Abstract
Fragments (414 bp) of the gene-encoding ribosomal protein L16 from Enterococcus faecium and Enterococcus faecalis that were resistant and susceptible to the oligosaccharide antibiotics avilamycin and evernimicin (SCH 27899) were sequenced and compared. The susceptible E. faecalis and E. faecium isolates had sequences that were similar to those of the type strains. All resistant E. faecalis isolates contained the same base pair variation [CGT (Arg-56) → CAT (His-56)]. The same variation and two additional variations [ATC (Ile-52) → ACC (Thr-52) and ATC (Ile-52) → AGC (Ser-52)] were found in the resistant E. faecium isolates. This study indicated that resistance to the oligosaccharides in enterococci is associated with variations in the ribosomal protein L16.
Multiply resistant enterococci have emerged as increasingly important nosocomial pathogens during the last decade (10, 12, 13). This has increased the interest in searching for new antibiotics or modifications of older antibiotics with activity against multiply resistant staphylococci and enterococci. One of these agents is evernimicin (SCH 27899) (Ziracin), an oligosaccharide antimicrobial agent belonging to the everninomicins that has been developed by Schering-Plough. This compound has shown excellent activity against enterococci, staphylococci, and streptococci of human origin (5, 6, 8, 11, 14) but was recently suspended by the company from any further clinical development. The everninomicins have been known since the 1960s (15) but have not previously gained any clinical interest.
Another oligosaccharide, avilamycin, has been used as a growth promoter for food animals in the European Union for several years, and resistance to avilamycin has frequently been found among Enterococcus faecium isolates from broilers in Denmark (2). Cross-resistance between avilamycin and evernimicin has been detected among Enterococcus faecalis and E. faecium isolates (1).
The mode of action of avilamycin and evernimicin is not well elucidated. It has been suggested that avilamycin acts by binding to the 30S part of the ribosome and thereby inhibiting the protein synthesis (16). However, recently Adrian and Klugman reported that single base-pair mutations in ribosomal protein L16 giving rise to an amino acid substitution (Ile-52 → Ser-52 or Thr-52) resulted in decreased susceptibility to evernimicin in Streptococcus pneumoniae (P. V. Adrian and K. P. Klugman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C110, 1998). Furthermore, McNicholas et al. (7) showed that evernimicin binds the 50S subunit and that the binding sites for avilamycin and evernimicin overlap on the 50S subunit.
This study was conducted to assess the effects of variations in the L16 sequence of E. faecalis and E. faecium isolates on susceptibility to the oligosaccharide antimicrobial agents avilamycin and evernimicin (SCH27899).
Bacterial isolates.
The bacterial isolates chosen for sequence analysis of L16 are shown in Table 1. The isolates were chosen on the basis of their susceptibility or resistance to avilamycin. Eleven avilamycin-resistant and 4 susceptible isolates of E. faecalis and 11 resistant and 6 susceptible E. faecium isolates were chosen. All isolates originated from different broiler farms or pig herds and were collected from the continuous surveillance of antimicrobial resistance among food animals in Denmark between 1995 and 1998 (2). The following reference strains were included: E. faecalis ATCC 19433, E. faecalis ATCC 29212, and E. faecium CCUG542.
TABLE 1.
