Abstract
Objective
The aim of this prospective observational study was to investigate the effects of a novel Wim Hof psychophysiological training program on stress responses and hormone release in healthy participants during an Antarctic expedition.
Methods
All members of an Antarctic expedition were included in the study. The participants were healthy volunteers allocated to an intervention group (n = 6) and a control group (n = 7). The intervention consisted of 8 weeks of Wim Hof training. The training program comprised three integrated parts: breathing exercises, cold exposure and meditation. Psychometric measures (the Beck Depression Inventory and the Trauma Symptom Checklist-40) and neuroendocrine measures (cortisol, melatonin) were assessed pre- and post-intervention.
Results
The results showed that the 8-week training program significantly reduced stress responses, as indicated by a reduction in depressive symptoms. A non-significant reduction in cortisol was also observed.
Conclusions
These data constitute preliminary findings indicating that the Wim Hof Method may positively affect stress symptoms and adaptability of the hormonal system to respond adequately to the circadian rhythm in healthy volunteers who participated in an Antarctic expedition.
Keywords: Cortisol, melatonin, psychophysiological training, stress, Wim Hof Method, depression, observational study, hormone release
Introduction
Recent research provides preliminary evidence for the effectiveness of a novel psychophysiological training program called the Wim Hof Method (WHM). The WHM is based on cold exposure and special meditation techniques and was developed by a Dutch athlete named Wim Hof who exposed himself to extreme cold temperatures. The WHM consists of three main components: 1) breathing exercises, 2) various types of cold exposure,1,2 and meditation related to mindful body awareness, including enhancement of willpower and self-awareness.3–6
There are only a few studies on this novel training program and its psychophysiological effects, but findings suggest that the WMH has positive effects on immune and endocrine functions.1,2,5,6 A recent neuroimaging study using functional magnetic resonance imaging analyses showed that the WHM training program activates primary control centers for descending pain/cold stimuli modulation in the periaqueductal gray, possibly initiating a stress-induced analgesic response. In addition, this study reported that the WHM activated the left anterior and right middle insula, which are associated with self-reflection, and that respiration practice may affect sympathetic activation. 2
The aim of this study was to investigate the effects of a WHM training program on psychological and neuroendocrine stress responses caused by extreme conditions during an expedition based at the J.G. Mendel Antarctic Station from January to March 2019.7,8 All participants were healthy individuals who were researchers at the Antarctic station. This research focused on psychometric measures related to long-term stress (Beck Depression Inventory [BDI-II] and the Trauma Symptom Checklist-40 [TSC-40]) and neuroendocrine measures (cortisol, melatonin). Measures were taken at the start and at the end of an 8-week Antarctic expedition in a WMH training group and a control group.
Methods
Participants
Participants in this prospective observational study comprised all members of the expedition. Participants were divided into two groups based on their interest in the WMH psychophysiological training: an intervention group and a control group.
The expedition physician ascertained that none of the participants had any serious comorbidities, were taking any chronic medication that affected the central nervous system or the hormonal system (including hormonal contraception), had any mental health issues or chronic disorders (including hormonal or immune system) disorders, or had colored hair, and that all had American Society of Anesthesiology health scale scores of 1 to 2. All participant details were de-identified and all participants provided signed informed consent related to this study. Potential adverse effects were explained to participants. The study was approved by the university hospital ethical committee (Medical Psychology and Psychopathology review board) of Charles University, First Faculty of Medicine; approval no. 1162017A1), Prague, on 16 January 2017. We have followed the relevant EQUATOR STROBE guidelines for observational studies. 9
Wim Hof psychophysiological training program
Approximately 1 month before the expedition, the intervention group underwent introductory WHM training led by a certified instructor. The instructor prepared a specific tailored program that followed the WHM procedure. The intervention consisted of 8 weeks of Wim Hof training and included three integrated parts: breathing exercises, cold exposure and meditation.
