Abstract
Objectives:
Despite improved care for rheumatoid arthritis (RA) patients, many still experience treatment failure with biologic disease-modifying antirheumatic drugs (bDMARDs) or targeted synthetic DMARDs [tsDMARDs; typically Janus kinase inhibitors (JAKi)], and eventually switch to other agents. We compared the efficacy of a second tumor necrosis factor inhibitor (TNFi) and non-TNF-targeted treatment as the second-line treatment in patients showing an insufficient response to the first TNFi.
Methods:
Patients were included if they had received at least one prescription for a TNFi, and at least one follow-up prescription for a second TNFi or non-TNF-targeted treatment after discontinuation of the first drug. In total, 209 patients were analyzed, including 69 with a second TNFi and 140 with a non-TNF-targeted treatment (106 non-TNFi biologics and 34 JAKi). Cox regression was used to estimate the hazard ratio (HR) for discontinuation.
Results:
The mean follow-up period after switching was 28.0 (range: 0–80) months and 24.4% of the 209 patients switched or discontinued the second drug. In multivariate Cox proportional hazard analysis, the non-TNF-targeted treatment group had a lower likelihood of discontinuing their treatment than the second TNFi group [HR = 0.326, 95% confidence interval (CI): 0.170–0.626, p = 0.001]. When analyzed separately, the risk of discontinuation was significantly lower in both the non-TNFi biologic (HR = 0.318, 95% CI: 0.160–0.633, p = 0.001) and JAKi (HR = 0.356, 95% CI: 0.129–0.980, p = 0.046) groups than in the second TNFi group.
Conclusion:
Our study supported switching to a non-TNF-targeted treatment instead of TNF cycling in patients with RA showing an inadequate response to initial TNFi.
Keywords: JAK inhibitor, rheumatoid arthritis, switching, treatment continuation, TNF inhibitor
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune inflammatory diseases, and is characterized by pathological synovial hypertrophy, joint inflammation, and structural damage.1,2 RA has an incidence of up to 2% worldwide and often causes substantial functional impairment and decreased health-related quality of life relative to the general population.3,4 Although conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), including methotrexate (MTX), remain the first-line therapy for RA, considerable advances have been made in the treatment of RA over the last few decades. The development of targeted treatments, such as biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs), represented by Janus kinase inhibitors (JAKis), showed a therapeutic revolution in the treatment of RA.5,6
Tumor necrosis factor inhibitors (TNFis) remain the first-line targeted treatment following csDMARD therapy failure,7–9 as these agents are effective in reducing the signs and symptoms of RA and inhibiting the progression of structural joint damage.10,11 Despite the marked treatment effect of TNFi, 40–50% of RA patients discontinue their first TNFi within 3 years of initiation.12,13 For patients discontinuing their first TNFi due to a lack of efficacy or intolerance, both cycling to another TNFi (cycling strategy) or switching to other targeted agent with a different mechanism of action (MOA) (swap strategy) may be considered as alternative strategies.7,8 An observational cohort study reported similar outcomes between RA patients who cycled to another TNFi and those who initiated non-TNFi biologic agent with a different MOA (abatacept). 14 A retrospective study reported that switching from first TNFi (infliximab) to either another MOA bDMARD (tocilizumab) or second TNFi (etanercept) in patients with RA showed no significant difference in efficacy, as measured by disease activity. 15 However, randomized controlled trials suggested that switching to non-TNFi biologic agents, such as rituximab, tocilizumab, and abatacept, could be an alternative option for RA patients with an inadequate response to one or more TNFis.16–18 Moreover, an observational study reported that, after failure of a first TNFi for RA, switching to a new MOA bDMARD (rituximab) was more effective than cycling to another TNFi. 19 Furthermore, another randomized trial showed that a swap strategy using non-TNFi biologic agents may be more effective than use of a second TNFi in patients with an insufficient response to the first TNFi. 20 With this background, the 2021 American College of Rheumatology (ACR) guideline conditionally recommended a swap strategy over cycling to another TNFi in patients who showed an insufficient response to a previous TNFi. 21 However, as this recommendation is based on very low-certainty evidence, the therapeutic choice after failure of the first TNFi is largely dependent on the experience of the treating physician or the patient’s preference. 21
Therefore, further studies are needed to better understand the outcomes of either cycling to another TNFi or switching to non-TNF-targeted treatment, such as a different MOA bDMARD or JAKi. Using data from the KOrean nationwide BIOlogics and targeted therapy (KOBIO) registry, a nationwide real-world prospective cohort to assess outcomes of RA patients treated with any targeted treatment, 8 this study was performed to compare the effectiveness of the cycling and swap strategies in terms of the drug discontinuation rate, and to clarify the predictors of discontinuation of second-line treatment in RA patients who discontinued their prior TNFi.
Patients and methods
Study design and population
This study was performed using data from the KOBIO registry, a nationwide multicenter, hospital-based observational registry maintained by the Korean College of Rheumatology (KCR). The aim of the registry was to prospectively assess the clinical manifestations and outcomes, including adverse events, of RA patients who had received any targeted treatment, such as bDMARDs or tsDMARDs. 8 All patients eligible for the study were classified as having RA by their treating rheumatologist, and fulfilled the 2010 ACR criteria for RA. 2 The RA patients were enrolled from 47 tertiary academic and community rheumatologic centers across the country and had follow-up assessments at approximately 12-month intervals. 8 For this study, subjects were identified from the baseline and follow-up data of the KOBIO registry.
Although the decision to switch targeted treatment with a certain drug or certain MOA was made solely by the treating rheumatologist, these decisions were based on the Korean National Health Insurance (KNHI) reimbursement criteria. The criteria for initiation and maintenance of targeted agents, including bDMARDs and JAKi, are summarized in Supplementary Table 1. If the maintenance criteria are met after 6 months of a targeted agent, that drug can be reimbursed for an additional 6 months. If the maintenance criteria are not satisfied or serious adverse events occur, treatments should be switched to other agents.