Origin, drug susceptibility, and observed mutations of Enterococcus isolates chosen for sequencing
| Species | Strain | Animal origin | Yr of isolation | MIC (μg/ml) of:
|
Nucleotide (amino acid) substitutiona | |
|---|---|---|---|---|---|---|
| Avilamycin | Evernimicin | |||||
| E. faecalis | ATCC 19433 | 0.5 | 0.25 | — | ||
| ATCC 29212 | Human | 0.5 | 0.19 | — | ||
| 96-30477-4 | Pig | 1996 | 1 | 0.25 | — | |
| 96-31228-1 | Pig | 1996 | 0.5 | 0.5 | — | |
| 98-30756-2 | Pig | 1998 | 1 | 0.38 | — | |
| 98-30993-2 | Pig | 1998 | 1 | 0.125 | — | |
| 96-30354-3 | Pig | 1996 | >64 | 4 | CGT (Arg-56) → CAT (His-56) | |
| 96-30972-3 | Pig | 1996 | 32 | 2 | CGT (Arg-56) → CAT (His-56) | |
| 96-31097-2 | Pig | 1996 | >64 | 3 | CGT (Arg-56) → CAT (His-56) | |
| 96-31439-4 | Pig | 1996 | >64 | 6 | CGT (Arg-56) → CAT (His-56) | |
| 97-30100-4 | Pig | 1997 | >64 | 8 | CGT (Arg-56) → CAT (His-56) | |
| 97-30356-3 | Pig | 1997 | >64 | 8 | CGT (Arg-56) → CAT (His-56) | |
| 97-30477-2 | Pig | 1997 | >64 | 2 | CGT (Arg-56) → CAT (His-56) | |
| 97-30616-1 | Pig | 1997 | >64 | 3 | CGT (Arg-56) → CAT (His-56) | |
| 97-30729-5 | Pig | 1997 | >64 | 8 | CGT (Arg-56) → CAT (His-56) | |
| 97-31152-4 | Pig | 1997 | >64 | 4 | CGT (Arg-56) → CAT (His-56) | |
| 98-30352-2 | Pig | 1998 | >64 | 4 | CGT (Arg-56) → CAT (His-56) | |
| E. faecium | CCUG542 | Human | 1 | 0.5 | — | |
| 95-08111 | Broiler | 1995 | 0.5 | 0.5 | — | |
| 98-31068-1 | Broiler | 1998 | 2 | 0.19 | — | |
| 98-30182-1 | Pig | 1998 | 1 | 0.75 | — | |
| 98-30309-1 | Broiler | 1998 | 1 | 0.19 | — | |
| 98-31131 | Broiler | 1998 | 0.5 | 0.38 | — | |
| 98-31134 | Broiler | 1998 | 0.5 | 0.125 | — | |
| 95-08170 | Broiler | 1995 | >64 | 3 | ATC (Ile-52) → AGC (Ser-52) | |
| 95-08182 | Broiler | 1995 | >64 | 6 | ATC (Ile-52) → ACC (Thr-52) | |
| 96-08080 | Broiler | 1996 | >64 | 4 | ATC (Ile-52) → ACC (Thr-52) | |
| 96-31456-1 | Broiler | 1996 | >64 | 3 | CGT (Arg-56) → CAT (His-56) | |
| 97-30342-1 | Broiler | 1997 | >64 | 3 | CGT (Arg-56) → CAT (His-56) | |
| 97-31173-1 | Broiler | 1997 | >64 | 6 | ATC (Ile-52) → ACC (Thr-52) | |
| 98-30070-1 | Broiler | 1998 | >64 | 8 | ATC (Ile-52) → ACC (Thr-52) | |
| 98-30223-1 | Broiler | 1998 | >64 | 8 | CGT (Arg-56) → CAT (His-56) | |
| 98-30327-1 | Broiler | 1998 | >64 | 4 | CGT (Arg-56) → CAT (His-56) | |
| 98-31106-1 | Broiler | 1998 | >64 | 6 | CGT (Arg-56) → CAT (His-56) | |
| 98-31132 | Broiler | 1998 | 64 | 3 | ATC (Ile-52) → ACC (Thr-52) | |
—, no mutation (strain is susceptible to both drugs).
Susceptibility testing.
Susceptibility to avilamycin was determined by culturing on Mueller-Hinton II agar plates containing twofold serial dilutions of antimicrobials (MIC determinations) at dilutions ranging from 0.25 to 128 μg/ml, according to NCCLS guidelines (9). The susceptibility to evernimicin (SCH27899) was determined by using the E-test according to the manufacturer's guidelines (AB Biodisk, Solna, Sweden).
PCR amplification and DNA sequencing.