The breathing exercises comprised a series of four rounds. Each round consisted of 30 deep breaths, after which participants exhaled and held their breath until the moment they felt the urge to breathe in. This phase was a retention phase. After the retention phase, participants took a deep breath and exhaled after 10 s. During the retention phase, participants were instructed to completely relax and keep their eyes closed. As each retention phase lasted 2 to 3 minutes, the four rounds were equivalent to 10 minutes of relaxed meditation. During the meditation, participants were instructed to focus on “body awareness,” including enhancement of willpower and self-awareness.
Cold exposures were performed on an individual basis in the shower with water coming straight from a creek or by immersion in the water of Prince Gustav Channel. Cold water exposure was preceded by 5 to 10 long deep breaths coupled with contraction of specific abdominal and pelvic muscle groups. After three initial monitored sessions at the station, participants were instructed to perform the exercises once a day on their own, fitting them around their work schedule. One session lasted approximately 20 minutes.
Psychometric measures
Trauma Symptom Checklist-40 (TSC-40)
The TSC-40 is a standardized and reliable measure of stress-related symptoms in adults. 10 The TSC-40 is a self-report scale with 40 items on six clinical subscales (Anxiety, Depression, Dissociation, Sexual Abuse Trauma Index, Sexual Problems and Sleep Disturbance). Respondents were asked to rate the relevance of each item on a 4-point Likert scale ranging from 0 (never) to 3 (often) during the last 2 months. The instrument has been standardized for both clinical and non-clinical populations. 11 The Czech version of the TSC-40 has high reliability and internal consistency (Cronbach’s alpha 0.91, test–retest reliability after 1 week 0.88) (data not published).
Beck Depression Inventory (BDI-II)
The BDI-II was used to assess depressive symptoms as a possible reaction to long-term stress conditions related to the Antarctic expedition.12,13 The BDI-II is a 21-item self-report instrument with good psychometric properties that is widely used to screen for depressive symptoms in adults and adolescents.14,15 Respondents are asked to rate their experiences during the last 2 weeks on a 4-point Likert scale ranging from 0 (rarely/none) to 3 (most of the time). The Czech version of the BDI-II has high reliability and internal consistency. 16
Hair cortisol and salivary melatonin analysis
Cortisol analysis of hair samples is a retrospective measure of long-term cortisol concentrations. Hair grows an average of 1 cm per month, but because of a “washout” effect, this method only enables retrospective analysis of cortisol concentrations for a maximum of 3 to 6 months. 17
Hair samples for all participants were collected before (on the day of departure from the home country) and after 8 weeks of the expedition and WHM practice. Clean scissors were used to cut small hair samples from the posterior vertex of each participant’s scalp because the variation of hair cortisol levels is relatively small in that location. Hair samples were stored in envelopes at room temperature according to the recommended procedure. 18 The temperatures inside the Antarctic station were between 18°C and 23°C.
In the laboratory, each ∼1 cm scalp hair sample (to assess cortisol concentrations in the month prior to sampling) was weighed and diced into pieces 1 mm long. The cortisol was extracted in methanol for 16 h at 80°C. The liquid was then evaporated under nitrogen and the sample dissolved in phosphate-buffered saline. The sample was frozen at −25°C until analysis. For analysis, the sample was processed using a Cortisol sal. ELISA kit (DiaMetra Srl Unipersonale, Segrate, Italy) according to the manufacturer’s recommendation. The obtained concentration was recalculated per mg of original hair. As previous findings indicate, this method of hair cortisol analysis provides reliable and valid results. 19
Melatonin saliva samples were collected after waking before (on the day of departure) and after 8 weeks of practicing the WHM, and processed using the Melatonin ELISA kit (ab213978, Abcam, Baria, Czech Republic) according to the manufacturer’s recommendation. Salivary samples were immediately frozen and kept at −20°C until the melatonin analysis was conducted.
Statistical analysis
To assess the difference between the WMH intervention group and the control group, we analyzed changes in all assessed variables at the beginning and at the end of the expedition (after 8 weeks of practice for the intervention group). To measure the effect of the WMH program, we calculated differences before and after the expedition for each variable (Δ – score differences); the same differences were calculated for the control group. To compare the assessed variables between the groups we used the non-parametric Mann–Whitney U test. The software package Statistica Version 6 (StatSoft, Tulsa, OK, USA) was used to perform all statistical analyses.
