hair loss (50–94%)” or “nearly all or all of scalp hair missing (95–100%).” For eyebrow hair loss, participants could select “full eyebrows on each eye,” “minimal gap(s) or minimal amount of thinning,” “large gap(s) or large amount of thinning” and “no or barely any eyebrow hairs.” Similarly, for eyelashes, response options included “full eyelashes,” “minimal gap(s) along eyelids,” “large gap(s) along eyelids” and “no or barely any eyelash hair.”
The Skindex-16 for AA [6] was included in the survey to evaluate disease burden. The wording of the Skindex-16 had slight wording changes to refer to scalp and AA, yielding three subscale scores, namely, Symptoms, Emotions and Functioning. Domain scores range from 0 to 100, with higher scores indicating a higher impact of AA. For the healthcare and treatment experience section, participants self-reported which treatments for AA they had ever used, and those they were currently using, as well as treatment satisfaction and reporting on impressions of their interactions with their HCP. A single-item question was also included to evaluate participants’ willingness to try a new, regulatory-approved treatment for AA (“If a new, regulatory-approved prescription treatment was available, would you be willing to try it, understanding the need to take it long-term?”; response options: yes, no, not sure).
Analyses
Descriptive statistics [e.g., mean, standard deviation (SD), frequency, etc.) were applied to characterize the overall sample in terms of their sociodemographic and clinical characteristics as well as AA signs, symptoms and HRQL. To further explore the relationship between AA severity, HRQL, healthcare and treatment experience, we conducted analysis of variance (ANOVA) and Chi-square tests. Post-hoc pairwise comparisons were performed using Tukey’s test (p < 0.05) adjusting for multiple comparisons.
Results
Patient Disposition and Demographics
A total of 1327 participants with self-reported AA completed the survey. The mean age of the sample was 39.7 (SD 12.3) years, and a little over half were female (58.4%). Approximately half were White, non-Hispanic (52.7%). Over two thirds were married (69.9%) and living with a partner or spouse (70.1%). There was variability among participants’ education levels, with 12.2% (n = 162) having a high school degree or less, 28.3% (n = 376) having an associate, technical or trade school degree, 44.5% (n = 591) having a college degree and 14.9% (n = 198) having a post-graduate degree. Participants had employer-provided insurance (49.7%) or Medicare (25.6%) [Electronic Supplementary Material (ESM) Table 1].
On average, participants had experienced signs and symptoms of AA for 11.5 (SD 12.5) years and were diagnosed by an HCP 10.5 (SD 12.2) years ago. Participants reported that their current hair loss was most often AA monolocularis (22.8%) or AA multilocularis/patchy (35.1%), with 7.9% reporting ophiasis, 12.1% reporting totalis, 18.7% reporting universalis and only 3.4% reporting no current hair loss (Table 1).
Table 1.
Clinical characteristics of survey participants
| Participant clinical characteristics | Summary values | |
|---|---|---|
| Description of hair loss, n (%) | Current | At worst |
| Alopecia areata monolocularis (single spot) | 303 (22.8) | 130 (9.8) |
| Alopecia areata multilocularis/patchy (multiple spots) | 466 (35.1) | 368 (27.7) |
| Ophiasis (wave-shaped hair loss at the circumference of the head) | 105 (7.9) | 198 (14.9) |
| Alopecia areata totalis (complete or near complete hair loss on the scalp) | 160 (12.1) | 299 (22.5) |
| Alopecia areata universalis (complete or near complete loss of all body hair) | 248 (18.7) | 332 (25.0) |
| No hair loss | 45 (3.4) | N/A |
| Time since first experienced symptoms of alopecia areata, years | ||
| Mean (SD) [range] | 11.5 (12.5) [0.4–67.7] | |
| Time since diagnosis by a healthcare professional, years | ||
| Mean (SD) [range] | 10.5 (12.2) [0.2–67.4] | |
| Number of episodes of hair loss in lifetimea | ||
| N: Mean (SD) [range] | 1311: 4.4 (6.7) [0–60] | |
| Have you ever had hair regrowth when you were not taking any treatments for alopecia areata?, n (%) | ||
| No | 668 (50.3) | |
| Yes | 659 (49.7) | |
| Partial regrowth | 534 (81.0) | |
| Full regrowth | 125 (19.0) | |
N/A Not available, SD standard deviation
aTrimmed to exclude observations with reported values of ≥ 61 episodes (n = 16)
Signs and Symptoms of AA
Participants reported their current scalp hair loss as categorized on the Scalp Hair PRO as being in “a limited area” (1–20% of scalp; 39.8%), a “moderate area” (21–49% of scalp; 26.2%), a “large area” (50–94%; 10.2%) and “nearly all or all” (95–100%; 21.3%), with very few reporting “no missing hair” currently (2.6%). Most participants had full eyebrows (26.6%) or minimal gaps or thinning in the eyebrows (38.8%), and 20.7% had no or barely any eyebrows. Participants experienced a wide range of eyelash hair loss severity, with 32.5% having full eyelashes, followed by no or barely any eyelashes (19.4%), large gap(s) (14.1%) and minimal gap(s) (34.1%) (Table 2).