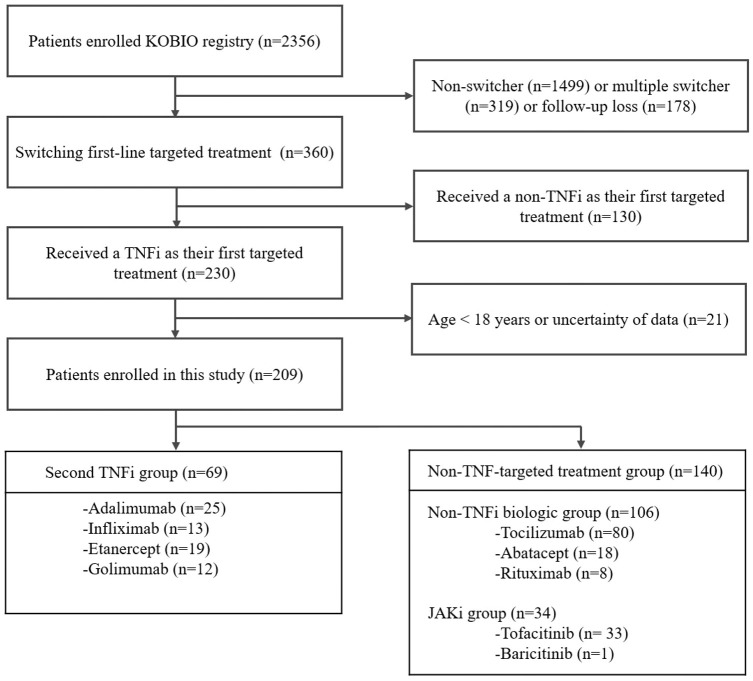
A total of 2356 RA patients receiving a bDMARD or tsDMARD were enrolled in the KOBIO registry from December 2013 to November 2020 (Figure 1). Of these, RA patients who did not switch their first targeted treatment (n = 1499) or multiple switcher (n = 319), or those who were lost to follow-up (n = 178) were excluded. Among patients switching their first targeted therapy, those receiving non-TNFi treatment as the first targeted therapy (n = 130) and those with missing follow-up data (n = 21) were also excluded. Finally, a total of 209 patients were analyzed in this study, consisting of 69 patients in the second TNFi group and 140 in the non-TNF-targeted treatment group (106 in the non-TNFi biologic group and 34 in the JAKi group). Patients were followed up at least once from the time of initiation of the subsequent TNFi or non-TNF-targeted treatment until discontinuation. This study adhered to all relevant principles of the Declaration of Helsinki. The study protocol and data collection forms were approved by the institutional review board or local ethics committee of all participating institutions, including that of Chonnam National University Hospital (Approval No. CNUH-2012-239). All participants provided written informed consent for enrollment in the KOBIO registry. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies were followed. 22
Figure 1.
Flow diagram for study enrollment.
Data collection
All patient data were transferred, by individual investigators, into the KOBIO web server (http://www.rheum.or.kr/kobio/). RA patients were interviewed using a structured questionnaire that captured sociodemographic data and concomitant medications. The following data were collected: age, sex, disease duration, education level, smoking status, blood pressure, body mass index (BMI), presence of hypertension and diabetes mellitus, and laboratory findings, such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), and anti-cyclic citrullinated peptide (CCP) antibody. Radiographs of the hands and feet were also obtained to evaluate erosion and joint space narrowing of these joints at the time of enrollment.
Disease activity was evaluated using validated composite measures, and physical examinations were based on the clinical finding of one or more tender and swollen joints (44 joints). The joint assessments were performed by trained investigators at each institution. The results of 10-cm visual analog scales (VAS) for patient global assessment (PGA) and physician global assessment (PhGA) were recorded. Quantitative assessments of RA disease activity, such as Disease Activity Score of 28 joints (DAS28), 23 were evaluated at the time of the initiation of the second TNFi or non-TNF-targeted treatment. Following 1 year of treatment, achievement of remission or low disease activity (LDA) was also evaluated.
Concomitant csDMARD use was also recorded after initiation of the second TNFi or non-TNF-targeted treatment. Use of csDMARDs was determined based on any use of oral or subcutaneous MTX, sulfasalazine, hydroxychloroquine (HCQ), leflunomide, tacrolimus, or cyclosporine. The first TNFi was also recorded and reasons for switching were classified into the following mutually exclusive categories: inefficacy, adverse events, or other (such as patient preference, financial issues, and concerns regarding safety or comorbidity).
The primary outcome was the discontinuation of second TNFi or non-TNF-targeted bDMARD/tsDMARD in patients with RA who discontinued their first TNFi. In addition, the change in DAS28-ESR score between baseline and 1 year, and the proportion of patients who achieved DAS28-ESR remission or LDA at l year, were obtained to assess the effectiveness of targeted therapies. Predictors of discontinuation of the second-line targeted treatment in RA patients were also evaluated.
Statistical analysis
Descriptive statistical analyses were performed. Values are shown as means ± standard deviation (SD) or percentages. Data were analyzed using the χ2 test for categorical variables and Mann–Whitney U test or one-way analysis of variance (ANOVA) for continuous variables. The cut-off value for LDA was defined as DAS28 ⩽ 3.2 and that for clinical remission was defined as DAS28 < 2.6.23,24 Kaplan–Meier curves were used to examine the duration of treatment, and the log-rank test was used to compare drug continuation between the TNFi cycling and new MOA non-TNF-targeted swapping groups. Multivariable Cox proportional hazards models were used to evaluate potential predictors of drug discontinuation. Variables significant at p < 0.5 in univariable analysis, along with age, sex, disease duration, and concomitant use of csDMARDs, were included in multivariable analysis to evaluate predictors of discontinuation of second-line targeted treatment. Furthermore, subgroup analyses were performed to determine values for predicting discontinuation of the second TNFi drug and non-TNF-targeted treatment. In these analyses, multivariable Cox regression analysis was performed including variables significant at p < 0.5 in univariable analysis, that is concomitant DMARDs, age, sex, and disease duration. Multivariable analysis used a backward inclusion methodology, and hazard ratio (HRs), 95% confidence interval (CIs), and p-values were used to interpret the results. In all analyses, p < 0.05 was taken to indicate statistical significance; the Bonferroni correction was applied when performing multiple comparisons of the 1-year treatment responses among the three groups. Statistical analyses were performed using SPSS for Windows software (ver. 21.0; SPSS Inc., Chicago, IL, USA).
