Abstract
Background
Diverting ileostomy during resection of rectal cancer is frequently performed in patients at risk of anastomotic failure. Clostridium difficile infection (CDI) is reported to be frequent in patients who receive ileostomy closure with a questionable association to postoperative anastomosis leak. The primary aim of this study was to determine the incidence of CDI following ileostomy closure in patients who underwent rectal cancer surgery; the secondary aim was to assess the rate of postileostomy closure CDI in patients who presented with leakage at the original colorectal anastomosis site.
Methods
Medical records of patients with rectal cancer who underwent ileostomy closure between January 2015 and December 2019 were retrospectively reviewed. All patients had previously received resection and anastomosis for primary rectal cancer with diverting ileostomy. Data regarding CDI incidence, preoperative status, perioperative management, and clinical outcomes were collected. CDI positivity was determined by direct real-time PCR and enzyme-linked fluorescent assays for detecting toxin A and B.Statistical analyses were computed for CDI risk factors.
Results
A total of 1270 patients were included and 208 patients were tested for CDI owing to colitis-related symptoms. The incidence of CDI was 3.6 per cent (46 patients). Multivariable analysis for CDI risk factors identified adjuvant chemotherapy (hazard ratio (HR) 2.28; P = 0.034) and colorectal anastomosis leakage prior to CDI (HR 3.75; P = 0.008). Finally, patients with CDI showed higher colorectal anastomosis leakage risk in multivariable analysis after ileostomy closure (HR 6.922; P = 0.001).
Conclusion
Patients with CDI presented with a significantly higher rate of colorectal anastomosis leakage prior to ileostomy closure.
Adjuvant chemotherapy and colorectal anastomosis leakage increase risk of Clostridium difficile infection. Patients with Clostridium difficile infection also have a higher risk of anastomosis leakage after rectal surgery.
Introduction
Clostridium difficile infection (CDI) is the most common healthcare-related colitis and results in longer hospitalizations and therefore increased costs1. Originally named Bacillus difficilis by Hall and O’Toole, owing to the difficulty they faced in isolating and culturing the bacteria2,3, C. difficile contains two large exotoxins (TcdA and TcdB) that induce the symptoms related to colitis such as diarrhoea, fever, and abdominal pain4.
From the 1990s to 2006, the incidence of CDI increased in the USA by threefold (84 cases per 100 000 population in 2005), and deaths caused by CDI in England increased eightfold3,5,6. The frequency of this endemic infection, including sporadic outbreaks, seems to have stabilized in the past decade; a decreased CDI burden was evident in the USA from 2011 to 2017 due to a decline in healthcare-associated infections7. Notably, however, the CDI rate is still consistently reported to be much higher after colorectal surgery versus the healthy population (1 to 2.2 per cent), and is twofold more likely following colorectal procedures than after surgeries not involving the gastrointestinal tract8. Furthermore, patients who undergo ileostomy closure have a reported CDI incidence of up to 4 per cent9–11.
Other than the longer duration hospital stay and higher financial costs12, postoperative morbidities related to CDI have been described in several studies, and a possible but as yet unconfirmed association with postoperative anastomosis leakage has been suggested. Recent studies have described a leak rate of 7 to 45 per cent in CDI-positive patients versus 2 to 4 per cent in negative patients who have received colorectal surgery13–16. This issue has generated more interest among clinicians, but additional clinical data are now needed to validate this association properly.
The primary aim of this study was to determine the incidence of CDI that develops following ileostomy closure in patients who underwent rectal cancer surgery; secondly, the study aimed to assess the rate of post-ileostomy closure CDI in patients who presented leakage at the original colorectal anastomosis site.