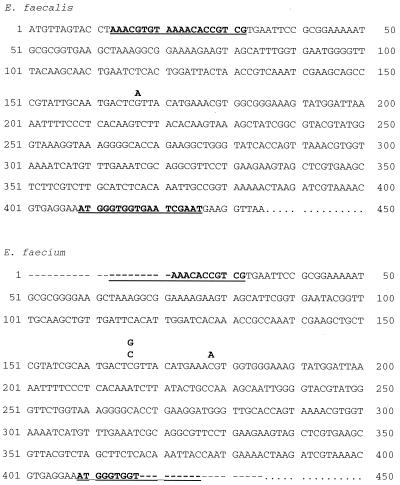
The sequence for L16 for Bacillus subtilis (accession number U43929) and S. pneumoniae (accession number AF126059) was retrieved from GenBank. The sequence from B. subtilis was used to search the database of The Institute for Genomic Research [TIGR] (http://www.tigr.org/cgi-bin/BlastSearch/blast.cgi?) for similar sequences for E. faecalis. Similarly, L16 sequences of Staphylococcus aureus, Streptococcus mutans, and Streptococcus pyogenes were also retrieved from the TIGR database. Based on a similar sequence of E. faecalis (V583), fragments (414 internal base pairs) of the L16 gene were amplified using the primers P1 (5′-AAACGTGTAAAACACCGTCG-3′) and P2 (5′CATTCGATTCACCACCCATT-3′) (Fig. 1). The amplification products were sequenced on an ABI 373A automatic sequencer using the AmpliTaq FS dye terminator kit (Applied Biosystems, Foster City, Calif.). The sequences were compared and analyzed using DNAsis software (Hitachi Software Engineering Co., Ltd.).
FIG. 1.
L16 DNA sequence of Enterococcus faecalis and Enterococcus faecium (CCUG 542). Position of primers are indicated (bold and underlined). The variations observed are also indicated.
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis of the 22 avilamycin-resistant E. faecalis and E. faecium isolates chosen for sequence analysis was performed as previously described (4).
Complete agreement between resistance and susceptibility to avilamycin and evernimicin (SCH27899) was found among the isolates. The MICs of avilamycin were between 0.5 and 2 μg/ml for the susceptible isolates and from 32 to >64 μg/ml for the resistant isolates (Table 1). For evernimicin (SCH27899) the MICs ranged from 0.125 to 0.75 μg/ml for the avilamycin-susceptible isolates and from 2 to 8 μg/ml for the avilamycin-resistant isolates. All oligosaccharide-susceptible E. faecalis isolates had a DNA sequence identical to the sequence retrieved from the TIGR database. The E. faecium type strain CCUG542 and the five oligosaccharide-susceptible E. faecium isolates all shared the same DNA sequence. This sequence showed 89% DNA homology and 95% amino acid homology to the sequence of E. faecalis.
Two different mutations associated with decreased susceptibility to evernimicin have previously been observed among S. pneumoniae isolates (Adrian and Klugman, 38th ICAAC). These mutations, ATC (Ile-52) → AGC (Ser-52) and ATC (Ile-52) → ACC (Thr-52), were detected among 1 and 5, respectively, of the 11 E. faecium isolates examined in this study (Table 1 and Fig. 1). In addition, another mutation [CGT (Arg-56) → CAT (His-56)] was detected in all the 11 resistant E. faecalis isolates and in the remaining 5 resistant E. faecium isolates.
All resistant E. faecium isolates were of different genotypes as determined by PFGE typing. This indicates that resistance has developed among several different clones. In contrast, all E. faecalis isolates belonged to the same indistinguishable SmaI PFGE type, even though all the isolates were from different herds and collected during a period of 3 years.
When the DNA sequence and translated amino acid sequence of the L16 gene were compared to those of B. subtilis, E. faecalis, S. aureus, S. pneumoniae, and S. pyogenes, it was observed that all three mutations were within an otherwise conserved 21-amino-acid area of L16. The observed conservation of this area in several bacterial species could indicate that this region is essential for the function of L16 in gram-positive bacteria. The conservation in amino acid sequence could perhaps indicate that oligosaccharide-resistant variants with changes in this region are less fit than the susceptible variants and that resistance thus will disappear over time.
Recently, a decrease in the occurrence of avilamycin-resistant E. faecium isolates has been detected along with a decreased consumption (3), indicating that resistance will decrease when the selective pressure is removed.
In conclusion, the observations in this study and the studies by Adrian and Klugman (38th ICAAC) and McNicholas et al. (7; P. M. McNicholas, P. A. Mann, D. J. Najarian, L. Miesel, R. S. Hare, K. J. Shaw, and T. A. Black. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-846, 1999) strongly suggest that oligosaccharide antimicrobial agents such as avilamycin and evernimicin (SCH27899) act by binding to ribosomal protein L16 and thereby probably interact with the peptidyltransferase activity.
Nucleotide sequence accession numbers.