Multiple imputation
The multiple imputations method was applied to address missing baseline data, using the Estimation Maximization (EM) algorithm; five imputed datasets were thus created. The complete variables included age at commencement, gender, disease duration, BMI, smoking, diabetes mellitus, hypertension, educational status, duration of TNFi treatment, reason for discontinuing first TNFi treatment, tender and swollen joint counts, PGA and PhGA levels, and elevated ESR/CRP and DAS28-ESR/CRP scores. Missing variables (number of missing data items) included erosion or joint space narrowing evident in X-ray (n = 65), RF positivity (n = 3), and anti-CCP positivity (n = 27).
Results
The baseline characteristics of the patients are shown in Table 1. At the time of enrollment, the mean age was 46.3 ± 12.9 years, and most of the patients were women (85.2%; n = 178). The mean time since initial diagnosis of RA (disease duration) was 71.1 ± 77.9 months. Of the total of 209 patients with RA, 69 were second TNFi cyclers (second TNFi group) and 140 comprised the non-TNF-targeted treatment group. The non-TNF-targeted treatment group was further subdivided into two subgroups: non-TNFi biologic switcher (non-TNFi biologic group; 106 patients) and JAKi switcher (JAKi group; 34 patients) groups. There were no significant differences in age or sex distribution among the three groups. In all patients, infliximab (33.5%) was the most commonly prescribed first TNFi, followed by adalimumab (30.6%), etanercept (27.8%), and golimumab (8.1%). In the second TNFi group, infliximab (40.6%) was the most common first TNFi drug, but adalimumab was the most commonly prescribed drug in both the non-TNFi biologic and JAKi groups (34.9% and 41.2%, respectively); these differences were statistically significant (p = 0.004). The most commonly reported reason for stopping prior TNFi was inefficacy (70.3%), followed by adverse events (26.3%), and other miscellaneous causes (mostly patient choice due to infection-related problems, family planning, or financial issues). There were no significant differences in the reason for discontinuation of the first TNFi among the groups. The rate of concomitant csDMARD use after switching was significantly lower in the non-TNFi biologic group (84.9%) than the TNFi and JAKi groups (95.7% and 94.1%, respectively) (p = 0.047).
Table 1.
Demographic and clinical features of RA patients receiving second-line targeted treatments.
| All patients n = 209 |
Second TNFi group n = 69 |
Non-TNFi biologic group n = 106 |
JAKi group n = 34 |
p-value | |
|---|---|---|---|---|---|
| Age at commencement, years | 46.3 ± 12.9 | 45.7 ± 12.1 | 46.9 ± 12.2 | 45.3 ± 16.4 | 0.688 |
| Men | 31 (14.8) | 8 (11.6) | 16 (15.1) | 7 (20.6) | 0.479 |
| Disease duration, months | 71.1 ± 77.9 | 70.7 ± 63.4 | 71.3 ± 85.4 | 71.4 ± 82.2 | 0.447 |
| BMI, kg/m2 | 22.3 ± 3.10 | 22.8 ± 3.25 | 22.3 ± 3.80 | 22.3 ± 3.09 | 0.715 |
| Current smoker | 14 (6.7) | 5 (7.2) | 8 (7.5) | 1 (2.9) | 0.630 |
| Diabetes mellitus | 27 (12.9) | 9 (13.0) | 16 (15.1) | 2 (5.9) | 0.378 |
| Hypertension | 53 (25.4) | 18 (26.1) | 26 (24.5) | 9 (26.5) | 0.961 |
| Education, years a | 12.0 ± 3.79 (n = 202) |
11.7 ± 3.88 | 12.0 ± 3.72 (n = 103) |
13.1 ± 3.79 (n = 30) |
0.281 |
| Duration of first TNFi treatment, months | 12.3 ± 10.7 | 14.2 ± 12.6 | 10.4 ± 9.13 | 14.2 ± 10.55 | 0.072 |
| First TNFi | 0.004 | ||||
| Etanercept | 58 (27.8) | 25 (36.2) | 27 (25.5) | 6 (17.6) | |
| Infliximab | 70 (33.5) | 28 (40.6) | 35 (33.0) | 7 (20.6) | |
| Adalimumab | 64 (30.6) | 13 (18.8) | 37 (34.9) | 14 (41.2) | |
| Golimumab | 17 (8.1) | 3 (4.3) | 7 (6.6) | 7 (20.6) | |
| Reason for discontinuing first TNFi | 0.205 | ||||
| Inefficacy | 147 (70.3) | 44 (63.8) | 78(73.6) | 25 (73.5) | |
| Adverse events | 55 (26.3) | 20 (29.0) | 27 (25.5) | 8 (23.5) | |
| Other | 7 (3.3) | 5 (7.2) | 1 (0.9) | 1 (2.9) | |
| Use of concomitant csDMARDs | 188 (90.0) | 66 (95.7) | 90 (84.9) | 32 (94.1) | 0.047 |
| Use of concomitant corticosteroids | 182 (87.1) | 60 (87.0) | 94 (88.7) | 28 (82.4) | 0.632 |
| Erosion or joint space narrowing on X-ray a | 85/144 (59.0) | 28/43 (65.1) | 44/75 (58.7) | 13/26 (50.0) | 0.463 |
| RF positivity a | 177/206 (85.9) | 54/67 (80.6) | 93/106 (87.7) | 30/33 (90.9) | 0.281 |
| Anti-CCP positivity a | 162/182 (89.0) | 46/59 (78.0) | 91/96 (94.8) | 25/27 (92.6) | 0.004 |
| Swollen joint count (44 joints) | 5.23 ± 6.84 | 5.72 ± 8.77 | 5.08 ± 5.80 | 4.71 ± 5.33 | 0.573 |
| Tender joint count (44 joints) | 6.45 ± 7.67 | 6.38 ± 9.16 | 6.37 ± 6.82 | 6.85 ± 7.10 | 0.266 |
| PGA | 5.79 ± 2.58 | 5.00 ± 2.58 | 6.00 ± 2.63 | 6.74 ± 2.59 | 0.004 |
| PhGA | 5.53 ± 2.55 | 4.88 ± 2.39 | 5.65 ± 2.71 | 6.44 ± 2.02 | 0.008 |
| Elevated ESR | 157 (75.1) | 51 (73.9) | 78 (73.6) | 28 (82.4) | 0.566 |
| Elevated CRP | 135 (64.6) | 40 (58.0) | 71 (67.0) | 24 (70.6) | 0.346 |
| DAS28-ESR a | 4.76 ± 1.72 | 4.45 ± 1.86 | 4.84 ± 1.66 | 5.12 ± 1.51 | 0.181 |
| DAS28-CRP a | 4.13 ± 1.57 | 3.78 ± 1.68 | 4.27 ± 1.51 | 4.39 ± 1.45 | 0.062 |
Anti-CCP, anti-cyclic citrullinated peptide; BMI, body mass index; CRP, C-reactive protein; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, Disease Activity Score-28; ESR, erythrocyte sedimentation rate; JAKi, Janus kinase inhibitor; PGA, patient global assessment; PhGA, physician global assessment; RF, rheumatoid factor; TNFi, tumor necrosis factor inhibitor.
