Abstract
Introduction
Hidradenitis suppurativa (HS) is considered to be the most burdensome dermatosis, with a well-documented negative influence on quality of life (QoL). The patient’s perception of the disorder, assessed as the self-reported severity, has been used in other dermatoses but not in HS. The aim of this study was to evaluate the usefulness of self-reported HS severity in clinical practice.
Methods
The study was performed on a group of 130 Spanish HS patients. HS severity was assessed for all the subjects. Hurley staging and patient self-reported severity were used. Moreover, QoL impairment was evaluated using the Dermatology Life Quality Index (DLQI) and the Hidradenitis Suppurativa Quality of Life 24 (HSQoL-24) questionnaire.
Results
The severity of HS according to the Hurley staging was most commonly assessed as Hurley II (47.7%), indicating moderate disease, followed by severe disease (Hurley III, 26.9%) and mild disease (Hurley I, 25.4%). According to the patient self-reported HS severity, most of the patients reported having mild disease (76 patients, 58.5%), followed by moderate disease (31 patients, 23.8%). Only 23 patients (17.7%) assessed their disease as severe. Moreover, men reported mild disease significantly more frequently than women (70.9% and 49.3%, respectively; p = 0.014).
The self-reported HS severity correlated positively with the effect of the disease on patient QoL assessed with DLQI (r = 0.288, p < 0.001). Likewise, a strong positive correlation was found between self-reported HS severity and QoL impairment assessed with HSQoL-24 (r = 0.404, p = 0.001). No statistically significant correlation between Hurley severity stage and DLQI or HSQoL-24 was found. Moreover, there were significant differences in both DLQI and HSQoL-24 total score between different self-reported HS severities. This was not seen for any of the QoL instruments or for Hurley severity staging.
Conclusion
The results show that self-assessment severity may reflect patients’ subjective feelings more adequately than popular objective instruments, and there should be a place for its use in daily clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00701-5.
Keywords: Hidradenitis suppurativa, Self-reported severity, Hurley, Assessment
Key Summary Points
| Hidradenitis suppurativa (HS) is a chronic inflammatory dermatosis with a well-documented negative influence on quality of life (QoL). |
| The patient’s perception of the disorder, assessed as the self-reported severity, has been used in other dermatoses, but not in HS. |
| The aim of the study was to assess the clinical usefulness of self-reported HS severity evaluation. |
| The self-reported severity correlated positively with QoL impairment assessed with the Dermatology Life Quality Index and Hidradenitis Suppurativa Quality of Life 24. Different HS severities had different effects on QoL. |
| The above-mentioned results were not found for Hurley staging, indicating that self-reported disease severity may more adequately reflect the patient’s perspective. |
Introduction
A patient-reported outcome (PRO) is defined as a type of patient health measurement that comes directly from the patient, without any interpretation of the results by a clinician or others [1]. In recent years, PROs have become an important part of a new holistic approach to the patient in both clinical and academic settings [1]. These subjective measurements allow physicians to gain an insight into the patient’s perspective and to understand the patient’s attitudes, burden, and feelings [2]. Dermatological disorders are often associated with disfigurement and a negative influence on the patient’s quality of life (QoL), which may not be adequately reflected by an assessment of the area and the severity of the disease [3–5]. Therefore, PROs are an important part of routine dermatological care and scientific research, and are currently commonly used as endpoints in clinical trials [6].
Hidradenitis suppurativa (HS) is a burdensome, debilitating, chronic inflammatory dermatosis for which QoL impairment is well documented [5, 7–9]. The severity of the disease is commonly assessed and evaluated by a clinician using many available instruments [10–12]. The aim of this study was to assess the usefulness of a self-reported hidradenitis suppurativa (HS) severity scale and to evaluate if there is a place for it in routine dermatology practice.
Methods
Study group
The study was performed on a group of hidradenitis suppurativa patients treated at the following hospitals in Spain between 2016 and 2017: University Hospital Miguel Servet (Zaragoza), Royo Villanova Hoaspital (Zaragoza), Barbastro Hospital (Huesca), Infanta Sofia Hospital (Madrid), Santa Creuu I Sant Pau Hospital (Barcelona), and Doctor Negrin Hospital (Las Palmas de Gran Canaria). All the patients were examined and assessed by a trained specialist in dermatology. Basic sociodemographic data were collected, including gender, age, weight, and height, as well as age at onset of the disease, its duration, and the number of affected areas. The study was conducted according to the guidelines of the Declaration of Helsinki of 1964 and its later amendments. The study was accepted by the Clinical Research Ethics Committee of Aragon (CEICA) on 10 February 2016 (number PI16/020), and by the corresponding committees in the other participating hospitals. Moreover, a signed consent was obtained for every patient before their inclusion in the study.