Methods
Patient selection
Consecutive patients with rectal cancer who underwent ileostomy closure between January 2015 and December 2019 at Asan Medical Center (a tertiary referral hospital affiliated to Ulsan University of Medicine, which performs more than 3500 colorectal surgeries annually), Seoul, Republic of Korea, were reviewed. These patients had received a diverting ileostomy either prior to or simultaneously with the surgical resection of rectal cancer, or during the postoperative course after primary surgery due to complications such as anastomosis failure. Patients who received palliative surgery were excluded from the cohort, as were any cases who underwent a total colectomy or total proctocolectomy as the presence of remnant colon can be a distinct influence on the microbiome. The hospital medical records were reviewed retrospectively to collect the relevant patient data for the analyses, including age, sex, BMI, alcohol or smoking history, diabetic mellitus (DM), hypertension (HTN), laboratory results (including haemoglobulin, albumin, and creatinine), perioperative neoadjuvant and adjuvant treatment, administered prophylactic antibiotics, and postoperative complications.
The study is reported in accordance with the STROBE guidelines and was approved by the Institutional Review Board of Asan Medical Center (approval number: 2020-0279)17. The requirement for patient informed consent was waived by the review board.
Patient treatments
All patients received curative surgery for rectal cancer. The types of surgery used in this population included low anterior resection (LAR) and ultra-low anterior resection (uLAR). Patients are usually treated using minimal invasive surgery, either laparoscopic or robotic. Patients unfit for minimal invasive surgery for bulky tumours were operated with low midline incisions. The standard method of anastomosis was done by double stapling in which distal rectum is transected by a linear stapler and anastomosed to proximal colon with a circular stapler. When necessary, colo-anal anastomosis was performed manually.
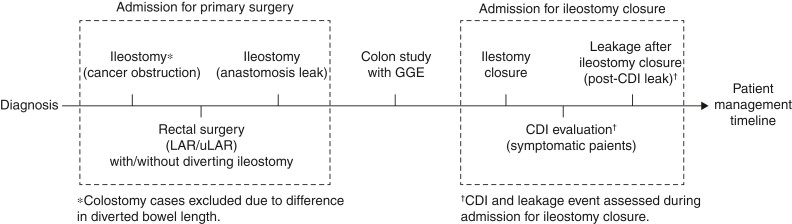
Patients who did not receive adjuvant chemotherapy were scheduled for ileostomy closure at 3 months after the primary surgery. Candidates for adjuvant chemotherapy were scheduled for ileostomy closure after the end of the regimen, which was generally 6 to 7 months after the primary surgery. All patients received contrast enema with gastrografin to assess the primary colorectal anastomosis. If there were signs of leakage in the contrast enema, ileostomy closure was postponed until future colon enema study showed no evidence of leakage. Patients were admitted 1 day prior to undergoing ileostomy closure and were subjected to midnight fasting only. On the day of the closure procedure, cefotetan or cefoxitin prophylactic antibiotics were given 30 minutes before the skin incision. The routine postoperative course following ileostomy closure included sips of water on postoperative day 1, a liquid diet on day 2, and a soft diet on days 3 to 4.Patients were considered for discharge when the soft diet was tolerable, with no sign of complications. For patients diagnosed with anastomosis leakage, a combination of third-generation cephalosporin with metronidazole, piperacillin/tazobactam, or vancomycin with meropenem were selected for empirical antibiotic treatment. A schematic timeline of patient management is depicted in Fig. 1.
Fig. 1.
Schematic timeline of patient management
GGE, gastrograffin enema; CDI, Clostridium difficile infection; LAR, lower anterior resection; uLAR, ultra-low anterior resection.
Assessment and management of Clostridium difficile infection
Patients were tested for a possible CDI when a daily excessive watery diarrhoea was observed in the postoperative admission period after ileostomy closure. In our centre, diarrhoea less than 10 times a day is considered natural after ileostomy closure. Patients with less prominent diarrhoea were also tested for CDI if they experienced fever, abdominal distention, ileus, or any other combination of symptoms that are not typical of low anterior resection syndrome. The two diagnostic modalities used for CDI were direct real-time PCR and enzyme-linked fluorescent assay for detecting toxin A and B in anaerobic cultures.
