Abstract
Objective:
To evaluate the effects and side effects of both inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus (SLE).
Methods:
This was a prospective, single-center, observational study. Patients with SLE planning to receive COVID-19 vaccines were recruited and matched 1:1 with healthy controls. The immunogenicity of the COVID-19 vaccines was assessed by a surrogate neutralization assay at 28 days after the second dose. The main outcome was the antibody response comparing SLE patients and controls. Other outcomes included reactogenicity, disease activity and predictors of antibody responses in patients with SLE.
Results:
Sixty-five SLE patients received 2 doses of COVID-19 vaccines (Comirnaty: 38; CoronaVac: 27) were recruited. Many of them were on systemic glucocorticoids (76%) and immunosuppressants (55%). At day 28 after the second dose of vaccines, 92% (Comirnaty: 100% vs CoronaVac: 82%, p = 0.01) of the patients had positive neutralizing antibody. However, compared to the age, gender, vaccine type matched controls, the level of neutralizing antibody was significantly lower (p < 0.001). The self-reported adverse reactions after vaccines in lupus patients were common but mild, and were more frequent in the Comirnaty group. There was no significant change in lupus disease activity up to 28 days after vaccination. The independent predictors of neutralizing antibody level included the dosage of systemic glucocorticoids, use of mycophenolate and type of vaccines.
Conclusions:
COVID-19 vaccines produced satisfactory but impaired humoral response in SLE patients compared to controls which was dependent on the immunosuppressive medications use and type of vaccines received. There was no new short-term safety signal noted. Booster dose is encouraged.
Keywords: COVID-19, SARS-CoV-2, systemic lupus erythematosus, vaccination
Introduction
The Coronavirus Disease 2019 (COVID-19) infection has been haunting the whole world since early 2020. Vaccination is believed to be a good strategy to control the pandemic. Currently, there are two COVID-19 vaccines with different mechanism of action available in Hong Kong–they are the inactivated virus vaccine CoronaVac® (Sinovac) and the mRNA-based vaccine Comirnaty® (BioNTech/Fosun). Both vaccines have obtained approval for emergency use by the World Health Organization and were widely deployed globally. 1 A mass voluntary vaccination program (CoronaVac: 2 doses, 28 days apart; Comirnaty: 2 doses, 21 days apart) for all Hong Kong residents has started in late February, 2021. 2
Systemic lupus erythematosus (SLE) is a chronic autoimmune rheumatic disease (ARD) characterized by systemic inflammation, requiring treatment with immunosuppressive medications and leading to multiple medical comorbidities. Patients with SLE are potentially at higher risk of more severe COVID-19 infection. 3 Initial work from the Global Rheumatology Alliance (GRA) showed a higher percentage of patients who were hospitalized had SLE versus those who were not hospitalized, and 56% of 85 SLE patients with COVID-19 infection required hospitalization. 4 Another study by the GRA identified medications commonly used to treat SLE (rituximab, azathioprine, cyclophosphamide and calcineurin inhibitors) were associated with increased mortality compared with methotrexate. 5 Therefore, although there is so far no clinical trial assessing directly its efficacy in patients with SLE, COVID-19 vaccination is advocated by international and local rheumatology organizations because the benefit probably out-weights its risk.6–11 At the same time, there are concerns regarding increased side effects, risk of inducing disease flare and reduced efficacy of these vaccines in lupus patients due to the underlying autoimmunity as well as immunosuppressive medication use. In fact, according to a web-based questionnaire study, uncertainty about COVID-19 vaccination was reported from 31.8% of 1,266 patients with systemic rheumatic diseases (38.9% with SLE), which could be a major barrier to vaccine uptake. 12
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new campaign. It is of urgent need to evaluate the real-world immunogenicity and safety of COVID-19 vaccines of different platforms in patients with SLE. In addition, it is important to know the clinical predictors of vaccine immunogenicity, so that better informed decision of who should be vaccinated with what type of vaccine could be made.
