Abstract
Background:
Adults with primary focal segmental glomerulosclerosis (FSGS) are frequently resistant to multiple immunosuppressive agents, which is associated with ongoing complications of nephrotic syndrome and a high risk of progression to end-stage renal disease (ESRD). Rituximab, a monoclonal antibody against the B cell CD20 antigen, has shown some preliminary evidence in treating nephrotic syndrome.
Objectives:
Describe the usage and the efficacy of rituximab for adults with FSGS in British Columbia (BC) (Canada) and perform a literature review of multi-immunosuppressive drug resistant FSGS in adult patients treated with rituximab to estimate the overall response rate.
Design:
Case series report and a literature review.
Patients:
For the case-series, all BC patients who received rituximab for a diagnosis of primary FSGS were included. The literature review included all cases of immunosuppressive-resistant FSGS patients treated with rituximab. We excluded transplant and pediatric patients in both groups.
Methods:
We describe all cases of adults with native-kidney FSGS resistant to conventional immunosuppressive medications from our provincial health database who were treated with rituximab from 2014 to 2018. A review of the existing literature was performed via PubMed, MEDLINE, and Embase using the following keywords: rituximab, focal segmental glomerulosclerosis, and FSGS up to August 2019.
Results:
We characterize four immunosuppressive-resistant FSGS patients who were treated with rituximab as part of our provincial program, all of whom showed a response to rituximab with a sustained remission. We found 29 specific cases in the literature of adults with native-kidney FSGS treated with rituximab after being resistant to other immunosuppressive medications, of whom 15 cases showed a response to rituximab. This has increased the total response rate from 15/29 (52%) to 19/33 (58%).
Limitations:
Literature on this topic is coming predominantly from case series. Prospective trials are needed to confirm efficacy, tolerability, and duration of remission.
Conclusions:
Due to the low number of currently reported cases and variable response rates, these four cases provide critical data to generate a more accurate understanding of the role of rituximab in adults with resistant FSGS. Adding these results to the confirmed literature cases of multiple-immunosuppressive-resistant FSGS patients treated with rituximab results in a total remission rate of 19/33 cases.
Keywords: focal segmental glomerular disease, FSGS, glomerulonephritis, immunosuppressant resistant, rituximab
Abrégé
Contexte:
Il arrive fréquemment que les adultes atteints d’une glomérulosclérose segmentaire focale primaire (focal segmental glomerulosclerosis—FSGS) soient résistants à de multiples agents immunosuppresseurs. Cette résistance est associée à des complications du syndrome néphrotique et à un risque élevé de progression vers l’insuffisance rénale terminale (IRT). Le rituximab, un anticorps monoclonal contre l’antigène CD20 des lymphocytes B, a montré quelques résultats préliminaires prometteurs pour le traitement du syndrome néphrotique.
Objectifs:
Décrire l’utilisation du rituximab et son efficacité chez les adultes atteints de FSGS en Colombie-Britannique (C.-B.) (Canada) et estimer le taux de réponse global par le biais d’une revue de la littérature portant sur les cas de FSGS résistante à plusieurs agents immunosuppresseurs chez des patients adultes traités par rituximab.
Conception de l’étude:
Rapport de série de cas et revue de la littérature.
Sujets:
Ont été inclus pour la série de cas tous les patients britanno-colombiens qui recevaient du rituximab pour un diagnostic de FSGS primaire. La revue de la littérature a porté sur tous les cas de patients atteints de FSGS résistante aux immunosuppresseurs et traités par rituximab. Les patients transplantés et les enfants ont été exclus des deux groupes.
Méthodologie:
Nous décrivons tous les cas répertoriés d’adultes atteints d’une FSGS résistante aux immunosuppresseurs conventionnels dans un rein natif et traités par rituximab dans la base de données de santé provinciale entre 2014 et 2018. Une revue de la littérature existante a été réalisée dans PubMed, Medline et Embase en utilisant les mots-clés suivants: Rituximab, focal segmental glomerulosclerosis (glomérulosclérose segmentaire focale) et FSGS jusqu’en août 2019.
Résultats:
Nous caractérisons les cas de quatre patients atteints de FSGS résistante aux immunosuppresseurs et traités par rituximab dans le cadre de notre program provincial; tous ont répondu au rituximab avec une rémission durable. Dans la littérature, nous avons répertorié 29 cas particuliers d’adultes atteints d’une FSGS de rein natif ayant été traités par rituximab après avoir été résistants à d’autres médicaments immunosuppresseurs. De ces 29 cas, 15 ont répondu au rituximab. Le taux de réponse total est ainsi passé de 52 % (15/29) à 58 % (19/33).
Limites:
La littérature sur ce sujet provient principalement de séries de cas. Des essais prospectifs sont nécessaires pour confirmer l’efficacité, la tolérance et la durée de la rémission.