Oral metronidazole (500 mg three times daily) was the treatment of choice for patients diagnosed with CDI. Although oral vancomycin is the first-choice treatment recommended by the guidelines of Infectious Diseases Society of America18, oral metronidazole is recommended first choice in South Korea for cost-related reasons. However, oral vancomycin is used in refractory CDI patients who show a poor response to metronidazole.
Statistical analysis
Categorical variables were analysed with a χ2 test. Continuous variables were expressed as the mean (s.d.) and analysed using ANOVA with a Tukey post hoc multiple comparison test to determine statistically significant differences between untested patients, patients who tested negative for CDI, and patients who tested positive for CDI. Multivariable analysis with a binary logistic regression model was used to identify independent risk factors for CDI and for leakage at colorectal anastomosis after ileostomy closure. The variables included were factors considered to have clinical relevance to CDI or leakage, and those with a P value < 0.1 in the univariable analysis. An abnormal BMI was defined as less than 18.5 or 25 or more, in accordance with the guidelines of the Korean Society for the Study of Obesity19, and abnormal serum results were defined in accordance with Asan Medical Center criteria as follows: anaemia (serum haemoglobin < 12 g/dL), hypoalbuminemia (serum albumin < 3.5 g/dL), and high creatinine (serum creatinine ≥ 1.4 mg/dL). All statistical analyses were performed using SPSS® version 21.0 (IBM, Armonk, NY, USA), with P values < 0.05 considered to indicate statistical significance.
Results
Clinical characteristics of study patients
Of the 1270 patients included in the study total cohort, 208 (16.4 per cent) were tested for CDI and 46 (3.6 per cent) showed a positive result. Almost all of the treated cancers in the cohort were adenocarcinomas (1254 patients), but a small number of cases with other malignancies such as neuroendocrine tumours (six patients) and gastrointestinal stromal tumours (10 patients) were also included. The mean age of the total patient population was 59.81 (±11.3) and 870 (68.5 per cent) patients were male. A LAR was performed in 326 (25.7 per cent) patients, and a uLAR in the remaining 944 (74.3 per cent) patients. Among the study population, leakage at colorectal anastomosis occurred in 71 (5.6 per cent) patients. In 50 (3.9 per cent) of these cases, this leakage was detected during the postoperative course just after the primary resection and anastomosis, and a diverting ileostomy was performed accordingly. The mean interval from the ileostomy diversion to ileostomy closure was 6.5 (±2.7) months. The mean duration of hospital stay for ileostomy closure was 6.73 (±4.2) days. The prophylactic antibiotics used included cefoxitin in 965 (76.0 per cent), cefotetan in 289 (22.8 per cent), ciprofloxacin in eight (0.6 per cent), and ceftriaxone in eight (0.6 per cent) patients. In 25 (2.0 per cent) cases, colorectal anastomosis leakage occurred after the ileostomy closure.
Risk factors for Clostridium difficile infection
Age, BMI, alcohol or smoking histories, DM, HTN, and serum haemoglobulin, albumin, and creatinine levels were comparable between the untested, the CDI-negative, and CDI-positive groups. Preoperative chemoradiotherapy was given to 770 (62.9 per cent) cases in the CDI-negative group (untested + tested negative cases) versus 23 patients (50 per cent) in the positive group. Adjuvant chemotherapy was given to 36 (78.3 per cent) patients in the CDI-positive group versus 852 cases (69.6 per cent) in the negative group (untested + tested negative cases), but this was not statistically significant (P = 0.417). Untested patients were more frequently administered with prophylactic cefotetan than their tested counterparts, but the use of different prophylactic antibiotics was comparable between the CDI-negative and CDI-positive groups. The interval between the generation and closure of the ileostomy did not differ between any of the groups (P = 0.231) (Table 1).
Table 1.