Methods
Study design and subjects
This was a prospective, single-center, observational study. We hypothesize that the immunogenicity of COVID-19 vaccines in patients with SLE is reduced compared to healthy controls, which may be related to the immunosuppressive medications used. The primary outcome was the neutralizing antibody level after two doses of COVID-19 vaccine at day 28 in SLE patients compared to controls. Secondary outcomes included (1) the rates of adverse reactions after the vaccines in SLE patients, (2) the change of SLE disease activity from baseline to one month after the second dose in patients with SLE, and (3) the clinical variables predicting the antibody response and adverse events in patients with SLE.
From June to August 2021, consecutive patients with a clinical diagnosis of SLE followed up at the rheumatology clinic of a regional hospital in Hong Kong were screened for eligibility when they came back for scheduled follow-up. Inclusion criteria were (1) SLE according to the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria, (2) age between 18 and 65 years, and (3) plan to receive COVID-19 vaccines. 9 The exclusion criteria were (1) history of allergic reaction to any component of the vaccines, (2) history of severe allergic reactions to any allergens, (3) history of allergic reaction to multiple classes of drugs, (4) severe neurological conditions (eg, transverse myelitis, Guillain-Barre syndrome), (5) patients with uncontrolled severe chronic diseases (including active SLE), (6) previous COVID-19 infection, and (7) pregnant and lactating women. Eligible participants would undergo COVID-19 vaccination according to the local mass vaccination program with type of vaccines at their own choice. Adjustment of dosing schedules of usual medications was not advised in accordance to the local recommendation. 10 Subjects without previous COVID-19 infection or any significant acute/chronic illness participated in another study investigating the immune protective response after COVID-19 vaccines were selected as controls. They were age and gender matched with the patients with SLE. Written informed consents were obtained from all participants. The study was approved by the local Research Ethical Committee. All patient details have been de-identified. The reporting of this study conforms to the checklist recommended by EULAR for reporting longitudinal observational registry studies in rheumatology derived from the STROBE guidelines. 13
Clinical assessments
Demographic data and medical history of the SLE patients were recorded at baseline (within 2 weeks prior to vaccination). SLE disease activity at baseline and 28 days after the second dose was assessed by a rheumatologist (HS) blinded to the type of vaccines the patient received using the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2k). 14 At baseline and 28 days after the second dose, all patients had blood (complete blood picture, liver and renal function tests, complement components (C3 and C4) and anti-dsDNA) and urine (routine microscopy and protein creatinine ratio) checked.
Incidence and severity of vaccine-emergent serious adverse events and adverse events were documented till 28 days after the second dose through a self-administered online questionnaire. Serious adverse events were defined as any adverse reaction resulting in: a life-threatening condition or death, a significant or permanent disability or hospitalization or prolongation of hospitalization.
Samples and assays
Blood was sampled from each patient at baseline and 28 (±2) days after the second dose of vaccination. The plasma fraction of the blood samples was used for SARS-CoV-2-specific neutralizing antibody measurement using a surrogate virus neutralization assay (SARS-CoV-2 surrogate neutralization test kit, GenScript USA, Inc, NJ). Sero-positivity after vaccination was defined according to the cut-off (30% inhibition) set by the assay manufacturer.
Statistical analysis
The seroconversion rate in patients with SLE was hypothesized to be 55% while that for controls would be 80% after two doses of vaccines. 15 Based on this difference, with a two-sided α of 0.05, about 50 patients in each group would be required to achieve a statistical power of 0.8 (G*Power version 3.1.9.2, Germany). To make up for the loss of follow-up rate of 30%, a sample size of 65 SLE patients was targeted.