Table 2.
Current signs and symptoms of alopecia areata
| Signs and symptoms of current alopecia areata | Total (N = 1327) |
|---|---|
| Scalp, n (%) | |
| No missing hair (0% of my scalp is missing hair; I have a full head of hair) | 34 (2.6) |
| A limited area (1–20% of my scalp is missing hair) | 528 (39.8) |
| A moderate area (21–49% of my scalp is missing hair) | 347 (26.2) |
| A large area (50–94% of my scalp is missing hair) | 135 (10.2) |
| Nearly all or all (95–100% of my scalp is missing hair) | 283 (21.3) |
| Eyebrows, n (%) | |
| I have full eyebrows on each eye | 353 (26.6) |
| I have a minimal gap(s) or a minimal amount of thinning in at least one of my eyebrows | 515 (38.8) |
| I have a large gap(s) or a large amount of thinning in at east one of my eyebrows | 185 (13.9) |
| I have no or barely any eyebrow hairs | 274 (20.7) |
| Upper and lower eyelashes, n (%) | |
| I have full eyelashes on each eyelid | 431 (32.5) |
| I have a minimal gap or minimal gaps along the eyelids | 452 (34.1) |
| I have a large gap or large gaps along the eyelids | 187 (14.1) |
| I have no or barely any eyelash hair | 257 (19.4) |
There was a significant relationship between the extent of current scalp hair loss and the current severity of other signs and symptoms associated with AA (all p < 0.001) (ESM Table 2). Participants who reported more severe scalp hair loss also tended to report greater severity of other AA signs and symptoms, including eyebrow and eyelash hair loss. In addition, participants who reported 50–94% or 95–100% of scalp hair loss were more likely to have AA for longer duration (p < 0.001) (ESM Table 3).
Healthcare and Treatment Experience
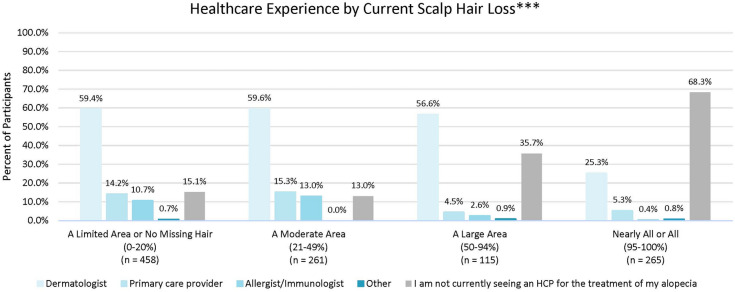
Most participants (71.7%) were diagnosed with AA by a dermatologist, and approximately half (58.0%) were still currently seeing a dermatologist for their AA. A quarter of the sample (24.5%) was not currently seeing an HCP for treatment for their AA. The highest proportion of patients not currently seeing an HCP for their AA were those with 95–100% of scalp hair missing. Conversely, the highest proportion currently seeing an HCP were those with 21–49% of scalp hair missing, of which 59.6% were seeing a dermatologist (p < 0.001) (Fig. 1). Approximately half of the participants (51.8%) felt their HCP adequately addressed their concerns, while 32.5% did not. As was expected, participants with no missing hair were more likely to report feeling their HCP adequately addressed their concerns with AA (p = 0.016).
Fig. 1.
Healthcare experience by current scalp hair Loss. ***p < 0.001 (Chi-square test)
Many participants had some experience with topical treatments (57.9%), injection treatments (41.3%), non-medical interventions (e.g. wigs) (34.6%), oral treatments (33.8%) and dietary changes or supplements (32.8%). Relatively fewer participants were currently using topical treatments (26.9%), injection treatments (13.2%) or oral treatments (24.3%) (ESM Table 4). Most participants currently on treatment had either 1–20% or 21–49% of scalp hair missing. Additionally, the highest proportion of patients (55.6%) reporting that they currently use non-medical interventions (e.g. wearing wigs or camouflage) were those with 95–100% of scalp hair missing (ESM Table 4).