Clinical characteristics of the study patients
| Not tested | CDI (−) | CDI (+) | P | |
|---|---|---|---|---|
| (n = 1062) | (n = 162) | (n = 46) | ||
| Sex | 0.004 | |||
| Male | 709 (66.8) | 129 (79.6) | 32 (69.6) | |
| Female | 353 (33.2) | 33 (20.4) | 14 (30.4) | |
| Mean (s.d.) age (years) | 60.0 (11.3) | 58.56 (10.8) | 59.85 (12.1) | 0.296 |
| Mean (s.d.) BMI (kg/m2) | 23.93 (3.2) | 23.79 (3.4) | 23.39 (3.4) | 0.491 |
| Alcohol consumption | 560 (52.8) | 95 (58.6) | 21 (45.7) | 0.217 |
| Smoking history | 532 (50.1) | 89 (54.) | 20 (43.5) | 0.327 |
| Diabetes mellitus | 172 (16.2) | 30 (18.5) | 4 (8.7) | 0.280 |
| Hypertension | 374 (35.2) | 57 (35.2) | 14 (30.4) | 0.801 |
| Mean (s.d.) haemoglobin | 12.68 (1.6) | 12.9 (1.5) | 12.59 (1.7) | 0.244 |
| Mean (s.d.) albumin | 3.78 (0.3) | 3.81 (0.4) | 3.69 (0.4) | 0.131 |
| Mean (s.d.) creatinine | 0.89 (0.4) | 0.92 (0.3) | 0.98 (0.8) | 0.185 |
| PCRT | 666 (62.7) | 104 (64.2) | 23 (50.0) | 0.194 |
| Adjuvant chemotherapy | 737 (69.4) | 115 (71.0) | 36 (78.3) | 0.417 |
| Type of surgery | 0.130 | |||
| LAR | 261 (24.6) | 51 (31.5) | 14 (30.4) | |
| uLAR | 801 (75.4) | 111 (68.5) | 32 (69.6) | |
| Prophylactic antibiotics | < 0.001 | |||
| Cefotetan | 276 (26.0) | 11 (6.8) | 2 (4.3) | |
| Cefoxitin | 771 (72.6) | 150 (92.6) | 44 (95.7) | |
| Ceftriaxone | 7 (0.7) | 1 (0.6) | 0 (0) | |
| Ciprofloxacin | 8 (0.8) | 0 (0) | 0 (0) | |
| Mean (s.d.) time to closure (months) | 6.45 (2.6) | 6.81 (2.6) | 6.72 (3.1) | 0.231 |
| Mean (s.d.) duration of hospital stay (days) | 6.03 (2.5) | 10.19 (7.9) | 10.78 (7.4) | < 0.001 |
| Leak* | 45 (4.2) | 16 (9.9) | 10 (21.7) | < 0.001 |
| Pre-CDI leak | 33 (3.1) | 11 (6.8) | 6 (13.0) | < 0.001 |
| Post-CDI leak | 14 (1.3) | 7 (4.3) | 4 (8.7) | < 0.001 |
Values are presented as a n (%) or as a mean (s.d.). *Leakage was assessed for anastomosis between colon and rectum. CDI, Clostridium difficile infection; PCRT, preoperative chemoradiotherapy; LAR, low anterior resection; uLAR, ultra-low anterior resection.
Confounding factors were adjusted in subsequent multivariable analysis for prognostic indicators of CDI. Patients who experienced anastomosis leakage prior to the detection of CDI presented with a significantly higher risk (hazard risk (HR) 3.753; P = 0.008) of developing this infection. Adjuvant chemotherapy was also found to be associated with a higher risk of CDI (HR 2.276; P = 0.034) (Table 2).
Table 2.