Conclusion:
Compte tenu du faible nombre de cas actuellement signalés et des taux de réponse variables, ces quatre cas fournissent des données cruciales qui permettent de mieux comprendre le rôle du rituximab chez les adultes atteints de FSGS résistante aux agents immunosuppresseurs. L’ajout de ces résultats confirmés dans la littérature aux cas de patients atteints de FSGS résistante à plusieurs immunosuppresseurs traités par rituximab a entraîné un taux de rémission total de 19 cas sur 33.
Introduction
Focal segmental glomerulosclerosis (FSGS) occurs when the kidney develops segmental glomerular scarring. 1 Primary FSGS has a low rate of spontaneous remission and adults with primary FSGS are frequently resistant to multiple immunosuppressive agents. 2 Left untreated, FSGS is associated with ongoing complications of nephrotic syndrome and a 50% risk of disease progression to end-stage renal disease (ESRD) in 6 to 8 years. 3 Conventional immunosuppressive treatment options such as corticosteroids, cyclophosphamide, mycophenolate mofetil (MMF), and calcineurin inhibitors have variable efficacy as well as significant long-term side-effects. 4
Rituximab, a monoclonal antibody against the B cell CD20 antigen, has shown some preliminary evidence in treating nephrotic syndrome. 5 However, much of the data on rituximab for FSGS treatment has been in the pediatric population.6,7 Given the substantial cost of rituximab compared to other treatment options, it is likely to remain a treatment of last resort for FSGS. 8 As such, we described the usage and the efficacy of rituximab for adults with FSGS in the province of British Columbia (Canada). We conducted a comprehensive literature review of multi-immunosuppressive drug-resistant FSGS in adult patients treated with rituximab to estimate an overall response rate.
Methods
In British Columbia, the provincial health database captures all patients with biopsy-proven glomerular disease and their associated immunosuppressive treatment. Rituximab is covered by the provincial government for FSGS resistant to other treatment options. We therefore sought to use this data infrastructure to describe all cases of adults (≥19 years) with native-kidney FSGS resistant to conventional immunosuppressive medications who were treated with rituximab from 2014 to 2018. Individual patients’ charts were accessed for demographic data, as well as patient’s complete medical and pharmacological history.
A detailed literature search for cases of immunosuppressive-resistant FSGS treated with rituximab up to August 2019 was performed via PubMed, MEDLINE, and Embase using the following keywords: rituximab, focal segmental glomerulosclerosis, and FSGS. Data on renal transplant patients and pediatric patients (age <19 years) were excluded, and only results for patients with primary FSGS resistant to at least 1 non-steroid immunosuppressive agent were included. For steroid-sensitive FSGS, only cases that were previously shown to be resistant to immunosuppressive agents other than rituximab where included.
As per KDIGO guidelines, complete remission (CR) for patients with primary FSGS is defined as a proteinuria <0.3 g/d (PCR < 30 mg/mmol or ACR < 30 mg/mmol). 9 Partial remission (PR) is defined as a 50% reduction in proteinuria to a proteinuria level less than 3.5 g/d (PCR < 350 mg/mmol or ACR < 350 mg/mmol). 9 For the purpose of this review, we defined a response to rituximab by (1) achieving CR or PR in patients with nephrotic range proteinuria at the time of rituximab treatment, or (2) maintaining CR/PR after tapering off corticosteroids in those patients with steroid-sensitive disease that were in remission on steroids at the time of rituximab treatment.
The dosage and regimen of rituximab, previous therapies, and markers of treatment response are summarized in Table 1.
Table 1.
A Description of Individual Adult Patients From the Existing Literature With Resistant FSGS Treated With Rituximab and Their Associated Outcome.