Except where otherwise indicated, data are shown as n (%) or mean ± standard deviation.aMissing data were excluded from the analyses.
With regard to clinical features, the TNFi group had significantly lower anti-CCP positivity than the non-TNFi biologic and JAKi groups (78.0%, 94.8%, and 92.6%, respectively) (p = 0.004). With regard to disease activity at the time of switching, both PGA and PhGA were higher in the JAKi group (6.74 ± 2.59 and 6.44 ± 2.02, respectively) than the TNFi group (5.00 ± 2.58 and 4.88 ± 2.39, respectively) and non-TNFi biologic group (6.00 ± 2.63 and 5.65 ± 2.71, respectively) (p = 0.004 and p = 0.008, respectively). Although both DAS28-ESR and DAS28-CRP tended to be higher in the JAKi group than in both the second TNFi group and non-TNFi biologic group, the differences were not statistically significant (all ps > 0.05). Furthermore, there were no significant group differences in the 44 tender-swollen joint count, 44 swollen joint count, levels of ESR and CRP, or presence of erosion or joint space narrowing on radiographic findings.
Table 2 shows a comparison of the response to treatment at 1 year among the groups. At the 1-year follow-up, the rate of continuation of their targeted treatment was significantly higher in the non-TNFi biologic group (80.2%) than the second TNFi group (62.3%) and JAKi group (76.5%) (p = 0.030). Furthermore, the proportion of patients who achieved remission or LDA was higher in the non-TNFi biologic group (72.9%) than the second TNFi group (46.5%) and JAKi group (44.0%) (p = 0.003). In addition, DAS28-ESR at 1 year was lower in the non-TNFi biologic group (2.79 ± 1.14) than the second TNFi group (3.37 ± 1.18) and JAKi group (3.24 ± 0.88) (p = 0.015). The change in DAS28-ESR score between baseline and 1 year was significantly greater in the non-TNFi biologic group (2.01 ± 2.00) and JAKi group (2.08 ± 1.47) than the second TNFi group (0.95 ± 1.63) (p = 0.006). However, the delta DAS28-ESR was not different between the non-TNFi biologic and JAKi groups (p > 0.05).
Table 2.
Comparison of treatment response at 1-year among groups.
| All patients n = 209 |
Second TNFi group n = 69 |
Non-TNFi biologic group n = 106 |
JAKi group n = 34 |
p a, e | p b, e | p c, e | p d | |
|---|---|---|---|---|---|---|---|---|
| Continuation of treatment at 1 year | 154 (73.7%) | 43 (62.3%) | 85 (80.2%) | 26 (76.5%) | 0.027 | 0.453 | 0.642 | 0.03 |
| Remission or LDA at 1 year f | 93/154 (60.8%) | 20/43 (46.5%) | 62/85 (72.9%) | 11/25 (44.0%) f | 0.009 | 0.999 | 0.007 | 0.003 |
| DAS28-ESR at 1 year f | 3.02 ± 1.14 (n = 154) | 3.37 ± 1.18 (n = 43) | 2.79 ± 1.14 (n = 85) | 3.24 ± 0.88 (n = 25) g | 0.027 | 0.999 | 0.075 | 0.015 |
| Delta DAS28-ESRf,h | 1.72 ± 1.88 (n = 154) | 0.95 ± 1.63 (n = 43) | 2.01 ± 2.00 (n = 85) | 2.08 ± 1.47 (n = 25) g | 0.009 | 0.018 | 0.874 | 0.006 |
DAS28, Disease Activity Score-28; ESR, erythrocyte sedimentation rate; JAKi, Janus kinase inhibitor; LDA, low disease activity; TNFi, tumor necrosis factor inhibitor.
Values were determined using the χ2 test or Mann–Whitney U test comparing the second TNFi group and non-TNFi biologic group.
Values were determined using the χ2 test or Mann–Whitney U test comparing the second TNFi group and JAKi group.
Values were determined using the χ2 test or Mann–Whitney U test comparing the non-TNFi biologic group and JAKi group.
Values were determined using the χ2 test or ANOVA comparing three groups.
Bonferroni-corrected p-values to account for multiple testing.
Patients who discontinued their second-line targeted treatment within 1 year were excluded.
Missing data were excluded (n = 1).
Delta DAS29-ESR was defined as the change in DAS28-ESR score between baseline and the 1-year follow-up.
The mean duration of follow-up after drug switching was 28.0 (range: 0–80) months. Overall, 156 (74.6%) patients continued their second targeted treatment, while 53 (24.4%) switched to another drug or discontinued treatment. In detail, 46 (66.7%) patients in the TNFi group, 83 (78.3%) in the non-TNFi biologic group, and 27 (77.4%) in the JAKi group continued their drug. Kaplan–Meier analysis showed that the duration of treatment after drug switching was significantly longer after non-TNF-targeted treatment swapping compared to second TNFi cycling (p = 0.015) [Figure 2(a)]. In fact, the second TNFi group showed the lowest rate of drug continuation among the three groups (p = 0.049), while there were no statistically significant differences in the duration of treatment between the non-TNFi biologic and JAKi groups (p > 0.05) [Figure 2(b)]. Although the data are not shown, there were no significant differences in rates of continuation between the different MOA biologic agents in the non-TNFi biologic group (p > 0.05).
Figure 2.
Kaplan–Meier curve of the duration of treatment. (a) Non-TNF-targeted treatment versus second TNFi. (b) Non-TNFi biologic agent versus JAKi versus second TNFi.
