Abstract
Background
Thrombophilia screening is widely done in clinical practice, and it is claimed that the extent of venous thromboembolism (VTE) recurrence risk in patients with common defects is still not fully understood.
Aim
We aimed to summarize data of all observational studies prospectively assessing the association of heterozygous factor V Leiden (FVL) mutation and recurrent VTE in patients with VTE, and to calculate pooled relative risks (RR), overall and in various subgroups.
Methods
We searched MEDLINE and EMBASE databases for cohort studies prospectively assessing VTE recurrence in patients with and without FVL mutation (PROSPERO: CRD42021182800). Data were extracted on cohort and study-level. The methodological quality was assessed using the Newcastle-Ottawa Scale (NOS). RR were calculated overall and in subgroups using a random-effects model.
Results
From 31 cohorts, 24 studies were finally included summarizing 13,571 patients. Heterozygous FVL mutation was identified in 2,840 individuals (21%). The methodological quality was estimated to be high in 20 studies (83%). The overall RR was 1.46 (95% CI: 1.31, 1.64), consistent across subgroups.
Conclusions
Pooling all high-quality epidemiological data, the risk of recurrent VTE was increased by 46% in patients with heterozygous FVL mutation. Against the background of established risk factors, the FVL mutation plays only a marginal role in the risk assessment for recurrent VTE.
Keywords: heterozygous factor V Leiden mutation, recurrent venous thromboembolism, prospective cohort studies, systematic review, risk factors
Introduction
Thrombophilia screening is still a popular tool in the workup of patients with venous thromboembolism (VTE) (1, 2). VTE is one of the most common cardiovascular diseases associated with high morbidity and mortality (3–7). More than 25% of unselected patients experience recurrent events, potentially resulting in a reduced quality of life or even death (8, 9). Thus, preventing recurrent VTE is an important goal of secondary prevention (4, 10–12). To accomplish this, high-risk patients must be identified (9, 13). Given the clustering of VTE in families or even in individuals, genetic factors are considered as promising targets (14–16). The most common inherited thrombophilia is heterozygous factor V Leiden (FVL) mutation, which is acknowledged as a relevant risk factor for first VTE (17, 18). Earlier investigations suggest a moderately increased risk only and current guidelines do not suggest thrombophilia testing in unselected patients (1, 19–26). However, the selection criteria are largely unclear and thrombophilia screening (including FVL mutation) is still frequently done in clinical practice (1, 2, 9, 20, 27–33). Besides, some authors claim that the knowledge is still limited, particularly within subgroups of patients, and that the presence of FVL mutation might sum up with other risk factors resulting in a modified treatment recommendation (14, 34, 35).
Various previous studies observed the association between the presence of FVL mutation and the risk of VTE recurrence and the results are conflicting. Some studies concluded that heterozygous FVL mutation increases the risk (10, 12, 36–41) and others do not (38, 42–48). In particular, some authors raise the question of whether FVL mutation increases the recurrence risk in specific subgroups such as men (36), young women without hormonal treatment (38), or cancer patients (49, 50). Indeed, FVL mutation was also detected in various genetic profiling studies (10, 23, 40, 41, 51–54), and it was included in one clinical prediction model (53). Thus, whether or not FVL mutation increases the risk of recurrent VTE to a relevant degree is not fully understood, and more data are needed to clarify this issue.
Aim
In a systematic review and meta-analysis, we aimed to summarize data of all observational studies prospectively assessing the association of heterozygous FVL mutation and recurrent VTE. We aimed to calculate relative risks (RR) overall and in various subgroups of patients. To set this into context, we observed the frequency of testing in Switzerland using a large claim-based dataset.
Methods
The study protocol was submitted to the PROSPERO international prospective register of systematic reviews (#CRD42021182800) and the manuscript was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (55).
Search Strategy, Screening, and Identification
A comprehensive search strategy for MEDLINE (1946 to February 03, 2022) and EMBASE (1974 to 2022 February 03) databases was developed, and the Ovid interface used (Supplementary List 1). The search strategy was based on three elements: heterozygous FVL mutation (patients); recurrent VTE (outcome); and prospective cohort study (study design). The search strategy was improved using keywords found in key publications and no limits were applied. The sensitivity was tested in eight key publications (100%). The literature search was completed by hand search using reference lists of articles retrieved. All included studies were checked for published errata. The last search run was done on the fourth of February 2022. All records were carefully assessed for eligibility by screening of title, abstract and full text by two reviewers in duplicate (D.E., M.N.).
Study Eligibility
The following inclusion criteria were applied: (a) prospective cohort studies, (b) patients tested for FVL mutation/ activated protein C resistance (APCR) at baseline, (c) objectively confirmed VTE, (d) recurrent VTE defined as primary outcome, and (e) numbers of recurrences or recurrence rates reported separately in patients with and without FVL mutation. Exclusion criteria were (1) retrospective studies, (2) case-control studies, case reports, and (3) studies conducted in close subgroups (e.g., children, perioperative VTE, upper extremity deep venous thrombosis, and homozygous FVL mutation). Articles based on the same cohort were compiled and the publication with the (a) highest number of patients, and (b) most complete clinical data were selected for meta-analysis.