| Reference | Sex | Ethnicity | FSGS Histological Subtype | Age a (Years) | Previous Therapies | Duration of Disease (years) |
Steroid-Sensitive | RTX Dosage | Proteinuria Prior to RTX | Proteinuria After RTX | SCr Prior to RTX (umol/L) | SCr After RTX (umol/L) | Albumin Prior to RTX (g/L) | Albumin After RTX (g/L) | Follow-up Time (Months) | Status at Last Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fernandez-Fresnedo et al. 5 | M | N/A | NOS | 19 | CST, TAC, MMF | 2 | No | 375 mg/m2 weekly × 4 | 16g/d | 14 g/d | 106.1 | 168.0 | — | — | 12 | No response |
| Fernandez-Fresnedo et al. 5 | M | N/A | Tip | 29 | CST, CYC, CsA, MMF | 3 | No | 375 mg/m2 weekly × 4 | 16.5g/d | 21 g/d | 79.6 | 70.7 | — | — | 12 | No response |
| Fernandez-Fresnedo et al. 5 | M | N/A | NOS | 34 | CST, CsA, TAC, MMF | 3 | No | 375 mg/m2 weekly × 4 | 9.4g/d | 11.3 g/d | 123.8 | 123.8 | — | — | 14 | No response |
| Fernandez-Fresnedo et al. 5 | M | N/A | NOS | 19 | CST, CsA, MMF | 1.1 | No | 375 mg/m2 weekly × 4 | 12g/d | 14 g/d | 79.6 | 97.2 | — | — | 18 | No response |
| Fernandez-Fresnedo et al. 5 | M | N/A | NOS | 55 | CST, CsA | 2 | No | 375 mg/m2 weekly × 4 | 9.8g/d | 10.5 g/d | 79.6 | 70.7 | - | - | 24 | No response |
| Fernandez-Fresnedo et al. 5 | M | N/A | Cellular variant | 51 | CST, CsA, MMF | 8.9 | No | 375 mg/m2 weekly × 4 | 12.7g/d | 9.0 g/d | 203.3 | 221 | — | — | 24 | No response. |
| Fernandez-Fresnedo et al. 5 | M | N/A | NOS | 20 | CST, CsA, TAC, MMF | 2.5 | No | 375 mg/m2 weekly × 4 | 12.9g/d | 3.2 g/d | 150.2 | 141.4 | — | — | 12 | Response PR with tapering of CST, TAC, and MMF. |
| Fernandez-Fresnedo et al. 5 | F | N/A | NOS | 23 | CST, CYC, CsA, MMF | 7.9 | No | 375 mg/m2 weekly × 8 | 23g/d | 3.9 g/d | 168.0 | 141.4 | — | — | 15 | No response |
| Ochi et al. 10 | F | N/A | Collapsing | 25 | CST, CsA, Mizoribine | 12 | No | 375 mg/m2 single dose | 6.5g/d | 6-8 g/d | 114.9 | — | — | — | — | No response |
| Ochi et al. 10 | M | N/A | NOS | 21 | CST, CsA, CYC, MMF | 9 | No | 375 mg/m2 single dose | 13g/d | 15-20 g/d | 185.6 | — | — | — | — | No response |
| Ochi et al. 10 | F | N/A | Perihilar | 27 | CST, CsA | 18 | Yes | 375 mg/m2 × 4 doses | 9.8g/d | 0-1 g/d | 44.2 | — | — | — | — | Response CR not on other IS agents |
| Ochi et al. 10 | F | N/A | Tip | 26 | CST, CsA, CYC, MMF | 19 | Yes | 375 mg/m2 × 3 doses | 3.6g/d | 0-1 g/d | 35.4 | — | — | — | — | Response CR not on other IS agents |
| Marasà et al. 11 | F | White | NOS | 22 | CST, CYC, Azathioprine | 1 | No | 375 mg/m2 and a second dose 32 months after | 4g/d | 0.27 g/d | 71.6 | 48.6 | 26.8 | 38 | 48 | Response CR and off other IS agents. |
| Kisner et al. 12 | M | N/A | N/A | 51 | CST, CsA | 0.6 | No | 1000 mg single dose | 1105.9 mg/mmol | 441.3 mg/mmol | 136.1 | — | — | — | 8 | No response |
| Kisner et al. 12 | M | N/A | N/A | 50 | CST, CsA, TAC, MMF | 2 | Yes | 1000 mg × 2 doses | 485.6 mg/mmol | 185.43 mg/mmol | 110.5 | — | — | — | 22 | Response PR maintained with CST, MMF, TAC. |
| Peters et al. 13 | M | N/A | N/A | 20 | CSA, CST, MMF, TAC | 8 | Yes | 1000 mg × 2 doses with 2 week interval between doses | 1g/d | “Nephrotic range proteinuria persisted” | 170 | “Renal function rapidly deteriorated” | — | — | 7 | No response |
| Cortazar et al. 14 | M | N/A | N/A | 56 | CST, CsA, TAC, MMF | N/A | Yes | Initially 1000 mg × 2 doses every 2-4 weeks and then every 4 months after | 463 mg/mmol | <113 mg/mmol | 97.2 | 88-132 | 32 | 40-50 | 83 | Response PR |
| Cortazar et al. 14 | M | N/A | N/A | 67 | CST, MMF | N/A | Yes | Initially 1000mg × 2 doses every 2-4 weeks and then every 4 months after | 441 mg/mmol | 226-452 mg/mmol | 114.9 | 111-133 | — | 30-40 | 70 | No response Based on proteinuria, however prednisone was tapered off. |