Descriptive statistics were presented for baseline demographic and clinical characteristics. The primary outcome was analyzed using Mann–Whitney-U test comparing the antibody titers of SLE and control groups. The sero-prevalence was compared by Chi-square test or fisher exact test between SLE patients and controls. The changes of SLE serology and disease activity before and after vaccination were analyzed by Wilcoxon signed-rank test. Regarding the predictors of immunogenicity in patients with SLE, Mann–Whitney-U test and Spearman correlation test were used initially. A multivariate linear regression analysis using biologically plausible variables with p < 0.1 in the univariate analyses in addition to age and gender was performed to identify the independent predictors of vaccine response. Subgroup analyses were performed to evaluate if there were any differences between the two types of vaccines. A 2-tailed probability value of p < 0.05 wass considered statistically significant. Calculation was performed using IBM SPSS Statistics Version 24 (IBM, Armonk, NY, USA).
Results
Patient disposition and characteristics
A total of 68 patients with SLE were recruited. Among them, 3 patients were subsequently excluded due to active lupus disease. At the end, 65 patients received two doses of COVID-19 vaccines, with 38 (58%) being Comirnaty and 27 (42%) being CoronaVac which was similar to the controls (57% Comirnaty, 43% CoronaVac, p = 0.859). Of these 65 patients, 61 (94%) were women and the mean age was 46.2 ± 10.4 years (Table 1). The baseline mean SLEDAI-2 K was 2.9 ± 2.0. The majority of them (75%) were on systemic glucocorticoids with a mean daily prednisolone dose of 4.9 ± 2.6 mg. More than half of the patients were on immunosuppressive agents (55%), namely: mycophenolate mofetil (26%), azathioprine (11%), cyclosporine A (11%), tacrolimus (8%), and methotrexate (2%). The baseline demographics, disease activity, and treatments were comparable between patients received the two vaccines (Table 1).
Table 1.
Demographics and baseline clinical characteristics of patients with SLE and controls.
| Controls | SLE | Comirnaty | CoronaVac | P value | |
|---|---|---|---|---|---|
| N | 50 | 65 | 38 | 27 | |
| Age, years | 44.7 ± 10.7 | 46.2 ± 10.4 | 44.5 ± 10.1 | 48.6 ± 10.6 | 0.124 |
| Female, N (%) | 47 (94) | 61 (93) | 36 (95) | 25 (93) | 1.000 |
| Type of vaccines: | |||||
| Comirnaty, N (%) | 37 (57) | 38 (59) | |||
| CoronaVac, N (%) | 28 (43) | 27 (42) | |||
| SLEDAI-2k | 2.9 ± 2.0 | 2.8 ± 1.7 | 3.0 ± 2.4 | 0.625 | |
| Anti-dsDNA, IU/ml | 177 ± 179 | 181 ± 170 | 171 ± 194 | 0.830 | |
| Proteinuria, g/24-hour | 0.25 ± 0.25 | 0.24 ± 0.20 | 0.27 ± 0.30 | 0.676 | |
| Systemic glucocorticoids, N (%) | 49 (75) | 29 (76) | 20 (74) | 0.836 | |
| Prednisolone dosage, mg/day | 4.9 ± 2.6 | 4.6 ± 1.5 | 5.3 ± 3.7 | 0.452 | |
| Mycophenolate mofetil, N (%) | 17 (26) | 11 (29) | 6 (22) | 0.543 | |
| Azathioprine, N (%) | 7 (11) | 4 (11) | 3 (11) | 1.000 | |
| Cyclosporine A, N (%) | 7 (11) | 4 (11) | 3 (11) | 1.000 | |
| Tacrolimus, N (%) | 5 (8) | 2 (5) | 3 (11) | 0.642 | |
| Methotrexate, N (%) | 1 (2) | 0 (0) | 1 (4) | 0.415 | |
SLEDAI-2k, Systemic Lupus Erythematosus Disease Activity Index 2000; SLE, Systemic lupus erythematosus.