Definition of Outcomes
Recurrent VTE was defined as objectively confirmed VTE. For deep venous thrombosis (DVT), one of the following imaging techniques must have been used: venography, duplex sonography, or compression ultrasonography. For pulmonary embolism (PE), ventilation-perfusion scan, spiral computed tomography, or pulmonary angiography should have been used (56–58).
Data Extraction
First, several characteristics were retrieved to summarize each cohort: name of cohort, country, setting (type of health care institution), time period of patient recruitment, inclusion criteria and all publications. Secondly, detailed data were extracted out of the selected publication for meta-analysis: first author, year of publication, age of patients (mean or median), total number of patients, number of female patients, number of FVL mutation patients (at baseline), location of initial VTE (isolated distal DVT, proximal DVT/PE or mixed DVT/PE), triggering factor first VTE (unprovoked, provoked, mixed), duration of anticoagulation (months), type of anticoagulation [Vitamin-K antagonist (VKA), direct oral anticoagulants (DOAC)], absolute number of patients with unprovoked VTE, number of cancer patients, observation period (months), total number of patients with recurrence, number of FVL mutation patients with recurrence, number of non-FVL mutation patients with recurrence and recurrence rate of FVL mutation patients.
Assessment of Methodological Quality
The methodological quality of the primary studies was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies (59). The following three domains were applied: (a) selection of patients, (b) comparability of study groups, and (c) outcome of interest. The questions were modified to fit the present research question (Supplementary List 2). The assessment was done in duplicate (D.E., M.N.) and discrepancies were resolved by discussion.
Frequency of FVL Testing
To set this analysis into context, we assessed the frequency and trends of testing for FVL mutation in the Swiss health care system. Health care claims data of Helsana, one of the largest Swiss health insurance companies were used. Approximately 15% of the Swiss population are insured with Helsana for obligatory basic insurance, and the population is considered representative (60, 61). All invoices submitted for reimbursement for FVL mutation (#6200.64) and APCR (#1086.00) of the list of analyses from the Federal Office of Public Health were retrieved between 2014 and 2020 (62).
Statistical Analysis
Using the extracted data, the relative risks (RR) and their 95% confidence intervals (CI) were calculated for each primary study. The RRs were then calculated using a random-effects model based on the Mantel-Haenszel estimator, and the corresponding 95% CI were computed. Heterogeneity between studies was assessed using Higgins' I2. All analyses were performed using the “meta,” “etaphor,” and “dmetar” packages for R. As a first sensitivity analysis, a leave-one-out analysis was performed to check for outliers. Studentized residuals and Cook's distance were calculated, and studies with studentized residuals outside of −1 and 1, and Cook's distances >50% of the lower tail of a Chi-square distribution with p (p = number of model coefficients) degrees of freedom were flagged as potentially influential outliers. These studies were excluded from the overall analysis. Furthermore, subgroup analysis was performed for the following subgroups: Year of publication (<2000, 2000–2010, >2010), location of the initial VTE (mixed, proximal DVT/PE), presence of triggering risk factors for the initial VTE (unprovoked, provoked, and mixed), the anticoagulation drug used (VKA, DOAC), and the presence of cancer (no cancer, mixed). A funnel plot was additionally created to assess publication bias.