The results of univariable and multivariable Cox proportional hazard analyses for discontinuation of targeted treatments used after TNFi failure are presented in Table 3. In multivariable Cox proportional hazard analysis adjusted for baseline demographic and clinical characteristics, patients with non-TNF-targeted treatments, including the non-TNFi biologic and JAKi groups, had a lower risk of discontinuation of treatment than the second TNFi group (HR = 0.326, 95% CI: 0.170–0.626, p = 0.001). Even when analyzed separately, the risk of discontinuation was significantly lower in both the non-TNFi biologic group (HR = 0.318, 95% CI: 0.160–0.633, p = 0.001) and JAKi group (HR = 0.356, 95% CI: 0.129–0.980, p = 0.046) than in the second TNFi group. Furthermore, after the inclusion of first TNFi and PGA, both of which had a p-value < 0.05 (Table 1), the results of the multivariable Cox regression analysis were unchanged.
Table 3.
Univariable and multivariable Cox proportional hazard models of baseline variables predictive of discontinuation of second-line targeted treatment.
| Univariable analysis | p | Multivariable analysis | p | |
|---|---|---|---|---|
| Age at commencement, years | 0.998 (0.977–1.319) | 0.831 | ||
| Men | 1.439 (0.723–2.865) | 0.300 | ||
| Disease duration, months | 1.002 (0.999–1.005) | 0.202 | ||
| BMI, kg/m2 | 0.981 (0.904–1.064) | 0.638 | ||
| Current smoker | 1.095 (0.395–3.035) | 0.861 | ||
| Diabetes mellitus | 0.613 (0.244–1.541) | 0.299 | ||
| Hypertension | 0.957 (0.511–1.789) | 0.890 | ||
| Education | 1.021 (0.949–1.098) | 0.585 | ||
| Duration of TNFi treatment, months | 1.018 (0.993–1.043) | 0.154 | ||
| First TNFi | ||||
| Etanercept | Reference group | |||
| Infliximab | 0.821 (0.433–1.554) | 0.544 | ||
| Adalimumab | 0.517 (0.249–1.077) | 0.078 | ||
| Golimumab | 0.528 (0.155–1.794) | 0.306 | ||
| Reason for discontinuing first TNFi | ||||
| Inefficacy | Reference group | |||
| Adverse events | 1.240 (0.674–2.279) | 0.489 | ||
| Other | 3.559 (1.251–10.122) | 0.017 | ||
| Use of concomitant csDMARDs | 0.563 (0.275–1.154) | 0.117 | 0.382 (0.170–0.858) | 0.020 |
| Use of concomitant corticosteroids | 0.899 (0.405–1.995) | 0.793 | ||
| Erosion or joint space narrowing on X-ray a | 1.560 (0.764–3.187) | 0.222 | ||
| RF positivity | 0.563 (0.289–1.096) | 0.091 | ||
| Anti-CCP positivity | 0.403 (0.187–0.871) | 0.021 | ||
| Swollen joint counts (44 joints) | 1.034 (1.002–1.068) | 0.036 | ||
| Tender joint counts (44 joints) | 1.024 (0.994–1.055) | 0.124 | ||
| PGA | 1.101 (0.988–1.228) | 0.082 | ||
| PhGA | 1.123 (1.005–1.255) | 0.040 | 1.199 (1.055–1.362) | 0.005 |
| Elevated ESR a | 0.869 (0.472–1.602) | 0.653 | ||
| Elevated CRP a | 1.255(0.681–2.204) | 0.498 | ||
| DAS28-ESR a | 1.153 (0.975–1.364) | 0.097 | ||
| DAS28-CRP a | 1.208 (1.016–1.437) | 0.033 | ||
| Second-line targeted treatment | ||||
| Second TNFi | Reference group | Reference group | ||
| Non-TNF-targeted treatment | 0.516 (0.299–0.889) | 0.017 | 0.326 (0.170–0.626) | 0.001 |
| Second-line targeted treatment b | ||||
| Second TNFi | Reference group | Reference group | ||
| Non-TNFi biologic agent | 0.500 (0.280–0.893) | 0.019 | 0.318 (0.160–0.633) | 0.001 |
| JAKi | 0.575 (0.246–1.343) | 0.201 | 0.356 (0.129–0.980) | 0.046 |
Anti-CCP, anti-cyclic citrullinated peptide; BMI, body mass index; CRP, C-reactive protein; sDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, Disease Activity Score-28; ESR, erythrocyte sedimentation rate; JAKi, Janus kinase inhibitor;GA, patient global assessment; PhGA, physician global assessment;; RF, rheumatoid factor; TNFi, tumor necrosis factor inhibitor.
Multivariable Cox regression analysis was performed based on a backward inclusion methodology [age, sex, disease duration, reason for discontinuing first TNFi, use of concomitant csDMARDs, anti-CCP positivity, swollen joint count (44 joints), PhGA, DAS28-CRP, and second-line targeted treatment were included].
Patients with missing data were excluded from the analyses.
Instead of a binary variable (TNFi versus non-TNF-targeted treatment), a more detailed variable (TNFi versus non-TNFi biologic agent versus JAKi) was used in the multivariable model.
BMI,Further subgroup analyses were performed to determine the predictor of discontinuation of the second TNFi drug and non-TNF-targeted treatment (Table 4). We found that anti-CCP positivity (HR = 0.273, 95% CI: 0.092–0.809, p = 0.019) was an independent predictor of discontinuation of the second TNFi drug, while use of concomitant csDMARDs (HR = 0.131, 95% CI: 0.027–0.634, p = 0.011) was a significant predictor of discontinuation of non-TNF-targeted treatment.
Table 4.
Multivariable Cox proportional hazard models of baseline variables predictive of discontinuation of the second-line TNFi or non-TNF-targeted treatment.
| Multivariate analysis | p | |
|---|---|---|
| In RA patients cycled to a second TNFi | ||
| DAS28-CRP | 1.284 (0.985–1.675) | 0.065 |
| Anti-CCP positivity (%) | 0.273 (0.092–0.809) | 0.019 |
| In RA patients switched to non-TNF-targeted treatment | ||
| DAS28-CRP | 1.262 (0.962–1.655) | 0.092 |
| Use of concomitant csDMARDs | 0.131 (0.027–0.634) | 0.011 |
Anti-CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, Disease Activity Score-28; RA, rheumatoid arthritis; TNFi, tumor necrosis factor inhibitor.