Participants were also asked to report their satisfaction with their current treatment. Most participants currently taking oral treatment reported being somewhat satisfied (50.0%) or very satisfied (31.3%). Participants taking topical treatments were mostly somewhat satisfied (57.8%) or very satisfied (24.8%). Most participants taking injections (70.8%) or other alternative treatments (75.0%) were at least somewhat satisfied with their current treatment. There was no difference in treatment satisfaction by severity of hair loss for oral treatments (p = 0.194) or injection treatments (p = 0.191), and minimal differences in satisfaction were seen for topical (p = 0.006) and other treatments (p < 0.001). Overall, participants with 95–100% of scalp hair missing were more likely to report lower satisfaction with topical or other alternative treatment options.
As a part of the survey, participants were also asked if they would be willing to try a new, regulatory-approved treatment for AA long-term. Most participants (72.7%) said they would be willing and 18.4% reported they were not sure. Participants with “1–20%” (72.7%), “21–49%” (82.1%) or “50–94%” (77.0%) of scalp hair missing were willing to try a new treatment, while 44.1% with “no missing scalp hair” and 62.2% with “95–100% of scalp hair missing” were willing to try.
Health-Related Quality of Life
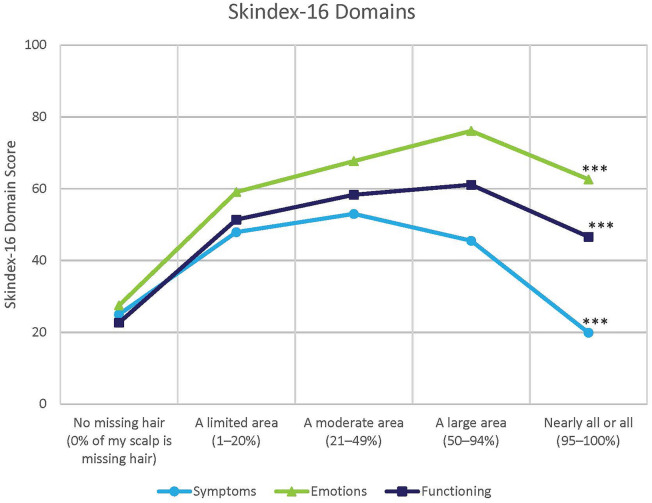
Participants reported impaired HRQL, as defined by the Skindex-16 for AA, for the Emotions domain (mean 63.0, SD 24.9), Functioning domain (mean 52.5, SD 27.0) and Symptoms domain (mean 42.4, SD 26.8). There was a non-linear relationship between HRQL and current AA hair loss (Fig. 2) with participants who had “21–49%” or “50–94%” of scalp hair missing reporting the largest impacts due to AA compared to participants with no missing scalp hair or 95–100% of scalp hair missing.
Fig. 2.
Health-related quality of life by current scalp hair loss. Asterisks indicate significant difference at ***p < 0.0001. Pairwise comparisons between group means were performed using the Tukey–Kramer test adjusting for multiple comparison
For current scalp hair loss, the highest burden on symptoms due to AA was seen for participants with “21–49% of scalp hair missing.” Participants with “50–94% of scalp hair missing” reported the highest burden due to Emotional impacts, and both “21–49%” and “50–94% of scalp hair missing” participants reported the most impact due to Functioning (p < 0.0001). When evaluating other key signs and symptoms of AA, participants with full eyebrows or eyelashes and those with no or barely any reported a significantly lower impact on Symptoms and Functioning (p < 0.0001). Participants with full eyebrows reported the lowest impact on Emotions, while there was no significance difference among participants with minimal to no or barely any eyebrow hairs (p < 0.0001) (ESM Fig. 1). No relationship was found between degree of eyelash hair loss and Emotional impacts (p = 0.2127) (ESM Fig. 2).
Discussion
Overall, the patient-reported impact of AA was highly variable, with a wide range of scores for the Skindex-16 for AA domains (Symptoms, Emotions and Functioning). AA has a consistent and significant emotional burden on patients with this disease. The significant emotional burden of AA shown in the study is consistent with previous research evaluating quality of life in this population[4] and with the FDA Voice of the Patient Report [2].
Participants who self-reported more severe AA were less likely to be currently seeing an HCP and receiving medical intervention for their AA. Participants with more severe AA were also more likely to have AA for longer. Patients with complete hair loss may have given up due to the lack of short- and long-term efficacy from the limited treatment options currently available. Additionally, complete hair loss may be less challenging to manage in terms of alternative strategies (i.e. wearing a wig). While the question still remains, these particular patients may have better adapted in terms of coping, especially due to the longer duration of disease, and be less willing to expend time and resources on seeking treatment options through an HCP. Future patient outreach on newly developed treatments may need to go beyond HCPs in order to reach patients who may benefit the most from treatments, but who may no longer be actively seeking treatments or consulting an HCP.