| Cortazar et al. 14 | F | N/A | N/A | 67 | CST, CsA | N/A | Yes | Initially 1000 mg × 2 doses every 2-4 weeks and then every 4 months after | 655 mg/mmol | 0 mg/mmol | 106 | 66-88 | 32 | 40-50 | 31 | Response CR |
| Cortazar et al. 14 | F | N/A | N/A | 54 | CST, CsA, TAC, MMF, Abatacept | N/A | Yes | Initially 1000 mg × 2 doses every 2-4 weeks and then every 4 months after | 554 mg/mmol | 0-113 mg/mmol | 70.7 | 44-88 | 22 | 37.5-40 | 13 | Response PR |
| Cortazar et al. 14 | F | N/A | N/A | 65 | CST, CsA, MMF | N/A | No | Initially 1000 mg × 2 doses every 2-4 weeks and then every 4 months after | 1559 mg/mmol | 226-339 mg/mmol | 144.4 | 155-177 | 23 | 30-40 | 29 | Response PR |
| Cortazar et al. 14 | F | N/A | N/A | 66 | CST, CYC, MMF | N/A | No | Initially 1000 mg × 2 doses every 2-4 weeks and then every 4 months after | 904 mg/mmol | 226-339 mg/mmol | 114.9 | 44-88 | 31 | 37.5-40 | 24 | Response PR |
| Wee Leng et al. 15 | M | N/A | NOS | 21 | CST, CYC, CsA, MMF | 3 | Yes | 500 mg × 4 doses 1 dose weekly, then 500mg × 6 doses 1 dose weekly | 1200 mg/mmol | 0-250 mg/mmol | 100-150 | 0-100 | 19 | — | — | Response PR |
| Jayaraman and Thomas 16 | M | White | N/A | 62 | CST, CsA, CYC | 2 | No | 375 mg/m2 × 4 doses total | 200-400 mg/mmol | 800-900 mg/mmol | 100-200 | 100-200 | 25-30 | 15-20 | 21 | No response |
| Ramachandran et al. 17 | M | N/A | Collapsing | 19 | CST, CYC, CsA, MMF, TAC | 1.5 | No | 375 mg/m2 × 4 doses 1 dose weekly | 5g/d | 1.2 g/d | 62-80 | 80 | <20 | 47 | 18 | Response PR |
| Kronbichler et al. 18 | F | N/A | N/A | 33 | CST, CsA, CYC, MMF, TAC | 29 | Yes | 375 mg/m2 × 4 doses 1 dose weekly, and then 23 months later another 4 doses 1 weekly | 689 mg/mmol | 4.6 mg/mmol | 67.2 | 68.9 | — | 48.5 | 55 | Response CR and off other IS agents. |
| Kronbichler et al. 18 | M | N/A | N/A | 32 | CST, CsA, MMF | 14 | Yes | 375 mg/m2 × 4 doses 1 dose weekly | 847.5 mg/mmol | 9.6 mg/mmol | 56.6 | 50.3 | 15.9 | 68 | 14 | Response CR and off other IS agents. |
| Kronbichler et al. 18 | M | N/A | N/A | 26 | CST, CsA, CYC, MMF | 2.6 | Yes | 375 mg/m2 × 4 doses 1 dose weekly | Not detectable | 6.4 mg/mmol | 112.3 | 79.6 | 38 | 40 | 29 | Response CR and off other IS agents. |
| Butterly et al. 19 | M | N/A | N/A | 32 | CST, MMF | N/A | N/A | 1400 mg | 1600 mg/mmol | 465 mg/mmol | — | — | <15 | 30 | 4 | No response |
| Barbour et al. 8 | F | Asian | NOS | 41 | CST, MMF, TAC, Galactose, CsA | 32 | No | 375 mg/m2 weekly × 4 doses and single 375mg/m2 with CD20 reconstitution | 4.95g/d | 2.1 mg/mmol (ACR) |
71 | 81 | 23 | 42 | 47 | Response CR and off other IS agents. |
| Barbour et al. 8 | M | White | NOS | 21 | CST, MMF, TAC, CsA, CYC, Infliximab, Galactose | 8 | No | 375 mg/m2 weekly × 4 doses and single 375mg/m2 with CD20 reconstitution | 8.69g/d | 55 mg/mmol (ACR) |
168 | 270 | 30 | 36 | 55 | Response PR and off other IS agents. |
| Barbour et al. 8 | F | White | NOS | 26 | CST, CYC, CsA, TAC | 12 | Yes | 1 g every 2 weeks for 2 doses and single 1g dose with CD20 reconstitution | 0.38g/d | 0.79 g/d | 63 | 70 | 35 | 38 | 30 | Response PR off CST and with tapering TAC. |
| Barbour et al. 8 | M | Asian | NOS | 19 | CST, Chlorambucil, Levamisole, CYC, MMF, TAC | 17 | No | 375 mg/m2 weekly × 4 doses and single 375 mg/m2 with CD20 reconstitution |
8.30g/d | 0.66 g/d | 233 | 91 | 12 | 43 | 40 | Response PR off CST and other IS agents. |
Note. FSGS = focal segmental glomerulosclerosis; RTX = rituximab; SCr = serum creatinine; N/A = not available; NOS = not otherwise specified; CST = corticosteroids; TAC = tacrolimus; MMF = mycophenolate mofetil; CYC = cyclophosphamide; CsA = cyclosporine.