Results
Of the 13 participants, 3 were women and 10 men. There were 6 participants in the intervention group (2 women and 4 men) and 7 in the control group (1 woman and 6 men). Participants were aged 27 to 60 years (mean age 36.4 years, standard deviation 10.1 years). One female participant from the intervention group discontinued the training program owing to personal reasons and was not included in the study.
Two participants (one from each group) had high school education and the other participants had university degrees. One male participant was a mild smoker; the other participants were non-smokers.
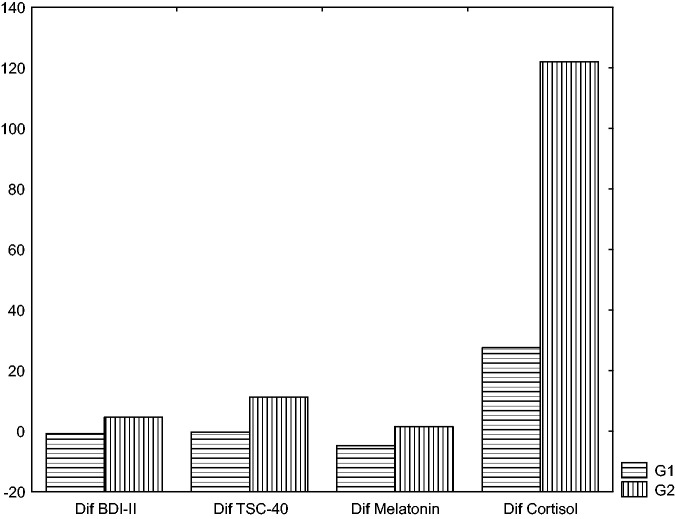
The results of the Mann–Whitney test showed no differences between the groups in all assessed variables at the beginning of the expedition (before WMH training). However, there were significant differences at the end of the expedition between the WMH intervention group and the control group in the levels of depressive symptoms and melatonin (p = 0.05, p = 0.03, respectively) (Table 1, Figure 1). Melatonin and depressive symptoms were significantly lower in the WMH group than in the control group. At the end of the expedition, cortisol levels and TSC-40 (stress) scores were higher in the control group; however, these differences were not significant. Hormone levels and scores on the psychometric measures for both groups are shown in Table 2.
Table 1.
Score differences between the intervention and control groups.
| Intervention |
Control |
U (MW test) | Z (MW test) | p-value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| BDI-II | −0.750* | 2.363 | 4.600* | 3.847 | 2 | −1.960* | 0.050* |
| TSC-40 | −0.250 | 0.957 | 11.333 | 14.640 | 4 | −0.707 | 0.48 |
| Melatonin | −4.800* | 5.020 | 1.500* | 3.391 | 3.5 | −2.100* | 0.036* |
| Cortisol | 27.560 | 65.062 | 122.050 | 133.064 | 6 | −0.98 | 0.327 |
*Statistically significant (p < 0.05). Melatonin (pg/mL); Cortisol (pg/mg). BDI-II, Beck Depression Inventory; TSC-40, Trauma Symptom Checklist-40); SD, standard deviation; MW, Mann–Whitney.
Figure 1.
Statistical differences between the intervention group (G1) and the control group (G2). The vertical axis describes the score differences between the groups at the end of the expedition.
Table 2.
Descriptive statistics for the intervention and control groups.
| Pre-intervention |
Post-intervention |
|||||||
|---|---|---|---|---|---|---|---|---|
| Intervention |
Control |
Intervention |
Control |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| BDI | 2.000 | 1.826 | 4.600 | 5.459 | 1.250 | 1.893 | 9.200 | 6.535 |
| TSC | 6.250 | 7.762 | 3.333 | 1.155 | 6.000 | 6.976 | 14.667 | 14.844 |
| Melatonin | 19.000 | 2.000 | 21.333 | 1.862 | 14.200 | 4.494 | 22.833 | 3.189 |
| Cortisol | 78.120 | 1.764 | 78.250 | 6.193 | 105.680 | 63.787 | 200.300 | 135.452 |
Melatonin (pg/mL); Cortisol (pg/mg). BDI-II, Beck Depression Inventory; TSC-40, Trauma Symptom Checklist-40); SD, standard deviation.