Immunogenicity
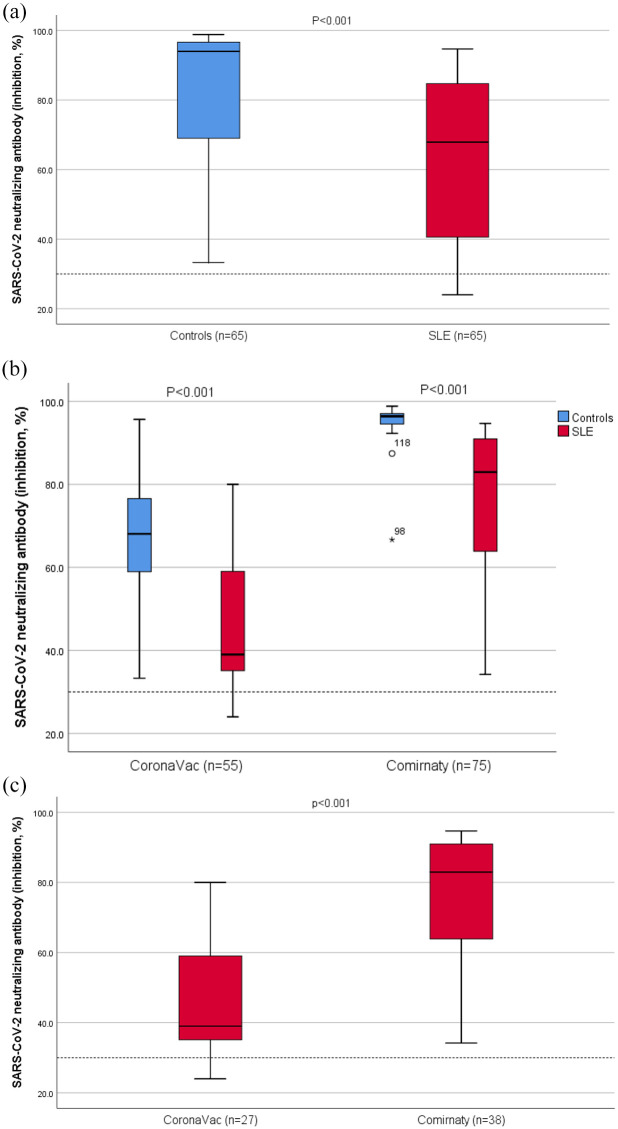
Among the 65 patients with SLE, 60 (92%) had positive neutralizing antibody at 28 days after the second dose, while all healthy controls (100%) were sero-positive (p = 0.058). Compared to controls, the neutralizing antibody level was significantly lower (mean 64.4 ± 22.9% vs 83.0 ± 18.0%, p < 0.001) in patients with SLE (Figure 1(a)). In subgroup analyses according to the type of vaccine received, the level of neutralizing antibody was significant lower in both vaccine groups (both p < 0.001) compared to controls (Figure 1(b)). When comparing 2 vaccine types, the day 28 neutralizing antibody level was significantly lower (mean 46.6% vs 77.0%, p < 0.001) in lupus patients received CoronaVac versus Comirnaty (Figure 1(c)). The post-vaccination sero-positivity rate was also significantly reduced (82% vs 100%, p = 0.01) in patients received CoronaVac. The clinical characteristics of the 5 patients who were sero-negative by the surrogate neutrolizing assay are shown in supplementary Table 1. Two of these 5 patients were tested positive for antibody against the receptor binding domain of S protein by a more sensitive assay (Elecsys Anti-SARS-CoV-2 S, Roche Diagnostics GmbH).
Figure 1.
Distribution of neutralizing antibody levels after COVID-19 vaccines comparing (a) SLE patients and matched controls, (b) SLE patients and matched controls in two vaccine subgroups, and (c) two vaccine types in SLE patients. Data for each group are presented as box plots: central values within boxes correspond to median; the range between the lower (Q1) and upper (Q3) bounds of the boxes is the IQR. Whiskers represent scores outside IQR and end in maximum (higher ‘calculated value’ = Q3 + 1.5 x IQR) and minimum (lower ‘calculated value’ = Q1 – 1.5 x IQR). Spots are outliers above the maximum or under the minimum values. Data regarding were analyzed using Mann–Whitney-U test. Dotted line denotes the cut-off level for positivity (30%).