Age at RTX Administration.
Ethics approval, including a waiver of individual patient consent, was granted from the University of British Columbia Research and Ethics Board.
Results
Case Reports
Of the 121 patients in British Columbia who received rituximab for the treatment of glomerulonephritis (GN) from January 2014 to April 2018, 4 were adults with primary FSGS in the native kidney. Clinical and laboratory data are summarized in Table 1.
Patient 1 is a 41-year-old female who had previously failed combination therapy with prednisone, MMF, tacrolimus, and galactose. Her creatinine was 71 µmol/L, albumin 23 g/L, and proteinuria 4.95 g/d while on therapeutic tacrolimus level and MMF. She was treated with rituximab 375 mg/m2 weekly for 4 doses followed by single 375 mg/m2 doses with CD20 reconstitution (approximately every 7 months). She achieved CR 3 months after her first rituximab dose. One year after the initiation of rituximab she was tapered off all other immunosuppressive agents and remained in CR. Two years after her first rituximab dose, we started tapering her rituximab dosage yearly. Fifty-seven months after her first rituximab dose, her serum creatinine was 81 µmol/L, albumin 42 g/L, and an ACR of 2.1 mg/mmol consistent with a CR.
Patient 2 is a 21-year-old male, who had previously failed tacrolimus, cyclosporine, and was on prednisone and MMF. His serum creatinine was 168 µmol/L, albumin 30 g/L, and proteinuria 8.69 g/d. He was treated with rituximab 375 mg/m2 weekly for 4 doses followed by single 375 mg/m2 doses with CD20 reconstitution (approximately every 6 months). He achieved PR 3 months after his first rituximab dose. Seventeen months after the initiation of rituximab, he was tapered off all other immunosuppressive and remained in partial remission. Thirty-three months after his first rituximab dose, we began tapering his rituximab dosage yearly. Fifty-five months after is first rituximab dose, his serum creatinine was 270 µmol/L, albumin 36 g/L, and an ACR of 55 mg/mmol consistent with a PR. Unfortunately, his chronic kidney disease continued to progress likely on the basis of sclerosis.
Patient 3 is a 26-year-old female who was steroid-sensitive, but unable to taper off prednisone without a disease flare despite concurrent use of cyclophosphamide, cyclosporine, or tacrolimus. At the time of being treated with rituximab 1 g every 2 weeks for 2 doses, she was on tacrolimus 3 mg BID and prednisone 20 mg/d, with serum creatinine 63 µmol/L, albumin 35 g/L, and proteinuria 0.38 g/d. She achieved PR 2 months after her first rituximab dose. Following the two rituximab doses, she received rituximab 1 g with CD 20 reconstitution (approximately every 8 months). Thirty months after rituximab treatment, she was off all immunosuppression. At that time, her serum creatinine was 70 µmol/L, albumin 39 g/L, and proteinuria 0.79 g/d consistent with a PR.
Patients 4 is a 19-year-old male. He was diagnosed at 2 years of age with biopsy proven minimal change disease. He was steroid-sensitive, but unable to taper off prednisone despite being treated with chlorambucil, levamisole, cyclophosphamide, tacrolimus, and MMF. At 17 years of age, he received 1 course of rituximab 375 mg/m2 weekly for 4 doses and remained relapse-free on no other immunosuppressive agent for 24 months. Two years later, he had a relapse with significant nephrotic syndrome and creatinine 233 µmol/L, albumin 12 g/L, and proteinuria 8.3 g/d. A repeat biopsy showed FSGS. He was on no immunosuppressive therapy at this point. He was treated with rituximab 375 mg/m2 weekly for 4 doses followed by single 375 mg/m2 doses with CD20 reconstitution (approximately every 11 months). He achieved PR 1 month after first rituximab dose. Twenty-three months after his first rituximab dose, we began tapering his rituximab dosage yearly. Forty months after his first rituximab dose, his serum creatinine was 91 µmol/L, albumin 43 g/L, and proteinuria of 0.66 g/day consistent with a PR.