During the intervention, we did not observe any adverse effects or unpleasant feelings in participants.
Discussion
To our knowledge, this is the first study to assess the effects of a novel WHM program in healthy participants under long-term stress conditions. The present findings indicate that the 8-week WHM training program for healthy participants during an Antarctic expedition significantly reduced depressive symptoms in comparison with a control group. These findings are in accordance with those of other studies that indicate a positive effect of meditation techniques on stress response. For example, a recent meta-analysis demonstrated a lowering of cortisol levels after mindfulness practice. 20 Other studies have confirmed the association between meditation practices and reduced depressive and anxiety symptoms in various populations.21,22
The present findings also show that melatonin in saliva samples was significantly lower in the intervention group than in the control group. These results suggest that WHM can improve the adaptability of the hormonal system so that it can respond adequately to circadian rhythm changes related to nocturnal increases and daylight decreases in endogenous melatonin secretion. 23
These results support similar previous findings of an association between melatonin changes and some meditation practices.24–27 For example, one study found that initial levels of plasma melatonin were higher in a group of advanced meditators than in a control group, and melatonin tended to decrease after 3 hours of morning meditation. 27 Another study reported increased total melatonin levels in 12-hour overnight urine samples after 1 week of regular meditation in healthy women compared with a non-meditating group. 24 Other studies indicate improvement in cardiovascular and respiratory performance and well-being, together with changes in plasma melatonin secretion in healthy participants who practiced yoga and meditation for 3 months. 26 Additionally, enhanced endogenous secretion of melatonin has been suggested to underly the effect of meditation in patients with cancer. 28
Limitations
Limitations of this study are the small sample, which was limited by the number of participants on the Antarctic expedition. Participants were not randomly divided into groups but allocated according to their interest in WHM. Additionally, the groups contained unequal numbers of women and men. Some studies indicate that hair cortisol concentrations may be affected by confounding factors such as gender, hair washing frequency, hair treatment, some anthropometric measures, systolic blood pressure, and other factors.17,29 In addition, there is no recent evidence on the effects of the Wim Hof program on normal healthy individuals in non-stressful (or non-extreme) conditions. Some of the above-mentioned variables were not examined in this study owing to the small number of participants, which made it difficult to conduct more detailed analyses.
Conclusion
This study provides preliminary results indicating that a novel WHM psychophysiological training program significantly reduced depressive symptoms and positively affected adaptability of the hormonal system to respond adequately to circadian rhythm changes in healthy participants on an Antarctic expedition.
Acknowledgement
The authors thank Libor Mattus, a certified instructor of the Wim Hof Method for his enthusiastic guidance throughout the WHM training program.
Footnotes
Author contributions: T.P.T., Z.B. and P.B. designed the research; T.P.T. and Z.B. performed the research; T.P.T., Z.B., P.B. and Z.V. performed the data analysis; T.P.T., Z.B., P.B., D.N. and Z.V. wrote the paper; P.B., D.N. and J.R. reviewed and edited the paper.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: The study was supported by the Charles University projects (Progress Cooperation SVV). The 2019 Antarctic expedition was supported by the Large Infrastructure project LM2015078 of the Ministry of Education, Youth and Sports of the Czech Republic.