Multivariable Cox regression analysis was performed based on a backward inclusion methodology [variables significant at p < 0.05 in univariable analysis (concomitant DMARDs, age, sex, and disease duration) were included].
TNFi,Discussion
In this real-world analysis of patients with RA, we found that switching to non-TNF-targeted treatment (both a non-TNFi biologic agent and JAKi) was associated with a lower risk of discontinuation of treatment and a better 1-year treatment response than cycling to a second TNFi. In addition, anti-CCP positivity was significantly associated with a lower risk of discontinuation of the second TNFi, while use of concomitant csDMARDs was associated with a lower risk of discontinuation of non-TNF-targeted treatment.
Our study was generally consistent with previous reports showing that switching to non-TNF-targeted drugs was more beneficial than cycling to a second TNFi. To date, observational and registry studies have suggested that RA patients swapping to non-TNFi treatments, such as rituximab, abatacept, tocilizumab, or tofacitinib, following an inadequate response to a first TNFi, showed a favorable outcome compared to those cycling to subsequent TNFis.14,25,26 In an Italian study, Favalli et al. 26 showed that RA patients swapping to different MOA bDMARDs (abatacept, rituximab, or tocilizumab) had a higher retention rate (HR = 2.258, 95% CI: 1.507–3.385) than those cycling to a second TNFi. Moreover, real-world data from the United States showed that during a median follow-up of 29.9 months, TNFi cyclers were more likely to be non-persistent (HR = 1.511, 95% CI: 1.196–1.908, p = 0.001) than new non-TNFi switchers, and switching to a non-TNF-targeted treatment (abatacept, anakinra, rituximab, tocilizumab or tofacitinib) showed a trend toward greater disease activity reduction. 27 A recent US study of large-scale claim-based data also found that, although the treatment cost tended to be lower for TNFi cycling, patients who swapped to a non-TNF-targeted treatment (abatacept, anakinra, rituximab, tocilizumab, or tofacitinib) had longer latencies to discontinuation than those who cycled to a second TNFi. 28 Moreover, in another US claim-based analysis, Bonafede et al. 29 showed that switching to non-TNF-targeted treatments (abatacept, tocilizumab, or tofacitinib) was associated with better outcomes, that is continuation at 12 months, than cycling to TNFi. In addition, a recent pragmatic randomized trial showed that non-TNFi biologic agents (abatacept, tocilizumab, and rituximab) achieved a better treatment response than a second TNFi in patients with an insufficient response to the first TNFi. 20 In summary, our study supported the use of non-TNF-targeted treatment in RA patients with initial TNFi failure.
To further investigate the efficacy of swap treatment, we stratified swap patients according to the MOA non-TNFi biologic agent used. However, we found no statistically significant differences in drug continuation among abatacept, rituximab, and tocilizumab in the non-TNFi biologic group. Although available data support the use of non-TNF-targeted treatment after first TNFi failure, there is no clear evidence of the superiority of any particular non-TNFi bDMARD. In RA patients with an inadequate response to TNFi, a prospective cohort study showed that short-term drug continuation was better in patients treated with rituximab or tocilizumab than in those treated with abatacept. 30 However, similar to our results, Favalli et al. 26 reported no significant differences in retention rates among these three non-TNFi bDMARDs in the swap group. Direct head-to-head randomized clinical trials comparing abatacept, tocilizumab, and tofacitinib are needed.
Furthermore, we found that switching to JAKi was associated with a lower risk of discontinuation of treatment than cycling to TNFi, while continuation of JAKi was similar to that of non-TNFi bDMARDs. As mentioned above, limited data are available for comparing a second TNFi with JAKi in RA patients with an inadequate response to a prior TNFi. As there have been no randomized trials directly comparing these drugs, a network meta-analysis was performed to compare bDMARDs, including TNFis (golimumab, abatacept, rituximab, and tocilizumab), with tofacitinib in RA patients showing an inadequate response to TNFi or treatment failure. 31 This analysis suggested that tofacitinib was comparable to non-TNFi bDMARDs (abatacept, rituximab, and tocilizumab) in terms of efficacy, based on the ACR response rates at Weeks 12 and 24, and improvement in the Health Assessment Questionnaire Disability Index (HAQ-DI) at Week 12; in this previous study, efficacy was not different between tofacitinib and golimumab as a second-TNFi. 31 In addition, although a claim-based analysis supported switching to non-TNF-targeted treatments, including tofacitinib, rather than cycling to TNFi, that study did not present a comparison between JAKi and non-TNFi biologic agents. 29 In our study, although the proportion of patients achieving remission or LDA at the l-year follow-up was not higher in the JAKi than second TNFi group, the JAKi group showed marked improvement in the DAS28-ESR score compared to the second TNFi group. This may have been due to the higher disease activity at the time of enrollment in the JAKi than TNFi group. Taken together, our results provide additional evidence supporting the use of JAKi as an effective therapy for patients with first TNFi treatment failure. However, as our study did not include upadacitinib, which showed greater improvement of disease activity than TNFi [28], further studies are needed to confirm our findings.
In this study, anti-CCP positivity and concomitant use of csDMARDs were associated with continuation of the second TNFi and non-TNFi-targeted treatment after initial TNFi failure, respectively. Previous studies aiming to identify predictors of the response to targeted treatments, such as bDMARDs and tsDMARDs, have not been fully validated and yielded inconsistent results. A large observational study indicated that patients with anti-CCP positivity showed greater clinical improvement after the initiation of TNFi than those negative for anti-CCP antibodies. 32 However, a meta-analysis showed that anti-CCP status was not associated with the response to TNFi. 33 In addition, other studies demonstrated that anti-CCP-negative patients showed better continuation of, or a greater response to, TNFi compared to patients who were anti-CCP-positive.34,35 Rather than predicting a response to TNFi, the presence of anti-CCP antibodies was a predictive factor of better responses to non-TNFi biologics, including abatacept and rituximab. 36 With regard to concomitant csDMARDs, studies have shown that the use of csDMARDs, such as MTX, was associated with clinical benefits, including improved continuation of TNFi treatment and disease activity compared to TNFi alone.37,38 In patients with high-disease activity, concomitant MTX use was associated with a higher remission rate (adjusted odds ratio 2.54) with tocilizumab treatment. 39 On the one hand, no significant differences were found in the clinical outcomes of abatacept between patients with and without concomitant MTX treatment. 40 However, these studies mostly focused on RA patients treated with first bDMARDs. Due to differences in characteristics between patients treated with first- and second-line targeted treatment, such as the presence of anti-drug antibodies, disease duration, and alterations of immunogenicity, predictors of the response to these drugs may be different. Although further studies are needed to confirm our results, this study provided useful information for clinical decision-making as it pertains to the choice of second-line targeted therapy for patients with RA.