Literature Search Results
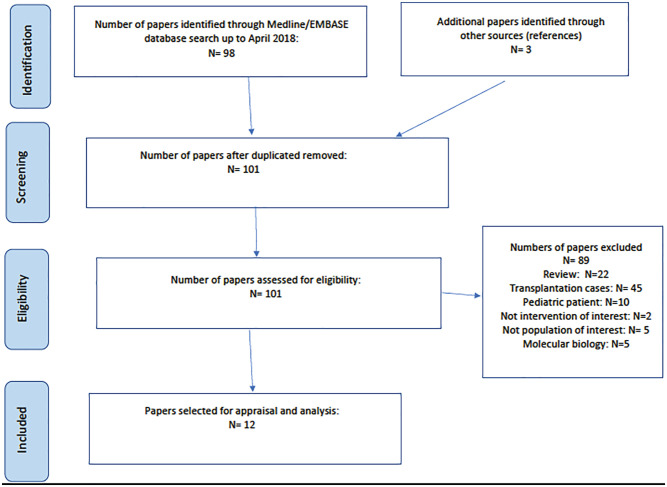
Figure 1 shows our literature strategy. Patients from 11 studies met our inclusion criteria, including a total of 29 patients with FSGS (Table 1).5,10-19 As per our definition, 15 of these cases showed a response to rituximab
Figure 1.
Flow diagram for the literature review.
The four new cases of immunosuppressive-resistant FSGS that were treated with rituximab and presented in this report increases the rate of response to this treatment from 15/29 (52%) to 19/33 (58%). From the total of the literature review and our 4 cases, of the 19 patients who showed response to treatment at the last follow-up time point, 8/19 (42%) were in complete remission and 11/19 (58%) were in partial remission.
Of the 31 patients with nephrotic range proteinuria at time of rituximab treatment, 17/31 (55%) responded to treatment; of which 7/17 (41%) achieved CR and 10/17 (59%) achieved PR. Of the 2 patients who were in remission (partial and complete) on steroids with or without other immunosuppressive agents, after rituximab both patients were able to maintain remission while tapering off steroids. Of the 2 patients, 1 patient was in complete remission and 1 was in partial remission.
Discussion
We performed a literature review of adult patients with FSGS resistant to multiple immunosuppressive agents to generate a more accurate assessment of the response rate to rituximab. Due to the low number of reported FSGS adult cases treated with rituximab to date, the 4 cases presented in this report provide meaningful data to support the role of rituximab in adults with multiple immunosuppressive-resistant FSGS. When our results were combined with the existing literature, the total response rate rises up to 58% (19/33) from originally 52% (15/29). We acknowledge that the literature to date for rituximab in adult FSGS patients is predominantly case series. This underscores the need for prospective clinical trials to better determine the efficacy, tolerability, and duration of remission of rituximab in FSGS.
There is evidence suggesting that rituximab induce remission in FSGS patients by two pathways. The first one is that CD20 positive B cells are involved in the immunopathogenesis of FSGS by the production of the circulating permeability factors, which disrupt podocyte integrity. This is thought to be the main cause of primary FSGS. 20 The second pathway is that rituximab reduces exposure to B-cell-induced local interleukine-4, which cause foot process effacement and proteinuria. 20 Our results demonstrate the potential efficacy of rituximab in both steroid resistant and steroid dependent FSGS. Patients 1, 2, and 4 were resistant to prednisone in combination with multiple other immunosuppressive agents, with persistent nephrotic range proteinuria and hypoalbuminemia. Rituximab resulted in proteinuria remission (complete remission in patient 1, partial remission in patient 2 and 4) that was sustained despite discontinuing all other immunosuppressive agents. Patient 3 had steroid-sensitive disease, but was not able to taper off prednisone despite concurrent use of other immunosuppressive agents. After rituximab, patient 3 was able to maintain proteinuric remission even after discontinuing prednisone and tapering of other immunosuppressive medications. By reporting all cases of adults with FSGS treated with rituximab in our province, we reduced the risk of bias from selective reporting of individual cases.
Currently, many adult FSGS patients either fail therapy with conventional immunosuppressive agents or only achieve proteinuria remission that is dependent upon the ongoing use of these medications.5,10,12,16-18 Long-term use of corticosteroids, cyclophosphamide, MMF, or calcineurin inhibitors is known to have multisystem side-effects. Some studies have looked at the long-term safety profile of rituximab in the rheumatoid arthritis population, the granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA) populations or systemic inflammatory and autoimmune disease population for up to 11 years.21-24 Results of these studies are quite consistent with an incidence ratio of infection of 3.76 to 7.1 per 100 patient-years, and malignancy rate similar to the one seen in general population.21,22,25 We propose that rituximab can be safely considered when other immunosuppressive options have been trialed and patients continue to have nephrotic range proteinuria or in patients who may have achieved proteinuric remission yet continue to rely on immunosuppressive agents with known long-term risks.