Multivariable analyses of prognostic factors for Clostridium difficile infection
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| Age ≥ 65 years | 1.673 | 0.863–3.244 | 0.128 |
| Sex, male | 0.818 | 0.3663–1.829 | 0.625 |
| Abnormal BMI (<18.5 or ≥ 25 kg/m2) | 0.843 | 0.4473–1.591 | 0.599 |
| Alcohol consumption | 0.850 | 0.4033–1.795 | 0.670 |
| Smoking history | 0.680 | 0.3073–1.507 | 0.342 |
| Diabetic mellitus | 0.511 | 0.1743–1.500 | 0.222 |
| Hypertension | 0.778 | 0.3853–1.571 | 0.484 |
| Serum haemoglobin < 12 | 0.558 | 0.2653–1.176 | 0.125 |
| Serum albumin < 3.5 | 1.894 | 0.8923–4.020 | 0.096 |
| Serum creatinine ≥1.4 | 0.996 | 0.2133–4.654 | 0.996 |
| PCRT | 0.552 | 0.2943–1.037 | 0.065 |
| Adjuvant chemotherapy | 2.276 | 1.0643–4.870 | 0.034 |
| Pre-CDI leak * | 3.753 | 1.4103–9.990 | 0.008 |
Leakage was assessed for anastomosis between colon and rectum. PCRT, preoperative chemoradiotherapy; CDI, Clostridium difficile infection.
Clinical outcomes of a Clostridium difficile infection
The mean duration of hospitalization was significantly longer for the patients who tested positive for CDI compared with the other groups in the study cohort (10.78 ± 7.4 versus 6.58 ± 3.9 days; P < 0.001). Notably, however, the duration of hospitalization was similar between the CDI-positive and CDI-negative patients among the tested population (10.78 ± 7.4 versus 10.19 ± 7.9; P = 0.626). CDI positivity was found to be associated with a higher incidence of colorectal anastomosis leakage versus either the untested or tested negative groups (P < 0.001 and P = 0.032, respectively) (Table 1). Patients who experienced anastomosis leakage prior to an ileostomy closure were treated with a wide range of empirical antibiotics. Among the 50 cases in the series in this category, 16 patients were treated with piperacillin/tazobactam, 15 with imipenem, 11 with third-generation cephalosporin with or without metronidazole, and eight with combinations of antibiotics, including vancomycin, tigecycline, meropenem, clindamycin, and moxifloxacin. Five patients (10.9 per cent) did not respond to oral metronidazole and were switched to oral vancomycin, subsequently showing improvement in their symptoms.
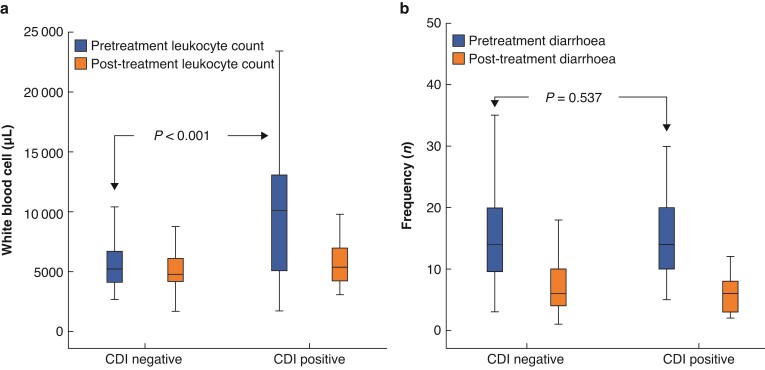
Among the 208 patients tested for CDI after ileostomy closure, an increased white blood cell count at the time of symptom onset was evident in 23 cases (50 per cent) with a mean value of 9921/µl in CDI-positive patients. Patients who tested negative for CDI presented with leukocytosis in 12 cases (7.4 per cent) with a mean value of 5810/µl. CDI-positive patients presented with a fever above 38.0°C in 23 (50 per cent) cases, while fever was noted in only 28 (17.3 per cent) patients who tested negative for CDI. After treatment with oral metronidazole, CDI-positive patients showed improvement in relation to their leukocytosis with a mean value of 5730/µl, while CDI-negative patients showed a value of 5127/µl (Fig. 2a). The mean daily frequency of diarrhoea prior to receiving oral metronidazole did not differ between the CDI-positive and CDI-negative groups (15.1 ± 8.7 and 14.3 ± 7.2 times, respectively). Both groups showed a decreased incidence of diarrhoea after taking oral metronidazole (6.3 ± 3.4 and 7.6 ± 5.4, respectively; Fig. 2b). Prominent symptoms of ileus (abdominal distention, nausea/vomiting) were identified in 21 CDI-positive patients (45.7 per cent) and were accompanied by a distinctive pattern of bowel dilatation, displaying a continued loss of haustra from the descending colon to the anastomosis site. CDI-negative patients presented with signs of ileus in 50 cases (30.9 per cent; P = 0.117).