This study had several strengths and limitations. We analyzed KOBIO data collected up to the end of 2020, and the KOBIO data included newer classes of biologic agents and JAKis. Furthermore, the KOBIO registry enrolled RA patients during routine clinical practice, and data were collected prospectively from both academic and community centers. Therefore, our data reflect the actual patterns of targeted treatment in recent years and represent various prescribing patterns and reasons for switching targeted treatments in real-world settings. However, our study also had several limitations. First, any open, non-randomized study has an inherent limitation in terms of the assignment of targeted treatments; selection bias is possible. Although all decisions to initiate or switch targeted treatments were based on the Korean National Health Insurance (KNHI) reimbursement criteria, the choice of targeted treatment could vary depending on the preference of the treating rheumatologist. Furthermore, Kearsley-Fleet et al. 41 suggested that increased class availability and higher expectations for bDMARDs in the more recent cohort could have led to selection bias. Therefore, because of differences in the availability of targeted treatments over time, selection bias might also be present in our study. At the time of cohort establishment, all targeted treatments (with the exception of JAKi) were approved as first-line targeted treatments after csDMARDs failure. In May 2017, JAKis were approved as first-line targeted agents that could be initiated after csDMARDs failure, but rituximab remained a second-line targeted agent. Thus, differences in availability among targeted treatments over time may have created selection bias. In an effort to avoid this, the non-TNF-targeted treatment group was divided into two subgroups (non-TNFi biologic and JAKi subgroups). Therefore, we believe that with exception of JAKi, the class availability and expectations for targeted treatments were generally consistent during the study period. Second, RA patients switching to non-TNFi-targeted treatments exhibited a higher anti-CCP-positive rate than the other patients, and those switching to non-TNFi bDMARDs received a first TNFi treatment for an average of 4 months less than the other patients. As this was an observational study, we could not strictly control the RA treatment; also, we may have failed to control for some confounding factors that can be better balanced in randomized controlled trials. In addition, the number of enrolled patients was relatively small, causing issues with certain analyses. Third, there are large disparities in the use of targeted drugs across countries. This discrepancy may be due to differences in healthcare systems and the accessibility of RA treatment among countries. Therefore, more recent data from around the world are needed to improve the generalizability of our results.
In conclusion, the results of this study showed that, after initial TNFi failure, switching to different MOA non-TNF-targeted treatments was associated with significantly better treatment outcomes and drug continuation than cycling to another TNFi. Although various targeted treatments, including TNFis, have revolutionized the treatment of RA, switching targeted drugs may be unavoidable. Therefore, understanding whether swapping to non-TNF-targeted treatment and cycling to another TNFi have different effects is important to establish evidence-based guidelines for switching to targeted treatments after first TNFi failure. This study provided useful data to develop better protocols for switching to targeted treatment of RA.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221091450 for Comparison of the efficacy and risk of discontinuation between non-TNF-targeted treatment and a second TNF inhibitor in patients with rheumatoid arthritis after first TNF inhibitor failure by Dong-Jin Park, Sung-Eun Choi, Ji-Hyoun Kang, Kichul Shin, Yoon-Kyoung Sung and Shin-Seok Lee in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank all of the rheumatologists and nurses who provided the data. In addition, they also thank for the support from the Korean College of Rheumatology (KCR) board members and the members of the KCR Clinical Trials Committee for establishing the national registry. The authors also thank the patients and their families for their participation.
Footnotes
Author contribution(s): Dong-Jin Park: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Sung-Eun Choi: Data curation; Formal analysis; Investigation; Methodology; Resources; Visualization.
Ji-Hyoun Kang: Data curation; Formal analysis; Investigation; Methodology; Resources; Visualization
Kichul Shin: Conceptualization; Data curation; Formal analysis; Project administration; Software; Supervision; Visualization.
Yoon-Kyoung Sung: Conceptualization; Data curation; Investigation; Project administration; Resources; Software; Supervision.
Shin-Seok Lee: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health and Welfare, Republic of Korea (grant nos. HI19C0481 and HC19C0052) and a grant (grant no. BCRI21029) by Chonnam National University Hospital Biomedical Research Institute. The KCR commissioned the KOBIO as a Korea nationwide project to investigate the safety of biologic agents in routine medical practice. KCR receives restricted grant from Korea pharmaceutical companies, presently AbbVie, BMS, Celltrion, Janssen, JW Pharmaceutical, and Pfizer. The investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions concerning analyses, interpretation, and publication are made autonomously of any industrial contribution.
ORCID iDs: Yoon-Kyoung Sung  https://orcid.org/0000-0001-6691-8939
https://orcid.org/0000-0001-6691-8939
Shin-Seok Lee  https://orcid.org/0000-0001-6810-7355
https://orcid.org/0000-0001-6810-7355
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Dong-Jin Park, Division of Rheumatology, Department of Internal Medicine, Chonnam National University Medical School & Hospital, Gwangju, Republic of Korea.
Sung-Eun Choi, Division of Rheumatology, Department of Internal Medicine, Chonnam National University Medical School & Hospital, Gwangju, Republic of Korea.
Ji-Hyoun Kang, Division of Rheumatology, Department of Internal Medicine, Chonnam National University Medical School & Hospital, Gwangju, Republic of Korea.
Kichul Shin, Division of Rheumatology, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Republic of Korea.
Yoon-Kyoung Sung, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Republic of Korea.
Shin-Seok Lee, Division of Rheumatology, Department of Internal Medicine, Chonnam National University Medical School & Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Republic of Korea.