To compare rituximab long-term side-effects to side-effects of previous immunosuppressive agents, one consideration is its efficacy and need for ongoing treatment. The dosing regimen of rituximab used at our center was largely based on dosing reported in previous studies.17,18,26 However, the dose which is commonly used in the adult literature was derived from doses used in refractory non-Hodgkin’s lymphoma. 26 To date, there has not been a study to determine the most efficacious dose and regimen in the adult FSGS population. A previous study of steroid-resistant FSGS cases has shown that there was an association between patients who received more doses of rituximab and a greater reduction of proteinuria. 5
From the available literature and based on our center’s experience, most patients received four 375 mg/m2 once-weekly doses of rituximab with stable remission status as far as several months from the time of rituximab administration. Additional single rituximab doses were given as infrequently as every 6 to 11 months thereafter as needed to maintain both CD20 depletion and proteinuria remission.5,10-19
One factor that may contribute to which patients are able to maintain remission after rituximab therapy is the rate of CD20 reconstitution. 22 Rituximab can reduce CD20-positive B cells in a single dose, selectively targeting the B cell population and potentially other immune cell populations affected by costimulatory pathways; however, the correlation between reconstitution of CD20-positive cells and FSGS relapse needs to be further studied. 27 At our institution, we used CD20 as the main marker to determine maintenance dosing because CD20 levels are early indicators of relapse in minimal change disease. 28 However, in the pediatric population despite there being an association between CD20 reconstitution with disease relapse, not all patients who had recovery of CD20 levels had worsening disease. 29 Since there are still individual differences in response to rituximab not associated with CD20 reconstitution, 29 the immunopathogenesis and response to rituximab in FSGS disease variants need to be further explored in future studies.
Although the optimal long-term rituximab maintenance therapy for particularly intractable cases is currently unknown, there is also potential for combined treatment using rituximab with other immunosuppressive medications.30-33 Two pediatric studies have shown that MMF therapy when given post-rituximab may help maintain remission.30,31 Other studies have previously shown that tacrolimus and rituximab efficacy in nephrotic syndrome increases when used in combination therapy with other agents such as MMF.32,33 Therefore, although we present cases of FSGS resistant to multiple immunosuppressive agents that have responded to prolonged rituximab treatment, the optimal duration and type of maintenance therapy will require ongoing study and development.
Conclusion
In conclusion, the preliminary results gained from this study support a role for rituximab in adults with FSGS when conventional therapies have failed. Moving forward, further trials are needed to confirm our findings in a larger number of patients, to identify patient characteristics that can predict response to treatment, and to determine the optimal rituximab dosing regimen in this population.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval, including a waiver of individual patient consent, was granted from the University of British Columbia Research and Ethics Board.
Consent for Publication: All authors provided consent for publication.
Availability of Data and Materials: Data generated and analyzed during this study are included in this published article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Judith G. Marin  https://orcid.org/0000-0001-9317-8809
https://orcid.org/0000-0001-9317-8809
References
- 1. De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29(3):759-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gbadegesin R, Lavin P, Foreman J, Winn M. Pathogenesis and therapy of focal segmental glomerulosclerosis: an update. Pediatr Nephrol. 2011;26(7):1001-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rydel JJ, Korbet SM, Borok RZ, Schwartz MM. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis. 1995;25(4):534-542. [DOI] [PubMed] [Google Scholar]
- 4. Beer A, Mayer G, Kronbichler A. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. Biomed Res Int. 2016;2016:4192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez-Fresnedo G, Segarra A, González E, et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4(8):1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prytuła A, Iijima K, Kamei K, et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol. 2010;25:461-468. [DOI] [PubMed] [Google Scholar]
- 7. Hoseini R, Sabzian K, Otukesh H, et al. Efficacy and safety of rituximab in children with steroid- and cyclosporine-resistant and steroid- and cyclosporine-dependent nephrotic syndrome. Iran J Kidney Dis. 2018;12(1):27-32. [PubMed] [Google Scholar]
- 8. Barbour S, Lo C, Espino-Hernandez G, Gill J, Levin A. The BC glomerulonephritis network: improving access and reducing the cost of immunosuppressive treatments for glomerular diseases. Can J Kidney Health Dis. 2018;5. doi: 10.1177/2054358118759551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cattran DC, Feehally J, Cook HT, et al. Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2(2):139-274. [Google Scholar]