Fig. 2.
a Box plot showing significantly increased leukocyte counts in Clostridium difficile infection (CDI)-positive patients prior to undergoing treatment, which were improved by oral metronidazole. CDI-negative patients did not present with leukocyte count increase. b The daily number of diarrhoea events was comparable between the CDI-negative and CDI-positive patients both pre- and post-treatment with oral metronidazole.
Potential risk factors for anastomosis leakage after ileostomy closure were analysed in the multivariable analyses (Table 3). Patients who tested positive for CDI show significantly higher risk of anastomosis leakage after ileostomy closure (HR 6.922; P = 0.001).
Table 3.
Multivariable analyses of risk factors for leakage after ileostomy closure*
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| Age ≥65 (years) | 0.271 | 0.091–0.809 | 0.019 |
| Sex, male | 0.382 | 0.122–1.195 | 0.098 |
| Abnormal BMI (< 18.5 or ≥ 25 kg/m2) | 0.551 | 0.220–1.377 | 0.202 |
| Diabetic mellitus | 2.103 | 0.799–5.539 | 0.132 |
| Hypertension | 1.951 | 0.804–4.734 | 0.139 |
| Serum haemoglobin < 12 | 1.132 | 0.415–3.086 | 0.809 |
| Serum albumin < 3.5 | 1.921 | 0.654–5.645 | 0.235 |
| Serum creatinine ≥ 1.4 | 0.390 | 0.045–3.384 | 0.393 |
| PCRT | 1.379 | 0.529–3.596 | 0.511 |
| Adjuvant chemotherapy | 0.608 | 0.233–1.583 | 0.308 |
| uLAR (versus LAR) | 1.547 | 0.538–4.454 | 0.418 |
| CDI-positive | 6.922 | 2.115–22.654 | 0.001 |
PCRT, preoperative chemoradiotherapy; uLAR, ultra-low anterior resection; LAR, low anterior resection; CDI, Clostridium difficile infection. *Leakage was assessed for anastomosis between colon and rectum.
Discussion
The present study findings indicate that anastomosis leakage prior to ileostomy closure is associated with a higher CDI rate after ileostomy closure, and that patients who develop CDI are more vulnerable to leakage at primary anastomosis after their ileostomy closure. While previous studies related to risk factors for CDI have reported comorbidities as significant variables, including DM, heart conditions, chronic renal disease, and old age, our current results revealed that only adjuvant chemotherapy and anastomosis leakage are independent risk factors for CDI.
Among the 46 cases in the current study cohort diagnosed with CDI, six (13 per cent) and four patients (8.7 per cent) experienced anastomosis leakage before and after ileostomy closure, respectively. All patients in the current series who received a diverting ileostomy due to anastomosis failure received extensive empirical antibiotic treatment. This could explain the high incidence of CDI in these cases as a previous exposure to antibiotics is the most well-known risk factor for this infeciton20,21. A previous study reported a 4.2 per cent rate of CDI after ileostomy closure versus 2.1 per cent for a right hemicolectomy and 1 per cent for an anterior resection. One of the reasons for the higher rate of CDI after ileostomy closure was anticipated to be the previous surgical procedure that the patients received before ileostomy closure that required antibiotic use9. Prior studies have also indicated that changes can occur in the bowel microbiome after stool diversion, which need to be considered22.