Quality of life
The effect of the disease on the quality of life (QoL) of each patient was assessed using the Dermatology Life Quality Index (DLQI) and the newly developed, HS-specific Hidradenitis Suppurativa Quality of Life 24 (HSQoL-24) questionnaire. DLQI is a widely used, user-friendly, dermatology-specific tool for assessing the impacts of skin diseases on quality of life. It was developed in 1994 by Finlay and Khan [13], and has been used since then for a variety of dermatoses, including psoriasis, atopic dermatitis, and HS [9, 14, 15]. It is a 10-item instrument, and the degree of impairment is assessed on a 4-point scale (0: not at all, 3: very much) for each item within a 7-day recall period. The maximum achievable score is 30 points, and the higher the score, the bigger the impact on QoL. HSQoL-24 is a new Spanish HS-specific questionnaire that was translated into and validated in English in 2021 [16, 17]. It consists of 24 items divided into six life domains. This instrument evaluates the impact on QoL in a 4-week recall period. Each item is scored on a 5-point Likert scale. The maximum achievable score is 96 points [16, 17].
HS severity
HS severity was assessed for all the subjects. Hurley staging and patient self-reported severity were used. Hurley staging was introduced by Hurley in 1989 [10]. It divides the severity of the disease into three stages from the mildest to the most severe (Hurley I to Hurley III, respectively). Moreover, a self-reported HS severity scale was used, in which patients were asked to evaluate the severity of their disease at the time of clinical evaluation on a verbal rating scale. The subjects were asked to assess their disease severity as mild, moderate, or severe (see Supplementary Table 1).
Statistical analysis
Statistical analysis of the obtained results was performed with the IBM SPSS Statistics v. 26 (SPSS INC., Chicago, IL, USA) software. All data were assessed for a normal or abnormal distribution. The minimum, maximum, mean, and standard deviation were calculated. Quantitative variables were evaluated using the Mann–Whitney U test and Spearman’s or Pearson’s correlations. For qualitative data, the chi-squared test was used.
Differences in total DLQI and total HSQoL-24 between patients with different severities according to the self-reported severity and the Hurley staging system were assessed via Kruskal–Wallis one-way analysis of variance on ranks. A two-sided p with a value lower than 5% was considered significant.
Results
The group consisted of 130 consecutive HS patients: 75 females (57.7%) and 55 males (42.3%). The patients were 37.3 ± 11.9 years old on average, with no age difference observed between the sexes. The population was characterized as overweight, with a mean BMI of 29.0 ± 5.6 kg/m2. Women tended to have a higher BMI than men (30.1 ± 6.0 kg/m2 and 27.5 ± 4.6 kg/m2, respectively; p = 0.023). The mean age at onset of the disease was reported as 23.1 ± 10.9 years old. The disease started significantly earlier in females than in males (21.8 ± 11.2 years old and 24.9 ± 10.5 years old, respectively; p = 0.021). The patients had suffered from HS for 14.1 ± 11 years on average, and the disease affected about 2.2 ± 1 skin areas (Table 1).
Table 1.