Safety
Almost all patients with SLE (94%) volunteered that they had some adverse reactions after the vaccines. After the first dose, 71%, 66%, and 5% of the patients reported local, systemic, and allergic reactions respectively. After the second dose, 66%, 63%, and 6% reported local, systemic, and allergic reactions, respectively. All reactions subsided within 4 days. There were no serious adverse events. No pericarditis was noted. SLE patients received Comirnaty had significant more local reactions after both doses (first dose: 95% vs 37%, p < 0.001; second dose: 90% vs 33%, p < 0.001) and systemic side effects after the first dose (82% vs 44%, p = 0.002) compared to CoronaVac (Table 2). There was no significant change in the SLEDAI-2k, anti-dsDNA level and proteinuria after vaccination. In fact, more patients had numerical improvement rather than deterioration in SLEDAI-2k, anti-dsDNA level, and proteinuria after vaccination (49% vs 39%, 43% vs 26% and 19% vs 16%, respectively). No patients had their medications titrated up in the immediate follow-up visit after vaccination. No significant change in SLE disease activity parameters was found in subgroup analyses according to vaccine types.
Table 2.
Adverse events following Comirnaty and CoronaVac vaccination in patients with SLE.
| After first dose vaccine | After second dose vaccine | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 65) | Comirnaty (n = 38) | CoronaVac (n = 27) | P value | All (n = 65) | Comirnaty (n = 38) | CoronaVac (n = 27) | P value | |
| No symptoms | 12 (19) | 1 (3) | 11 (41) | < 0.001 | 14 (22) | 4 (11) | 10 (37) | 0.010 |
| Local reaction | 46 (71) | 36 (95) | 10 (37) | < 0.001 | 43 (66) | 34 (90) | 9 (33) | < 0.001 |
| Redness | 7 (11) | 5 (13) | 2 (7) | 0.690 | 9 (17) | 9 (24) | 0 (0) | 0.008 |
| Swelling | 8 (12) | 7 (18) | 1 (4) | 0.126 | 12 (19) | 12 (32) | 0 (0) | 0.001 |
| Pain | 43 (66) | 34 (90) | 9 (33) | < 0.001 | 39 (60) | 30 (79) | 9 (33) | < 0.001 |
| Induration | 7 (11) | 7 (18) | 0 (0) | 0.036 | 7 (11) | 7 (18) | 0 (0) | 0.036 |
| Systemic reaction | 43 (66) | 31 (82) | 12 (44) | 0.002 | 41 (63) | 27 (71) | 14 (52) | 0.114 |
| Headache | 6 (9) | 4 (11) | 2 (7) | 1.000 | 8 (12) | 7 (18) | 1 (4) | 0.126 |
| Palpitation | 5 (8) | 4 (11) | 1 (4) | 0.393 | 4 (6) | 2 (5) | 2 (7) | 1.000 |
| Tiredness | 29 (44) | 22 (58) | 7 (26) | 0.011 | 24 (37) | 19 (50) | 5 (19) | 0.010 |
| Dizziness | 7 (11) | 4 (11) | 3 (11) | 1.000 | 9 (14) | 7 (18) | 2 (7) | 0.285 |
| Muscle pain | 21 (32) | 17 (45) | 4 (15) | 0.011 | 15 (23) | 14 (37) | 1 (4) | 0.002 |
| Joint pain | 8 (12) | 7 (18) | 1 (4) | 0.126 | 6 (9) | 3 (8) | 3 (11) | 0.686 |
| Low-grade fever | 5 (8) | 4 (11) | 1 (4) | 0.393 | 5 (8) | 5 (13) | 0 (0) | 0.071 |
| Chills or high-grade fever | 1 (2) | 1 (3) | 0 (0) | 1.000 | 4 (6) | 3 (8) | 1 (4) | 0.636 |
| Nausea | 2 (3) | 1 (3) | 1 (4) | 1.000 | 1 (2) | 1 (3) | 0 (0) | 1.000 |