- 10. Ochi A, Takei T, Nakayama K, et al. Rituximab treatment for adult patients with focal segmental glomerulosclerosis. Intern Med. 2012;51:759-762. [DOI] [PubMed] [Google Scholar]
- 11. Marasà M, Cravedi P, Ruggiero B, et al. Refractory focal segmental glomerulosclerosis in the adult: complete and sustained remissions of two episodes of nephrotic syndrome after a single dose of rituximab. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-205507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kisner T, Burst V, Teschner S, Benzing T, Kurschat CE. Rituximab treatment for adults with refractory nephrotic syndrome: a single-center experience and review of the literature. Nephron Clin Pract. 2012;120(2):c79-85. [DOI] [PubMed] [Google Scholar]
- 13. Peters HPE, van de Kar NCAJ, Wetzels JFM. Rituximab in minimal change nephropathy and focal segmental glomerulosclerosis: report of four cases and review of the literature. Neth J Med. 2008;66:408-415. [PubMed] [Google Scholar]
- 14. Cortazar FB, Rosenthal J, Laliberte K, Niles JL. Continuous B-cell depletion in frequently relapsing, steroid-dependent and steroid-resistant nephrotic syndrome. Clin Kidney J. 2019;12(2):224-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wee Leng G, Mustafar R, Kamaruzaman L, et al. Intravenous rituximab in severe refractory primary focal segmental glomerulosclerosis. Acta Med Indones. 2018;50(3):237-243. [PubMed] [Google Scholar]
- 16. Jayaraman VK, Thomas M. Abatacept experience in steroid and rituximab-resistant focal segmental glomerulosclerosis. BMJ Case Rep. 2016. 2016. doi: 10.1136/bcr-2016-214396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramachandran R, Rajakumar V, Duseja R, Sakhuja V, Jha V. Successful treatment of adult-onset collapsing focal segmental glomerulosclerosis with rituximab. Clin Kidney J. 2013;6(5):500-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kronbichler A, König P, Busch M, Wolf G, Mayer G, Rudnicki M. Rituximab in adult patients with multi-relapsing/steroid-dependent minimal change disease and focal segmental glomerulosclerosis: a report of 5 cases. Wien Klin Wochenschr. 2013;125(11-12):328-333. [DOI] [PubMed] [Google Scholar]
- 19. Butterly SJ, Pillans P, Horn B, Miles R, Sturtevant J. Off-label use of rituximab in a tertiary Queensland hospital. Intern Med J. 2010;40(6):443-452. [DOI] [PubMed] [Google Scholar]
- 20. Gauckler P, Shin JI, Alberici F, et al. Rituximab in adult minimal change disease and focal segmental glomerulonephritis- what is known and what is still unknown? Autoimmun Rev. 2020;19:102671. [DOI] [PubMed] [Google Scholar]
- 21. Vikse J, Jonsdottir K, KvalÃ,y JT, Wildhagen K, Omdal R. Tolerability and safety of long-term rituximab treatment in systemic inflammatory and autoimmune diseases. Rheumatol Int. 2019;39(6):1083-1090. [DOI] [PubMed] [Google Scholar]
- 22. van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: Final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol. 2015;42(10):1761-1766. [DOI] [PubMed] [Google Scholar]
- 23. Merkel PA, Niles JL, Mertz LE, Lehane PB, Pordeli P, Erblang F. Long-term safety of rituximab in granulomatosis with polyangiitis and in microscopic polyangiitis. Arthritis Care Res (Hoboken). 2021;73(9):1372-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAdoo SP, Medjeral -Thomas N, Pusey CD. Long-term follow-up of a combined rituximab abd cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. 2018;33:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Daalen EE, Rizzo R, Kronbichler A, et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2017;76(6):1064-1069. [DOI] [PubMed] [Google Scholar]
- 26. Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25:850-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawara Y, Itabashi M, Kojima C, et al. Successful therapeutic use of a single-dose of rituximab on relapse in adults with minimal change nephrotic syndrome. Clin Nephrol. 2009;72(1):69-72. [DOI] [PubMed] [Google Scholar]
- 28. Papakrivopoulou E, Shendi AM, Salama AD, Khosravi M, Connolly JO, Trompeter R. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology (Carlton). 2016;21(10):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guigonis V, Dallocchio A, Baudouin V, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269-1279. [DOI] [PubMed] [Google Scholar]
- 30. Ito S, Kamei K, Ogura M, et al. Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2011;26(10):1823-1828. [DOI] [PubMed] [Google Scholar]
- 31. Filler G, Huang Sharma AP. Should we consider MMF therapy after rituximab for nephrotic syndrome. Pediatr Nephrol. 2011;26(10):1759-1762. [DOI] [PubMed] [Google Scholar]
- 32. Wu B, Mao J, Shen H, et al. Triple immunosuppressive therapy in steroid-resistant nephrotic syndrome children with tacrolimus resistance or tacrolimus sensitivity but frequently relapsing. Nephrology (Carlton). 2015;20(1):18-24. [DOI] [PubMed] [Google Scholar]
- 33. Basu B, Mahapatra TKS, Mondal N. Mycophenolate mofetil following rituximab in children with steroid-resistant nephrotic syndrome. Pediatrics. 2015;136(1):e132-e139. [DOI] [PubMed] [Google Scholar]