Patient characteristics
| Characteristic | Whole group (n = 130) | Women (n = 75) | Men (n = 55) | P |
|---|---|---|---|---|
| Sex | ||||
| Number of men (%) | 55 (42.3) | NA | NA | NA |
| Number of women (%) | 75 (57.7) | |||
| Age | ||||
| Mean ± SD (years) | 37.3 ± 11.9 | 37.3 ± 11.9 | 37.4 ± 11.9 | NS |
| Body mass index (BMI) | ||||
| Mean ± SD (kg/m2) | 29.0 ± 5.6 | 30.1 ± 6.0 | 27.5 ± 4.6 | 0.023 |
| Age at onset of the disease | ||||
| Mean ± SD (years old) | 23.1 ± 10.9 | 21.8 ± 11.2 | 24.9 ± 10.5 | 0.021 |
| Duration of the disease | ||||
| Mean ± SD (years) | 14.1 ± 11.0 | 15.6 ± 11.2 | 12.1 ± 10.5 | |
| Number of locations | ||||
| Mean ± SD | 2.2 ± 1.0 | 2.2 ± 1.0 | 2.3 ± 1.1 | NS |
SD standard deviation, NS not significant, NA not applicable, n number of participants
According to Hurley staging, the severity of HS in the majority of patients was assessed as Hurley II, indicating moderate disease, followed by severe disease (Hurley III) and mild disease (Hurley I). No differences in Hurley severity assessment between the sexes were found. According to the patient self-reported HS severity, most of the patients reported having mild disease (76 patients, 58.5%), followed by moderate disease (31 patients, 23.8%). Only 23 patients (17.7%) assessed their disease as severe. Moreover, men reported mild disease significantly more frequently than women (p = 0.014). This difference was not observed for other HS severities (Table 2). On average, HS had a moderate effect on the patient’s life, with a mean DLQI score of 8.4 ± 7.2 points. The perceived effect was significantly greater for women than for men (p = 0.001). The impairment of QoL assessed with HSQoL-24 was considered to be serious, with a mean global score of 44.1 ± 19.2 points. Similar results were visible for every life domain aside from personal, for which QoL impairment was assessed as moderate (24.9 ± 21.7 points). As also seen for the DLQI, women scored significantly higher for the HSQoL-24 global score (p < 0.001), as well as for every life domain excluding the clinical and occupational domains (Table 3).
Table 2.
Hidradenitis suppurativa severity
| Characteristics | Whole group (n = 130) | Women (n = 75) | Men (n = 55) | P |
|---|---|---|---|---|
| Hurley stages, number of participants (%) | ||||
| I | 33 (25.4) | 21 (28.0) | 12 (21.8) | NS |
| II | 62 (47.7) | 31 (41.3) | 31 (56.4) | NS |
| III | 35 (26.9) | 23 (30.7) | 12 (21.8) | |