| Vomiting | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Cough | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Sore throat | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Rhinorrhea | 3 (5) | 2 (5) | 1 (4) | 1.000 | 2 (3) | 1 (3) | 1 (4) | 1.000 |
| Nasal congestion | 2 (3) | 1 (3) | 1 (4) | 1.000 | 0 (0) | 0 (0) | 0 (0) | |
| Diarrhea | 4 (6) | 2 (5) | 2 (7) | 1.000 | 7 (11) | 2 (5) | 5 (19) | 0.117 |
| Loss of appetite | 2 (3) | 1 (3) | 1 (4) | 1.000 | 7 (11) | 3 (8) | 4 (15) | 0.437 |
| Epigastric discomfort | 1 (2) | 1 (3) | 0 (0) | 1.000 | 2 (3) | 1 (3) | 1 (4) | 1.000 |
| Allergic reaction | 3 (5) | 2 (5) | 1 (4) | 1.000 | 4 (6) | 2 (5) | 2 (7) | 1.000 |
| Difficulty in breathing | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (3) | 0 (0) | 1.000 | |
| Swelling of face | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (3) | 0 (0) | 1.000 | |
| Rash | 3 (5) | 2 (5) | 1 (4) | 1.000 | 2 (3) | 0 (0) | 2 (7) | 0.169 |
SLE, Systemic lupus erythematosus.
Results are presented as n (%).
Predictors of immunogenicity
Univariate analyses showed that the use of mycophenolate mofetil was associated with significantly lower neutralizing antibody level (mean 52.1 ± 20.7% vs 68.7 ± 22.3%, p = 0.009) at day 28 after vaccination. The antibody level was also correlated with SLEDAI (rho = -0.30, p = 0.015), proteinuria (rho = -0.54, p = 0.043) and dosage of systemic glucocorticoids (rho = -0.247, p = 0.087). Multivariate analysis showed the independent predictors of neutralizing antibody level included the dosage of systemic glucocorticoids (β = -2.01, 95% CI = -3.66 - -0.37, p = 0.018), use of mycophenolate mofetil (β = -15.2, 95% CI -24.4 - -6.0, p = 0.002) and type of vaccines (Comirnaty, β = 28.8, 95% CI: 20.1–37.5, p < 0.001) after adjusted for age, gender and SLEDAI-2k (Table 3).
Table 3.
Multivariate linear regression analysis for neutralizing antibody activity.
| Beta | 95% confidence interval | p value | |
|---|---|---|---|
| Age | –0.022 | –0.425 – 0.381 | 0.914 |
| Gender | 8.16 | – 7.39 – 23.7 | 0.296 |
| SLEDAI-2k | –1.96 | –4.22 – 0.31 | 0.088 |
| Prednisolone dosage | –2.01 | –3.66 - –0.37 | 0.018 |
| Mycophenolate mofetil | –15.2 | –24.4 - –6.0 | 0.002 |
| Type of vaccines: Comirnaty | 28.8 | 20.1 – 37.5 | < 0.001 |
SLEDAI-2k, Systemic Lupus Erythematosus Disease Activity Index 2000.
Discussion
To our understanding, this is the first prospective study assessing the immunogenicity and safety of inactivated and mRNA COVID-19 vaccines focusing on patients with SLE. We found an impaired antibody response in lupus patients compared to matched healthy population. Adverse reactions were common but all mild and transient. There was no evidence of worsening of lupus disease control after vaccination in short term. Although higher disease activity appeared to be associated with lower antibody response, multivariate analysis revealed the independent determinants were the immunosuppressive medications used, particularly systemic glucocorticoids and mycophenolate. mRNA vaccine induced a better humoral response but was associated with more reactogenicity compared to inactivated vaccine in lupus patients.