With regard to patients who experienced anastomosis leakage after ileostomy closure, it is more difficult to determine in these cases whether CDI was the cause of the leakage. Colorectal anastomosis leak in patients with diverting ileostomy could be inapparent until ileostomy closure is performed when only then faecal material may cause clinical symptoms. Therefore, one should be cautious in suggesting CDI as the cause of leakage. However, there are increasing reports from various studies supporting this issue. One study reported a 6.69 per cent anastomosis leakage rate in patients with postoperative CDI versus 3.06 per cent in CDI-negative cases from a total study population of 56 631 patients who had undergone a colectomy14. Another study described 19 cases of anastomosis leakage out of 320 patients, of which 13 cases were CDI-positive (P < 0.001)15. A Japanese group also reported seven patients (3.8 per cent) with anastomosis leakage from a cohort of 185 colorectal surgery cases and indicated that CDI was significantly associated with leakage (P = 0.001). Finally, few case reports of anastomosis rupture related to CDI have been published13,16. Although the number of cases of anastomosis leakage after ileostomy closure is very small, the current findings indicate a higher rate of leakage in CDI-positive patients versus negative cases (8.7 per cent versus 1.4 per cent, respectively). Symptomatic C. difficile colonization is known to induce inflammation, secretions, bowel dilatation, and oedemas. The activity of C. difficile toxins has also been shown to stimulate an inflammatory host response that induces the degradation of collagen and other components of the extracellular matrix23,24. For these reasons, concerns regarding integrity of the anastomosis are well-founded in patients diagnosed with CDI, and patients at risk of these infections must be closely monitored.
There have been extensive studies to date on the risk factors for CDI such as old age, DM, smoking history, use of steroids, impaired kidney function, and a poor nutrition status25–27. Moreover, in an era where chemotherapy has become a mainstay treatment for patients with cancer, adjuvant chemotherapy is reported to increase the risk of CDI in oncology patients28,29. These results are consistent with the present study’s findings, which indicate adjuvant chemotherapy as one of two independent risk factors for the development of CDI. Although no clear risk factors for CDI other than adjuvant chemotherapy can yet be proposed for selecting candidates for a more thorough observation, conventional risk factors suggested by previous studies should also be considered when patients experience excessive diarrhoea, leukocytosis, fever, or any other symptoms related to colitis.
This study had some limitations, principally stemming from its retrospective nature. Although the medical records of the study subjects were reviewed as completely as possible, data for some patients were missing. Also, the heterogenous nature of management for involved patients in testing for CDI, either the indications used for performing tests or the testing modalities used, is likely to have influenced the incidence rate of CDI in the present cohort. Nevertheless, the current data indicate a clear trend regarding the onset of CDI in a single tertiary centre among a homogenous group of patients with rectal cancer who had all undergone an ileostomy closure.
Disclosure. The authors declare no conflict of interest.
Data availability
Raw data were generated at Asan Medical Center. Derived data supporting the findings of this study are available on request from the corresponding author.
Contributor Information
Young Il Kim, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Chang Sik Yu, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Yang Soo Kim, Department of Infectious Diseases, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Chan Wook Kim, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Jong Lyul Lee, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Yong Sik Yoon, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
In Ja Park, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Seok-Byung Lim, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
Jin Cheon Kim, Division of Colon and Rectal Surgery, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic of Korea.