| Self-reported HS severity, number of participants (%) | ||||
| Mild | 76 (58.5) | 37 (49.3) | 39 (70.9) | 0.014 |
| Moderate | 31 (23.8) | 22 (29.3) | 9 (16.4) | NS |
| Severe | 23 (17.7) | 16 (21.3) | 7 (12.7) | NS |
N number of participants; NS not significant
Table 3.
Quality of life impairment
| Characteristic | Whole group (n = 130) | Women (n = 75) | Men (n = 55) | P |
|---|---|---|---|---|
| DLQI | ||||
| Total score (mean ± SD) | 8.4 ± 7.2 | 10.1 ± 7.3 | 6.1 ± 6.4 | 0.001 |
| HSQoL-24 (mean ± SD) | ||||
| Global | 44.1 ± 19.2 | 49.8 ± 19.4 | 36.3 ± 16.1 | < 0.001 |
| Psychosocial | 46.6 ± 21.5 | 52.9 ± 20.6 | 38.1 ± 19.8 | < 0.001 |
| Economic | 39.6 ± 36.8 | 49.3 ± 38.5 | 26.4 ± 29.8 | 0.001 |
| Occupational | 45.5 ± 32.4 | 47.3 ± 33.2 | 43.1 ± 31.5 | NS |
| Relationships | 51.4 ± 31.6 | 58.9 ± 35.3 | 41.1 ± 22.2 | 0.001 |
| Personal | 24.9 ± 21.7 | 28.5 ± 22.3 | 20.0 ± 19.9 | 0.026 |
| Clinical | 46.8 ± 24.6 | 50.3 ± 27.3 | 42.0 ± 19.7 | NS |
DLQI Dermatology Life Quality Index, HSQoL-24 Hidradenitis Suppurativa Quality of Life 24, n number of participants, SD standard deviation, NS not significant
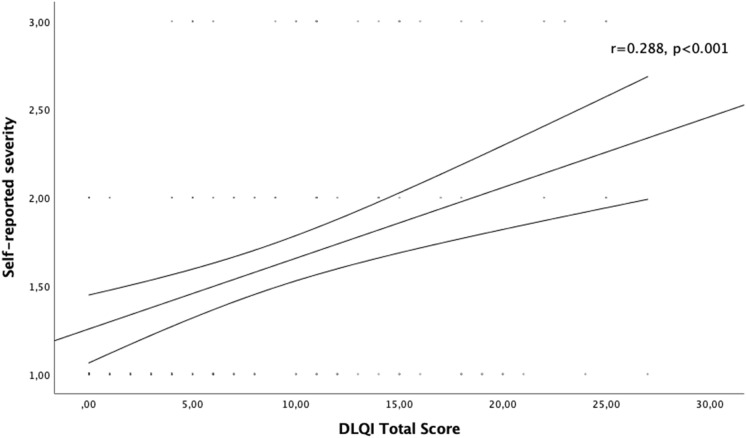
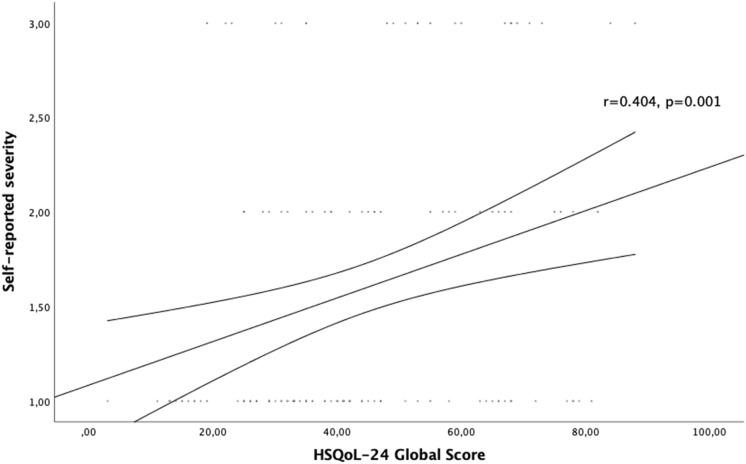
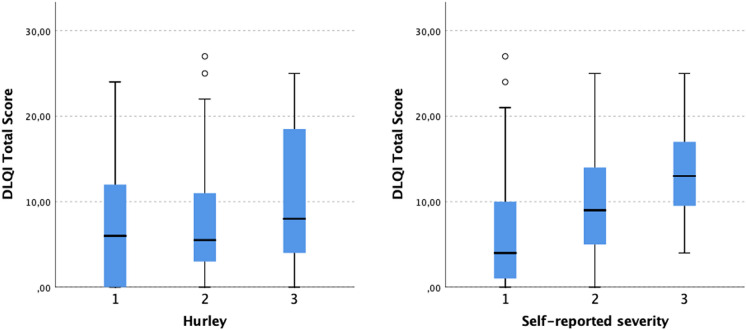
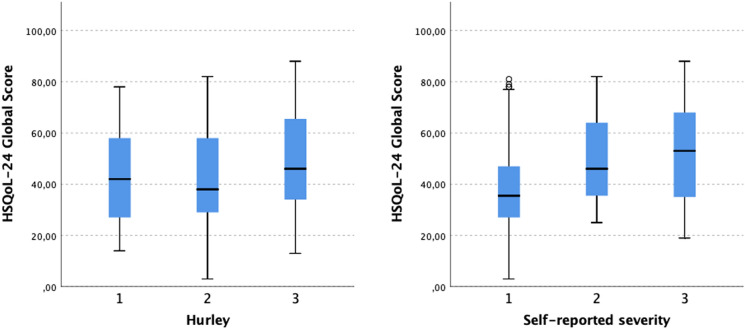
The self-reported HS severity correlated positively with the effect of the disease on patient QoL as assessed with DLQI (r = 0.288, p < 0.001) (Fig. 1). Likewise, a strong positive correlation was found between self-reported HS severity and QoL impairment as assessed with HSQoL-24 (r = 0.404, p = 0.001) (Fig. 2). No statistically significant correlation was found between Hurley severity stage and DLQI or HSQoL-24. Moreover, different self-reported HS severities showed significantly different DLQIs and HSQoL-24 total scores (Figs. 3 and 4). This was not seen for any of the QoL instruments or for Hurley severity staging (Table 4). The kappa value for the agreement between Hurley stage and self-reported severity was 0.153.
Fig. 1.
Correlation of self-reported disease severity with DLQI
Fig. 2.
Correlation of self-reported disease severity with HSQoL-24
Fig. 3.
Differences in DLQI total score between different self-reported HS severities and between different HS severities assessed with Hurley staging
Fig. 4.
Differences in HSQoL-24 global score between different self-reported HS severities and between different HS severities assessed with Hurley staging
Table 4.