Our results largely concur with the published literature on COVID-19 vaccines, mainly mRNA, in patients with ARD. A post-marketing study from a nationwide mass vaccination database (n = 596,618), which included 2.7% of participants with ‘immunosuppression’ (definition and underlying disease not mentioned), reported that the mRNA vaccine was equally effective for a wide range of COVID-19 related outcomes. 16 In an initial research letter, the immunogenicity of mRNA COVID-19 vaccine in 123 patients with rheumatic diseases (20% with SLE) was reported. 17 After the first dose, 74% patients had a detectable antibody response. However, those on mycophenolate or rituximab were less likely to develop an antibody response (p = 0.001 and p = 0.04, respectively). Of note, only 27.3% of patients on mycophenolate had detectable antibody. In a case-control study with 632 patients with autoimmune diseases (5% with SLE), seroconversion rates after first dose of mRNA or adenoviral vector COVID-19 vaccines were significantly lower in patients than controls. 18 After the second vaccination (n = 125), seroconversion exceeded 80% in all patient treatment subgroups, except those treated with anti-CD20. Another multicenter study showed that the seropositive rate in patients with ARD (n = 686, 15% with SLE) was 86% after 2 doses of mRNA vaccine which was significantly lower than controls, and the risk factors for reduced immunogenicity included older age and treatment with glucocorticoids, mycophenolate, rituximab and abatacept. 19 In a study of 264 ARD patients (10% with SLE) without control group, 86% of patients mounted humoral response after second dose of mRNA vaccine and 59% of those with negative response were treated with B cell-depleting agents. 20 Similarly, in another uncontrolled study, 89% of patients with SLE (n = 54) were able to mount a serological response, while only 24% of those on rituximab had detectable antibodies. 21 With regard to safety, a web-based survey of 696 patients with SLE showed that 45% and 53% of respondents reported side effects after first and second dose of vaccines respectively with no difference according to vaccine types (Pfizer-BioNTech 57%, Sinovac 22%, AstraZeneca 10% and Moderna 8%). 12 Only 3% of patients reported a medically confirmed flare after vaccination. In another large survey of 2860 patients with ARD (14% with SLE) who received COVID vaccination, adverse events were typical of those reported in the general population and disease flares requiring medication changes occurred in 4.6%. 22 Consistent immunogenicity and safety findings were reported in two meta-analyses, however, studies focusing primarily on SLE patients were scarce and data of in-activated vaccines was very limited.23,24 In a recent phase 4 trial conducted in Brazil comparing 910 adults with ARD (25.5% with SLE, 38.2% on glucocorticoids, 13.1% on myophenolate) and 182 matched healthy controls, both the rates of anti-SAR-CoV-2 IgG seroconversion (70.4% vs 95.5%, p < 0.001) and neutralizing antibody positivity (56.3% vs 79.3%, p < 0.001) were significantly lower in the ARD group 6 weeks after the second dose of CoronaVac. 15 The short-term immunogenicity overall was regarded acceptable. Adverse reactions were more frequently reported in ARD patients (50.5% vs 40.1%, p = 0.011), although there were no moderate/severe adverse events. In a recent study done in the US, the IgG antibodies against SARS-CoV-2 spike receptor-binding domain in 90 SLE patients were significant lower than controls (n = 20) after receiving Pfizer-BioNTech, Moderna and J&J vaccines, although the controls were not gender or vaccine type matched. 25 The use of any immunosuppressant or prednisone was also found to be associated with decreased antibody response and pre-/post-vaccination SLEDAI scores were similar.
There are limitations of the current study. First, in accordance to the local COVID vaccination program guideline, elderly patients and patients with complicated allergy history, severe neurological or uncontrolled chronic diseases were not included in the study which could restrict the generalizability of the results. Similarly, the effect of some medications such as high dose systemic glucocorticoids or anti-B cell therapies were not studied. Second, the lack of randomization could induce bias although the characteristics of the patients were balanced between the two vaccine groups. Third, the health status of the participants in the control group was self-reported and detailed clinical data was not available. Fourth, the cut-off level of positivity of the neutralizing antibody assay is arbitrary and should be further validated. Finally, the T-cell response and the persistence of immunogenicity which have important bearing in the vaccine efficacy as well as the long-term effect on SLE disease activity were not examined. The actual protective effect of vaccines against COVID-19 infection can only be tested in areas with high incidence.