The sequences obtained in this study have been deposited in GenBank under the accession numbers AF291861, AF291862, AF291863, AF291864, and AF291865.
Acknowledgments
We are grateful to René Hendriksen, Betina Elemark, Dorte Nielsen, and Christina Aaby Svendsen for technical assistance. The E-test was supplied by Schering-Plough Research Institute, Bloomfield, N.J.
This study was supported by a grant from Schering-Plough.
REFERENCES
- 1.Aarestrup F M. Association between decreased susceptibility to a new antibiotic for treatment of human diseases, evernimicin (SCH 27899), and resistance to an antibiotic used for growth promotion in animals, avilamycin. Microb Drug Resist. 1998;4:137–141. doi: 10.1089/mdr.1998.4.137. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M, Bager F, Madsen M, Jensen N E, Meyling A, Wegener H C. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS. 1998;106:606–622. doi: 10.1111/j.1699-0463.1998.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 3.Aarestrup F M, Bager F, Andersen J S. The association between the use of avilamycin for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs. Epidemiological study and changes over time. Microb Drug Resist. 2000;6:71–75. doi: 10.1089/mdr.2000.6.71. [DOI] [PubMed] [Google Scholar]
- 4.Jensen L B, Ahrens P, Dons L, Jones R N, Hammerum A M, Aarestrup F M. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J Clin Microbiol. 1998;36:437–442. doi: 10.1128/jcm.36.2.437-442.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones R N, Low D E, Pfaller M A. Epidemiologic trends in nosocomial and community-acquired infections due to antibiotic-resistant gram-positive bacteria: the role of streptogramins and other newer compounds. Diagn Microbiol Infect Dis. 1999;33:101–112. doi: 10.1016/s0732-8893(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S A, Jones R N, Erwin M E. Antimicrobial activity of SCH27899 (Ziracin), a novel everninomicin derivative, tested against Streptococcus spp.: disk diffusion/etest method evaluations and quality control guidelines. The Quality Control Study Group. Diagn Microbiol Infect Dis. 1999;33:19–25. doi: 10.1016/s0732-8893(98)00105-9. [DOI] [PubMed] [Google Scholar]
- 7.McNicholas P M, Najarian D J, Mann P A, Hesk D, Hare R S, Shaw K J, Black T A. Evernimicin binds exclusively to the 50S ribosomal subunit and inhibits translation in cell-free systems derived from both gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:1121–1126. doi: 10.1128/aac.44.5.1121-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashio S, Iwasawa H, Dun F Y, Kanemitsu K, Shimada J. Everninomicin, a new oligosaccharide antibiotic: its antimicrobial activity, post-antibiotic effect and synergistic bactericidal activity. Drags Exp Clin Res. 1995;21:7–16. [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Schaberg D R, Culver D H, Gayes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72–76. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 11.Schouten M A, Voss A, Hoogkamp-Korstanje J A. Antimicrobial susceptibility patterns of enterococci causing infections in Europe. Antimicrob Agents Chemother. 1999;43:2542–2546. doi: 10.1128/aac.43.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spera R V, Farber B F. Multidrug-resistant Enterococcus faecium. An untreatable nosocomial pathogen. Drugs. 1994;48:678–688. doi: 10.2165/00003495-199448050-00003. [DOI] [PubMed] [Google Scholar]
- 13.Swartz M N. Hospital-acquired infections: diseases with increasingly limited therapies. Proc Natl Acad Sci USA. 1994;91:2420–2427. doi: 10.1073/pnas.91.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban C, Mariano N, Mosinka-Snipas K, Wadee C, Chahrour T, Rahal J J. Comparative in-vitro activity of SCH 27899, a novel everninomicin, and vancomycin. J Antimicrob Chemother. 1996;37:361–364. doi: 10.1093/jac/37.2.361. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein M J, Wagman G H, Oden E M, Luedemann G M, Sloane P, Murawski A, Marquez J. Purification and biological studies of everninomicin B. Antimicrob Agents Chemother. 1965;34:821–827. [PubMed] [Google Scholar]
- 16.Wolf H. Avilamycin, an inhibitor of the 30 S ribosomal subunits function. FEBS Lett. 1973;36:181–186. doi: 10.1016/0014-5793(73)80364-3. [DOI] [PubMed] [Google Scholar]