Differences in QoL impairment between different HS severities
| Severity assessment | DLQI score (mean ± SD) | p | HSQoL-24 score (mean ± SD) | p | |
|---|---|---|---|---|---|
| Hurley | |||||
| I | 7.6 ± 7.2 | 42.8 ± 18.9 | |||
| II | 7.4 ± 6.3 | NS | 42.6 ± 19.2 | NS | |
| III | 10.91 ± 8.3 | 47.8 ± 19.8 | |||
| Self-assessed | |||||
| Mild | 6.4 ± 6.8 | 39.4 ± 18.6 | |||
| Moderate | 9.7 ± 6.8 | < 0.001 | 49.1 ± 17.3 | 0.005 | |
| Severe | 13.3 ± 6.5 | 52.7 ± 19.8 | |||
NS not significant, SD standard deviation
Discussion
Hidradenitis suppurativa (HS) is a chronic, debilitating, recurrent inflammatory skin disorder of unknown pathogenesis that affects the pilosebaceous unit [18]. It is characterized by the formation of deep-seated inflammatory nodules, predominantly in intertriginous locations such as the groin, armpits, and anogenital area [19]. In the course of the disease, nodules progress into abscesses, sinuses, and scarring [19]. The prevalence of the disease has been reported to peak in young adults between 20 and 40 years of age. The exact incidence varies greatly among the available studies, and is currently estimated at 0.03–1% [20, 21]. Due to the resulting continuous purulent discharge, foul smell, and disease-associated pain, HS is considered the most burdensome form of dermatosis, and has a well-documented negative impact on patient QoL. This disease is associated with a high incidence of depression, anxiety, stigmatization, alexithymia, workplace challenges, and even suicide ideation [22–26]. Moreover, it negatively affects patients’ partners and families [27].
The clinical severity of the disease may be assessed using a variety of instruments. Among the most frequently used are the Hurley staging system [10], the International Hidradenitis Suppurativa Severity Score (IHS4) [11], the Sartorius score [28], and the Physician Global Assessment (PGA) [29]. It is worth underlining that all of the previously mentioned scoring systems are objective and are designed to be used by a physician. The patient’s perception of the disorder, assessed as the self-reported severity, has been used for other dermatoses. Self-reported AD severity questionnaires include the Patient-Oriented SCORAD (PO-SCORAD) and Self-Administered Eczema Area and Severity Index (SA-EASI) [30, 31]. Similarly to their use in AD, patient-reported outcome measurements (PROMs) are also commonly used in psoriasis. The self-assessed Simplified Psoriasis Index (saSPI) is an instrument that combines the psychosocial impact of psoriasis, its current severity, and past history and interventions [32]. The severity assessment includes the extent of the disease and the choice of sentences that best describe the overall state of psoriasis at the time of examination [32]. The maximum achievable score for the severity of the disease is 50 points, and the higher the result, the more severe the AD. Moreover, it was proven that the results correlate strongly with the Psoriasis Severity and Area Index (PASI) [32]. Regarding HS, to the best of our knowledge, there are only three reports of attempts to use self-reported severity measurements in the literature [33–35]. The first, published by Cusack et al. [34], was performed on a group of 6 patients diagnosed with HS who were treated with etanercept [34]. The patients were supposed to assess the severity of the disease at the beginning and end of the therapy. Moreover, at the end of the treatment, all patients had to estimate the percentage improvement in HS [34]. In 2015, Deckers et al. [35] tried, for the first time, to determine the capability of patients to self-assess their Hurley stage using pictures. They found that in 76% of the cases, the results were similar to those given by a physician [35]. Moreover, a substantial agreement (with a weighted kappa of 0.63) was achieved, which was comparable to that seen in similar studies of psoriasis and atopic dermatitis [35]. The only available validation of a self-reported severity tool was published in 2019 by Senthilnathan et al. [33]. The tool consisted of 10 color photographs of different Hurley stages which 24 patients were supposed to choose from. The results, although worse than in the previous study, showed moderate agreement between assessments performed by patients and those performed by physicians (a weighted kappa of 0.57), indicating that patients may be able to assess their severity adequately [33].
The above-mentioned results clearly show that self-reported severity is a reliable tool for gaining the patient’s perspective and new insight into the disease. Contrary to Hurley staging, self-reported severity correlated positively with QoL impairment. Moreover, differences in QoL impairment between different self-assessed severities of the disease were found. This may indicate that self-reported severity reflects the patient’s subjective feelings more adequately than objective severity measures such as Hurley staging.