Interestingly, there was a consistent non-linear relationship between HRQL domains (including Symptoms, Functioning and Emotions) and AA severity, with a higher impact due to AA for participants with a “21–49%” or “50–94% of scalp hair missing” compared to those with no missing hair or “95–100% of scalp hair missing.” Future research might explore how the duration and severity of AA may result in coping strategies resulting in patients with the most severe AA adapting or coping better, resulting in less reliance on healthcare resources.
There were several limitations that should be considered when interpreting the results of the current study. First participants self-reported their clinical and disease characteristics Second, participants may not have been able to report on all aspects of their AA experience with a high degree of accuracy when the information was highly retrospective. For example, signs and symptoms at their worst and number of hair loss episodes were likely difficult for some participants to recall with a high degree of accuracy. Third, due to the large sample size, some statistical differences that are reported may represent smaller effects; however the large sample size for this study did allow for the opportunity to evaluate trends across key subgroups (i.e. AA severity by impact).
Conclusion
Despite the above-mentioned limitations, the current study provides valuable insights into the experiences of US patients with AA. The results suggest that these patients are highly impacted by AA and are only moderately satisfied with current treatments options, and that the majority are likely to be interested in considering future treatments, should they become available.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the research participants for participating in the study.
Funding
This research study was funded by Eli Lilly and Company. Eli Lilly and Company will be funding the journal’s Rapid Service Fees.
Medical Writing/Editorial Assistance
Dawn Ri’chard of Evidera provided editorial assisting for the manuscript, which was funded by Eli Lilly and Company.
Authorship
The authors received no financial support for the research, authorship, and/or publication of this article. The authors have given their approval for this version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors provided critical feedback and helped shape the research, analysis and manuscript.
Disclosures
Amy DeLozier, Peter Wright and Emily Edson-Heredia are employees and shareholders of Eli Lilly and Company. Katelyn Cutts and Heather Gelhorn are employees of Evidera, a research organization who conducted the study under contract by Eli Lilly and Company. Maryanne Senna has served on advisory boards and/or have been a consultant for Arena Pharmaceuticals, Concert Pharmaceuticals Inc., Eli Lilly and Company, Pfizer Inc, and Follica, Inc. She is a clinical trial investigator for Concert Pharmaceuticals Inc., Eli Lilly and Company and Follica, Inc. Jerry Shapiro is a consultant or clinical trial investigator for Pfizer Inc. and a consultant for Eli Lilly and Company. Antonella Tosti is a consultant for DS Laboratories, Monat Global, Almirall, Tirthy Madison, Leo Pharmaceuticals, Bristol Myers Squibb and P&G; and is is a compensated consultant/advisory board member for Eli Lilly and Company, sponsor of the study.
Compliance with Ethics Guidelines
The study was approved by an institutional review board (IRB) Ethical & Independent Review Services (E&I study number: 20154-01; 15 September 2020) prior to the initiation of any participant recruitment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Li SJ, Huang KP, Joyce C, Mostaghimi A. The impact of alopecia areata on sexual quality of life. Int J Trichol. 2018;10(6):271–274. doi: 10.4103/ijt.ijt_93_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER). The voice of the patient—alopecia areata. Public Meeting: 11 September 2017; Report: March 2018. https://www.fda.gov/media/112100/download. Accessed 1 June 2020.
- 3.Juarez-Rendon KJ, Rivera Sanchez G, Reyes-Lopez MA, et al. Alopecia Areata. Current situation and perspectives. Arch Argent Pediatr. 2017;115(6):e404–e411. doi: 10.5546/aap.2017.eng.e404. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright T, Endean N, Porter A. Illness perceptions, coping and quality of life in patients with alopecia. Br J Dermatol. 2009;160(5):1034–1039. doi: 10.1111/j.1365-2133.2008.09014.x. [DOI] [PubMed] [Google Scholar]
- 5.Davey L, Clarke V, Jenkinson E. Living with alopecia areata: an online qualitative survey study. Br J Dermatol. 2019;180(6):1377–1389. doi: 10.1111/bjd.17463. [DOI] [PubMed] [Google Scholar]
- 6.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–110. doi: 10.1177/120347540100500202. [DOI] [PubMed] [Google Scholar]
- 7.Wyrwich KW, Kitchen H, Knight S, et al. Development of clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) measures for eyebrow, eyelash and nail assessment in alopecia areata. Am J Clin Dermatol. 2020;21(5):725–732. doi: 10.1007/s40257-020-00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.