To conclude, the overall immunogenicity and safety of COVID-19 vaccines was satisfactory in patients with SLE, although the antibody response was dampened compared to healthy controls. No new safety concerns were found. There seems to be differential effects and side effects with the two vaccine platforms. Important determinants of post-vaccination antibody activity in lupus patients included systemic glucocorticoid dosage, the use of mycophenolate and the type of vaccines. Booster dose may be helpful but should be formally investigated.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221089586 for Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus by Ho So, Tena Li, Vivien Chan, Lai-Shan Tam and Paul KS Chan in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to express our gratitude to all recruited patients and medical staff.
Footnotes
Author contribution(s): Ho So: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Tena Li: Data curation.
Vivien Chan: Data curation.
Lai-Shan Tam: Conceptualization; Writing – original draft.
Paul KS Chan: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval information: The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee, No. 2021-0392.
ORCID iD: Ho So  https://orcid.org/0000-0001-7113-9390
https://orcid.org/0000-0001-7113-9390
Data sharing statement: Data can be shared upon request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ho So, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong.
Tena Li, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong.
Vivien Chan, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong.
Lai-Shan Tam, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, 9/F, Lui Che Woo Clinical Sciences Building, Shatin, Hong Kong.
Paul KS Chan, Department of Microbiology, The Prince of Wales Hospital, The Chinese University of Hong Kong, 30-32 Ngan Shing Street, Shatin, Hong Kong.
References
- 1. Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021; 397: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID-19 Vaccination Programme. https://www.covidvaccine.gov.hk/en/
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021; 80: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bijlsma JW. EULAR December 2020 view points on SARS-CoV-2 vaccination in patients with RMDs. Ann Rheum Dis 2021; 80: 411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 2021; 105: e56–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. England PH. Coronavirus (COVID-19) vaccination information for public health professionals. https://www.gov.uk/coronavirus [Google Scholar]
- 9. Aringer M, Costenbader K, Daikh D, et al. 2019. European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus.. Ann Rheum Dis 2019; 78: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 10. So H, Mok CC, Yip RML. The Hong Kong society of rheumatology consensus recommendations for COVID-19 vaccination in adult patients with autoimmune rheumatic diseases. J Clin Rheum Immunol 2021; 21: 7–14. [Google Scholar]
- 11. Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology 2021; 60: 3496–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felten R, Dubois M, Ugarte-Gil MF, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol 2021; 3: e243–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zavada J, Dixon WG, Askling J. Launch of a checklist for reporting longitudinal observational drug studies in rheumatology: a EULAR extension of STROBE guidelines based on experience from biologics registries. Ann Rheum Dis 2014; 73: 628. [DOI] [PubMed] [Google Scholar]
- 14. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 15. Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med 2021; 27: 1744–1751 [DOI] [PubMed] [Google Scholar]
- 16. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. New Engl J Med 2021; 384: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. Epub ahead of print 23 March 2021. DOI: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021; 3: e778–e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021; 80: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 20. Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021; 80: 1317–1321. [DOI] [PubMed] [Google Scholar]
- 21. Ammitzbøll C, Bartels LE, Bøgh Andersen J, et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol 2021; 3: 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 global rheumatology alliance vaccine survey. RMD Open 2021; 7: e001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramirez GA, Asperti C, Cucca V, et al. Challenges to vaccination against SARS-CoV-2 in patients with immune-mediated diseases. Vaccines 2021; 9: 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kroon FPB, Najm A, Alunno A, et al. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis 2021; 81: 422–432. [DOI] [PubMed] [Google Scholar]
- 25. Izmirly PM, Kim MY, Samanovic M, et al. Evaluation of immune response and disease status in SLE patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2022; 74: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221089586 for Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus by Ho So, Tena Li, Vivien Chan, Lai-Shan Tam and Paul KS Chan in Therapeutic Advances in Musculoskeletal Disease