We understand that our study has some limitations. Firstly, only Hurley staging was used to assess severity, which may not be the most detailed and reliable measurement. Nevertheless, it is necessary to underline that Hurley staging is still one of the most commonly used HS staging systems worldwide. Secondly, the self-reported severity assessment was not previously validated nor tested on smaller groups.
Conclusion
In conclusion, to the best of our knowledge, this is the first study to assess the usefulness of the self-reported severity of HS. The results of our study show that self-reported severity may be adequate for HS severity assessment and there should be a place for its use in daily clinical practice in the future. Nevertheless, further studies with validated objective tools for the assessment of HS are necessary before introducing this PROM into daily clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
The study was funded by a research grant from Wroclaw Medical University (SUBZ.C260.22.056). No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Servando E Marrón, Lucía Tomas Aragones and Yolanda Gilaberte-Calzada. The first draft of the manuscript was written by Piotr K Krajewski and Jacek C Szepietowski and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Piotr K Krajewski, Servando E Marrón, Lucía Tomas Aragones, Yolanda Gilaberte-Calzada and Jacek C Szepietowski have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted according to the guidelines of the Declaration of Helsinki of 1964 and its later amendments. The study was accepted by the Clinical Research Ethic Committee of Aragon (CEICA) on 10 February 2016 (number PI16/020), by the corresponding committees in the other participants hospitals. Moreover, a signed consent was obtained for every patient, before including in the study.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request and with permission of all the authors. The data are not publicly available due to reasons of sensitivity and other reasons
References
- 1.Apfelbacher CJ, Nelson PA. Patient-reported outcome measures and qualitative research in dermatology: the quest for authenticity. Br J Dermatol. 2017;176(2):285–287. doi: 10.1111/bjd.15251. [DOI] [PubMed] [Google Scholar]
- 2.Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book. 2018;38:122–134. doi: 10.1200/EDBK_200383. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrov D, Szepietowski JC. Instruments to assess stigmatization in dermatology. Postepy Hig Med Dosw (Online) 2017;71:901–905. doi: 10.5604/01.3001.0010.5607. [DOI] [PubMed] [Google Scholar]
- 4.Shen MX, Chen X. Minimally important change: approach to interpretation of patient-reported outcomes. Br J Dermatol. 2020;183(1):8–9. doi: 10.1111/bjd.18711. [DOI] [PubMed] [Google Scholar]
- 5.Krajewski PK, Matusiak Ł, von Stebut E, Schultheis M, Kirschner U, Nikolakis G, et al. Quality-of-life impairment among patients with hidradenitis suppurativa: a cross-sectional study of 1795 patients. Life (Basel). 2021;11(1):34. doi: 10.3390/life11010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copley-Merriman C, Zelt S, Clark M, Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient. 2017;10(2):203–213. doi: 10.1007/s40271-016-0196-6. [DOI] [PubMed] [Google Scholar]
- 7.Mac Mahon J, Kirthi S, Byrne N, O’Grady C, Tobin AM. An update on health-related quality of life and patient-reported outcomes in hidradenitis suppurativa. Patient Relat Outcome Meas. 2020;11:21–6. [DOI] [PMC free article] [PubMed]
- 8.Chernyshov PV, Finlay AY, Tomas-Aragones L, Poot F, Sampogna F, Marron SE, et al. Quality of life in hidradenitis suppurativa: an update. Int J Environ Res Public Health. 2021;18(11):6131. doi: 10.3390/ijerph18116131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernyshov PV, Zouboulis CC, Tomas-Aragones L, Jemec GB, Svensson A, Manolache L, et al. Quality of life measurement in hidradenitis suppurativa: position statement of the European Academy of Dermatology and Venereology task forces on Quality of Life and Patient-Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. 2019;33(9):1633–1643. doi: 10.1111/jdv.15519. [DOI] [PubMed] [Google Scholar]
- 10.Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. Dermatol Surg. 1989;729:39.