References
- 1. O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;28:1219–1227 [DOI] [PubMed] [Google Scholar]
- 2. Hall IC, O’Toole E. Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 1935;49:390–402 [Google Scholar]
- 3. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008;359:1932–1940 [DOI] [PubMed] [Google Scholar]
- 4. Sun X, Savidge T, Feng H. The enterotoxicity of Clostridium difficile toxins. Toxins 2010;2:1848–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals 1996–2003. Emerg Infect Dis 2006;12:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Office for National Statistics . Deaths Involving Clostridium difficile: England and Wales: 2011. Newport: Office for National Statistics, 2012 [Google Scholar]
- 7. Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM et al. Trends in US burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020;382:1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect 2007;8:557–566 [DOI] [PubMed] [Google Scholar]
- 9. Randall JK, Young BC, Patel G, Fitzgerald A, George BD. Is Clostridium difficile infection a particular problem after reversal of ileostomy? Colorectal Dis 2011;13:308–311 [DOI] [PubMed] [Google Scholar]
- 10. Skancke M, Vaziri K, Umapathi B, Amdur R, Radomski M, Obias V. Elective stoma reversal has a higher incidence of postoperative Clostridium difficile infection compared with elective colectomy: an analysis using the American College of Surgeons National Surgical Quality Improvement program and targeted colectomy databases. Dis Colon Rectum 2018;61:593–598 [DOI] [PubMed] [Google Scholar]
- 11. Harries RL, Ansell J, Codd RJ, Williams GL. A systematic review of Clostridium difficile infection following reversal of ileostomy. Colorectal Dis 2017;19:881–887 [DOI] [PubMed] [Google Scholar]
- 12. Wilson MZ, Hollenbeak CS, Stewart DB. Impact of Clostridium difficile colitis following closure of a diverting loop ileostomy: results of a matched cohort study. Colorectal Dis 2013;15:974–981 [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto T, Hyakudomi R, Tajima Y. [Clostridium difficile infection: a possible cause of anastomotic leakage after colorectal surgery]. Nippon Daicho Komonbyo Gakkai Zasshi 2018;71:63–69 [Google Scholar]
- 14. Baker S, Velasco-Gonzalez C, Green H, Margolin DA. Anastomotic leak rate is doubled in patients diagnosed with Clostridium difficile infection after colectomy: a retrospective review using the NSQIP database. J Am Coll Surg 2019:229(Suppl 1):S51 [Google Scholar]
- 15. Calu V, Toma EA, Enciu O, Miron A. Clostridium difficile infection and colorectal surgery: is there any risk? Medicina 2019;55:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velez DR, Ahmeti M. Clostridioides difficile enteritis induced anastomotic rupture: a case report and literature review. Case Rep Surg 2020;2020:9794823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499 [DOI] [PubMed] [Google Scholar]
- 18. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:987–994 [DOI] [PubMed] [Google Scholar]
- 19. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK et al. Korean society for the study of obesity guideline for the management of obesity in Korea. J Obes Metab Syndr 2019;28:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC. Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg 1995;169:480–483 [DOI] [PubMed] [Google Scholar]
- 21. Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis 1994;18:S265–S272 [DOI] [PubMed] [Google Scholar]
- 22. Kissmeyer-Nielsen P, Christensen H, Laurberg S. Diverting colostomy induces mucosal and muscular atrophy in rat distal colon. Gut 1994;35:1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamoto S, Horton KM, Fishman EK. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics 1999;19:887–897 [DOI] [PubMed] [Google Scholar]
- 24. Fletcher JR, Pike CM, Parsons RJ, Rivera AJ, Foley MH, McLaren MR et al. Clostridioides difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota. Nat Commun 2021;12:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections—an overview of the evidence base and challenges in data synthesis. J Glob Health 2017;7:010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998;40:1–15 [DOI] [PubMed] [Google Scholar]
- 27. Davies K, Lawrence J, Berry C, Davis G, Yu H, Cai B et al. Risk factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficile infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Health 2020;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neemann K, Freifeld A. Clostridium difficile-associated diarrhea in the oncology patient. J Oncol Pract 2017;13:25–30 [DOI] [PubMed] [Google Scholar]
- 29. Raza S, Baig MA, Russell H, Gourdet Y, Berger BJ. Clostridium difficile infection following chemotherapy. Recent Pat Antiinfect Drug Discov 2010;5:1–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Asan Medical Center. Derived data supporting the findings of this study are available on request from the corresponding author.