ORCID iDs: Tereza Petraskova Touskova https://orcid.org/0000-0001-9343-8850
References
- 1.Buijze G, De Jong H, Kox M, et al. An add-on training program involving breathing exercises, cold exposure, and meditation attenuates inflammation and disease activity in axial spondyloarthritis–A proof of concept trial. PloS One 2019; 14: e0225749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muzik O, Reilly KT, Diwadkar VA. “Brain over body”–A study on the willful regulation of autonomic function during cold exposure. NeuroImage 2018; 172: 632–641. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso R, De Souza E, Camano L, et al. Meditation in health: an operational definition. Brain Res Protoc 2004; 14: 58–60. [DOI] [PubMed] [Google Scholar]
- 4.Ospina MB, Bond K, Karkhaneh M, et al. Meditation practices for health: state of the research. Evid Rep Technol Assess (Full Rep) 2007; 1–263. [PMC free article] [PubMed] [Google Scholar]
- 5.Kox M, Stoffels M, Smeekens SP, et al. The influence of concentration/meditation on autonomic nervous system activity and the innate immune response: a case study. Psychosom Med 2012; 74: 489–494. [DOI] [PubMed] [Google Scholar]
- 6.Kox M, Van Eijk LT, Zwaag J, et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A 2014; 111: 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milan FA, Elsner RW, Rodahl K. Thermal and metabolic responses of men in the Antarctic to a standard cold stress. J Appl Physiol 1961; 16: 401–404. [DOI] [PubMed] [Google Scholar]
- 8.Carrère S, Evans GW, Stokols D. Winter-over stress: physiological and psychological adaptation to an Antarctic isolated and confined environment. In: Harrison AA, Clearwater YA, McKay CP. (eds) From Antarctica to outer space: life in isolation and confinement. New York: Springer-Verlag, 1991, pp.229–237. [Google Scholar]
- 9.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bulletin of the World Health Organization 2007; 85: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott DM, Briere J. Sexual abuse trauma among professional women: validating the Trauma Symptom Checklist-40 (TSC-40). Child Abuse Negl 1992; 16: 391–398. [DOI] [PubMed] [Google Scholar]
- 11.Gold SR, Milan LD, Mayall A, et al. A cross-validation study of the Trauma Symptom Checklist: the role of mediating variables. Journal of Interpersonal Violence 1994; 9: 12–26. [Google Scholar]
- 12.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull 1996; 52: 597–617. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn-Munro G, Blackburn-Munro R. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol 2001; 13: 1009–1023. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio 1996; 78: 490–498. [Google Scholar]
- 15.Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment 1998; 10: 83. [Google Scholar]
- 16.Ociskova M, Prasko J, Kupka M, et al . Psychometric evaluation of the Czech Beck Depression Inventory-II in a sample of depressed patients and healthy controls. Neuro Endocrinol Lett 2017; 38: 98–106. [PubMed] [Google Scholar]
- 17.Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 2017; 77: 261–274. [DOI] [PubMed] [Google Scholar]
- 18.Gow R, Thomson S, Rieder M, et al. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 2010; 196: 32–37. [DOI] [PubMed] [Google Scholar]
- 19.Russell E, Koren G, Rieder M, et al. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 2012; 37: 589–601. [DOI] [PubMed] [Google Scholar]
- 20.Pascoe MC, Thompson DR, Jenkins ZM, et al. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J Psychiatr Res 2017; 95: 156–178. [DOI] [PubMed] [Google Scholar]
- 21.Pascoe M, Crewther SG. A systematic review of randomised control trials examining the effects of mindfulness on stress and anxious symptomatology. In: Anxiety Disorders. USA: SM Group, 2017, pp.1–23.
- 22.Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A 2007; 104: 17152–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev 2005; 9: 41–50. [DOI] [PubMed] [Google Scholar]
- 24.Massion AO, Teas J, Hebert JR, et al. Meditation, melatonin and breast/prostate cancer: hypothesis and preliminary data. Med Hypotheses 1995; 44: 39–46. [DOI] [PubMed] [Google Scholar]
- 25.Tooley GA, Armstrong SM, Norman TR, et al. Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol 2000; 53: 69–78. [DOI] [PubMed] [Google Scholar]
- 26.Harinath K, Malhotra AS, Pal K, et al. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med 2004; 10: 261–268. [DOI] [PubMed] [Google Scholar]
- 27.Solberg EE, Holen A, Ekeberg Ø, et al. The effects of long meditation on plasma melatonin and blood serotonin. Med Sci Monit 2004; 10: CR96–CR101. [PubMed] [Google Scholar]
- 28.Srinivasan V, Spence D, Pandi-Perumal S, et al. Melatonin, environmental light, and breast cancer. Breast Cancer Res Treat 2008; 108: 339–350. [DOI] [PubMed] [Google Scholar]
- 29.Meyer JS, Novak MA. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012; 153: 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]