References
- 1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010; 376: 1094–1108. [DOI] [PubMed] [Google Scholar]
- 2. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 3. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008; 58: 15–25. [DOI] [PubMed] [Google Scholar]
- 4. Salaffi F, Carotti M, Gasparini S, et al. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016; 68: 1–25. [DOI] [PubMed] [Google Scholar]
- 6. Pope J, Sawant R, Tundia N, et al. Comparative efficacy of JAK inhibitors for moderate-to-severe rheumatoid arthritis: a network meta-analysis. Adv Ther 2020; 37: 2356–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease-modifying antirheumatic drug prescription patterns among rheumatologists in Europe and Japan. Rheumatol Ther 2020; 7: 517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park DJ, Choi SJ, Shin K, et al. Switching profiles in a population-based cohort of rheumatoid arthritis receiving biologic therapy: results from the KOBIO registry. Clin Rheumatol 2017; 36: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease-modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatol Ther 2020; 7: 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004; 50: 1051–1065. [DOI] [PubMed] [Google Scholar]
- 11. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004; 50: 1400–1411. [DOI] [PubMed] [Google Scholar]
- 12. Du Pan SM, Dehler S, Ciurea A, et al. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009; 61: 560–568. [DOI] [PubMed] [Google Scholar]
- 13. Gomez-Reino JJ, Carmona L. BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 2006; 8: R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrold LR, Reed GW, Kremer JM, et al. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis 2015; 74: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wakabayashi H, Hasegawa M, Nishioka Y, et al. Which subgroup of rheumatoid arthritis patients benefits from switching to tocilizumab versus etanercept after previous infliximab failure? A retrospective study. Mod Rheumatol 2012; 22: 116–121. [DOI] [PubMed] [Google Scholar]
- 16. Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005; 353: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 17. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67: 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006; 54: 2793–2806. [DOI] [PubMed] [Google Scholar]
- 19. Harrold LR, Reed GW, Magner R, et al. Comparative effectiveness and safety of rituximab versus subsequent anti-tumor necrosis factor therapy in patients with rheumatoid arthritis with prior exposure to anti-tumor necrosis factor therapies in the United States Corrona registry. Arthritis Res Ther 2015; 17: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA 2016; 316: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 21. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021; 73: 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 23. Prevoo ML, van Thof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 24. Prevoo ML, van Gestel AM, van Thof MA, et al. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 1996; 35: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 25. Finckh A, Ciurea A, Brulhart L, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis 2010; 69: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Favalli EG, Biggioggero M, Marchesoni A, et al. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford) 2014; 53: 1664–1668. [DOI] [PubMed] [Google Scholar]
- 27. Wei W, Knapp K, Wang L, et al. Treatment persistence and clinical outcomes of tumor necrosis factor inhibitor cycling or switching to a new mechanism of action therapy: real-world observational study of rheumatoid arthritis patients in the United States with prior tumor necrosis factor inhibitor therapy. Adv Ther 2017; 34: 1936–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karpes Matusevich AR, Duan Z, Zhao H, et al. Treatment sequences after discontinuing a tumor necrosis factor inhibitor in patients with rheumatoid arthritis: a comparison of cycling versus swapping strategies. Arthritis Care Res (Hoboken) 2020; 73: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 29. Bonafede MM, Curtis JR, McMorrow D, et al. Treatment effectiveness and treatment patterns among rheumatoid arthritis patients after switching from a tumor necrosis factor inhibitor to another medication. Clinicoecon Outcomes Res 2016; 8: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gottenberg JE, Morel J, Perrodeau E, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ 2019; 364: l67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vieira MC, Zwillich SH, Jansen JP, et al. Tofacitinib versus biologic treatments in patients with active rheumatoid arthritis who have had an inadequate response to tumor necrosis factor inhibitors: results from a network meta-analysis. Clin Ther 2016; 38: 2628–2641. [DOI] [PubMed] [Google Scholar]
- 32. Harrold LR, Litman HJ, Connolly SE, et al. Comparative effectiveness of abatacept versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis who are anti-CCP positive in the United States corrona registry. Rheumatol Ther 2019; 6: 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lv Q, Yin Y, Li X, et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFalpha agent treatment in patients with rheumatoid arthritis: a meta-analysis. PLoS ONE 2014; 9: e89442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin CT, Huang WN, Tsai WC, et al. Predictors of drug survival for biologic and targeted synthetic DMARDs in rheumatoid arthritis: analysis from the TRA clinical electronic registry. PLoS ONE 2021; 16: e0250877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Potter C, Hyrich KL, Tracey A, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis 2009; 68: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottenberg JE, Courvoisier DS, Hernandez MV, et al. Brief report: association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: results from the pan-European registry analysis. Arthritis Rheumatol 2016; 68: 1346–1352. [DOI] [PubMed] [Google Scholar]
- 37. Kristensen LE, Saxne T, Nilsson JA, et al. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther 2006; 8: R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmalzing M, Behrens F, Schwaneck EC, et al. Does concomitant methotrexate confer clinical benefits in patients treated with prior biologic therapy? Analysis of data from a noninterventional study of rheumatoid arthritis patients initiating treatment with adalimumab. Medicine (Baltimore) 2020; 99: e20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kojima T, Yabe Y, Kaneko A, et al. Importance of methotrexate therapy concomitant with tocilizumab treatment in achieving better clinical outcomes for rheumatoid arthritis patients with high disease activity: an observational cohort study. Rheumatology (Oxford) 2015; 54: 113–120. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi N, Kojima T, Kida D, et al. Concomitant methotrexate has little effect on clinical outcomes of abatacept in rheumatoid arthritis: a propensity score matching analysis. Clin Rheumatol 2019; 38: 2451–2459. [DOI] [PubMed] [Google Scholar]
- 41. Kearsley-Fleet L, Davies R, De Cock D, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018; 77: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221091450 for Comparison of the efficacy and risk of discontinuation between non-TNF-targeted treatment and a second TNF inhibitor in patients with rheumatoid arthritis after first TNF inhibitor failure by Dong-Jin Park, Sung-Eun Choi, Ji-Hyoun Kang, Kichul Shin, Yoon-Kyoung Sung and Shin-Seok Lee in Therapeutic Advances in Musculoskeletal Disease