- 11.Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748. [DOI] [PubMed] [Google Scholar]
- 12.Goldfarb N, Lowes MA, Butt M, King T, Alavi A, Kirby JS. Hidradenitis suppurativa area and severity index revised (HASI-R): psychometric property assessment. Br J Dermatol. 2021;184(5):905–912. doi: 10.1111/bjd.19565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. [DOI] [PubMed]
- 14.Mattei PL, Corey KC, Kimball AB. Psoriasis area severity index (PASI) and the dermatology life quality index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–7. [DOI] [PubMed]
- 15.Misery L, Seneschal J, Reguiai Z, Merhand S, Heas S, Huet F, et al. The impact of atopic dermatitis on sexual health. J Eur Acad Dermatol Venereol. 2019;33(2):428–432. doi: 10.1111/jdv.15223. [DOI] [PubMed] [Google Scholar]
- 16.Marron SE, Gomez-Barrera M, Tomas Aragones L, Goni-Navarro A, Vilarrasa-Rull E, Diaz-Diaz RM, et al. Quality of life in hidradenitis suppurativa: validation of the HSQoL-24. Acta Derm Venereol. 2021;101(8):adv00529. [DOI] [PMC free article] [PubMed]
- 17.Marrón SE, Gómez-Barrera M, Tomás-Aragonés L, Díaz Díaz RM, Vilarrasa Rull E, Madrid Álvarez MB, et al. Development and preliminary validation of the HSQoL-24 tool to assess quality of life in patients with hidradenitis suppurativa. Actas Dermosifiliogr (Engl Ed). 2019;110(7):554–60. [DOI] [PubMed]
- 18.Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 19.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 20.Kirsten N, Petersen J, Hagenström K, Augustin M. Epidemiology of hidradenitis suppurativa in Germany—an observational cohort study based on a multisource approach. J Eur Acad Dermatol Venereol. 2020;34(1):174–9. [DOI] [PubMed]
- 21.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Matusiak Ł. Profound consequences of hidradenitis suppurativa: a review. Br J Dermatol. 2020;183(6):e171–e177. doi: 10.1111/bjd.16603. [DOI] [PubMed] [Google Scholar]
- 23.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90(3):264–268. doi: 10.2340/00015555-0866. [DOI] [PubMed] [Google Scholar]
- 24.Głowaczewska A, Szepietowski JC, Matusiak Ł. Prevalence and associated factors of alexithymia in patients with hidradenitis suppurativa: a cross-sectional study. Acta Derm Venereol. 2021;101(11):adv00598. [DOI] [PMC free article] [PubMed]
- 25.Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138(1):52–57. doi: 10.1016/j.jid.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GBE, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Włodarek K, Głowaczewska A, Matusiak Ł, Szepietowski JC. Psychosocial burden of hidradenitis suppurativa patients’ partners. J Eur Acad Dermatol Venereol. 2020;34(8):1822–7. [DOI] [PubMed]
- 28.Sartorius K, Lapins J, Emtestam L, Jemec GB. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol. 2003;149(1):211–3. [DOI] [PubMed]
- 29.Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–55. [DOI] [PubMed]
- 30.Housman TS, Patel MJ, Camacho F, Feldman SR, Fleischer AB, Jr, Balkrishnan R. Use of the self-administered eczema area and severity index by parent caregivers: results of a validation study. Br J Dermatol. 2002;147(6):1192–1198. doi: 10.1046/j.1365-2133.2002.05031.x. [DOI] [PubMed] [Google Scholar]
- 31.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Arch Dermatol. 2004;140(12):1513–1519. doi: 10.1001/archderm.140.12.1513. [DOI] [PubMed] [Google Scholar]
- 32.Chularojanamontri L, Griffiths CE, Chalmers RJ. The Simplified Psoriasis Index (SPI): a practical tool for assessing psoriasis. J Invest Dermatol. 2013;133(8):1956–1962. doi: 10.1038/jid.2013.138. [DOI] [PubMed] [Google Scholar]
- 33.Senthilnathan A, Kolli SS, Cardwell LA, Richardson I, Feldman SR, Pichardo RO. Validation of a hidradenitis suppurativa self-assessment tool. J Cutan Med Surg. 2019;23(4):388–90. [DOI] [PubMed]
- 34.Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa. Br J Dermatol. 2006;154(4):726–729. doi: 10.1111/j.1365-2133.2005.07067.x. [DOI] [PubMed] [Google Scholar]
- 35.Deckers IE, Mihajlović D, Prens EP, Boer J. Hidradenitis suppurativa: a pilot study to determine the capability of patients to self-assess their Hurley stage. Br J Dermatol. 2015;172(5):1418–1419. doi: 10.1111/bjd.13502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request and with permission of all the authors. The data are not publicly available due to reasons of sensitivity and other reasons