Results
Cohort and Study Identification and Selection
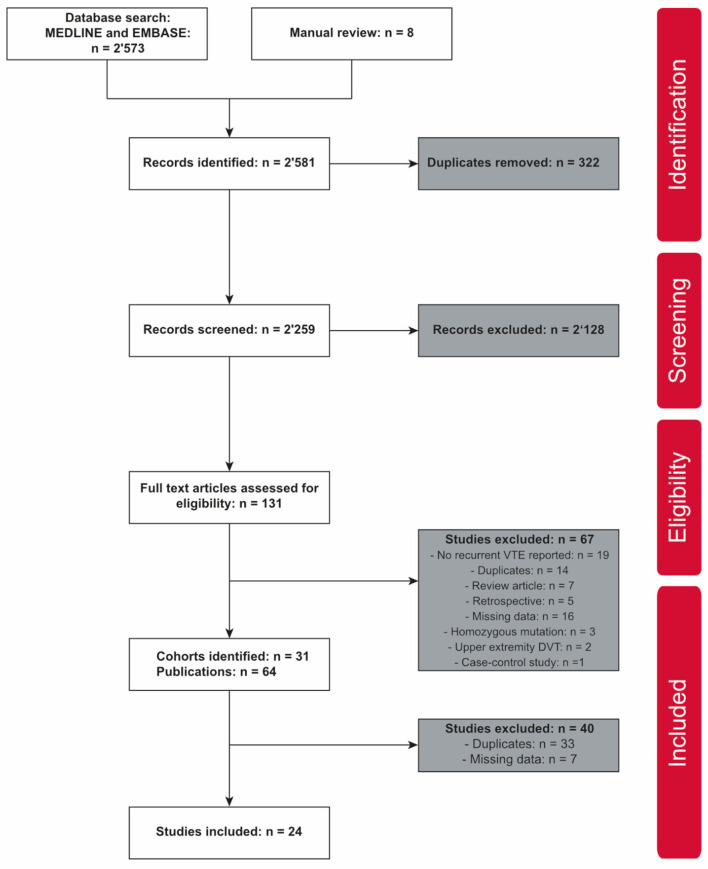
The literature search retrieved a total of 2,581 publications, 2,573 accessed in MEDLINE and EMBASE databases, and eight identified by manual review (Figure 1). After removing duplicates, the title and abstract of the 2,259 remaining publications were screened, giving 131 publications for full-text screening (including 100 journal articles and 21 conference abstracts). A total of 67 publications were excluded with reasons. Eventually, 31 different prospective cohort studies were identified (Table 1; Figure 1). Per cohort, the publication with the most complete clinical data was selected for further analysis. No publication with sufficient data were identified in seven cohorts (44, 45, 47, 54, 80, 101, 104). Twenty-four publications were finally considered for meta-analysis (Figure 1) (17, 36–41, 46, 48, 63–65, 67, 69–71, 73, 75, 78, 82–84, 86, 103).
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of prospective cohort studies including patients with VTE.
| Name of cohort | Time period of patient recruitment | Country | Setting | Inclusion criteria | Identified publications |
|---|---|---|---|---|---|
| PHS: Physicians' Health study | 1982 to 1983 | USA | Male physicians residing in the United States | VTE; U.S. male physicians 40–84 years | (63) |
| DURAC trial: Duration of Anticoagulation study | April 1988 to April 1991 | Sweden | 16 secondary/ tertiary hospitals, Department of internal Medicine | First DVT/PE; age > 15 and <71 | (64) |
| LETS: Leiden Thrombophilia Study | January 1988 to December 1992 | Netherlands | 3 anticoagulation clinics | First DVT including arm thrombosis; age <70 | (52, 65, 66) |
| Padua* | January 1986 to June 1994 | Italy | Thrombosis unit of the University of Padua | First DVT | (67, 68) |
| Extended anticoagulation trial | October 1994 to April 1997 | Canada, USA | 13 secondary/tertiary hospitals | First unprovoked proximal; DVT/PE; received OAC ≥ 3 months | (69) |
| EPCOT: European Prospective Cohort on Thrombophilia study | March 1994 to September 1997 | Spain, Italy, Germany, UK, Netherlands, Sweden, France, Austria | 9 anticoagulation clinics | In this subcohort: First DVT/PE before study entry | (70) |
| LIST: The Linköping Study on Thrombosis | February 1998 to January 2000 | Sweden | Linköping University Hospital (emergency department) | VTE; age ≥ 18 | (71, 72) |
| THRIVE III: Ximelagatran in VTE | November 1999 to October 2000 | 18 countries: Europe, Argentina, Brazil, Canada, Israel, Mexico, South Africa | 142 secondary/tertiary hospitals | DVT/PE; age ≥ 18; received OAC for 6 months without recurrence | (17) |
| ELATE: The Extended Low-intensity Anticoagulation for unprovoked Thrombo-embolism | December 1998 to May 2001 | Canada, USA | 16 secondary/tertiary hospitals | Unprovoked proximal DVT/PE; received OAC ≥ 3 months; warfarin therapy during follow-up | (73) |
| CVTE: The Cambridge Venous Thromboembolism Study | August 1997 to January 2002 | United Kingdom | Addenbrooke's Hospital Cambridge (thrombosis center) | First DVT/PE | (44, 52, 74) |
| Bologna* | February 1995 to February 2002 | Italy | S. Orsola-Malpighi University Hospital Bologna (thrombosis center) | First DVT/PE; received OAC ≥ 3 months | (75–77) |
| Salamanca* | June 1997 to June 2002 | Spain | Thrombosis and Hemostasis Section of the University Hospital of Salamanca | First DVT/PE | (46) |
| PORtromb project: Oporto thrombophilia study | October 1997 to November 2002 | Portugal | Sao Joao University hospital (outpatients unit) | First DVT including arm thrombosis; age <40 | (45) |
| PREVENT: Prevention of Recurrent Venous Thromboembolism trial | July 1998 to December 2002 | USA, Canada, Switzerland | 52 secondary/tertiary hospitals | Documented unprovoked VTE; age ≥ 30; received OAC ≥ 3 month | (47) |
| Italy1* | May 1991 to April 2003 | Italy | Emergency departments of 3 secondary/tertiary hospitals | First proximal DVT/PE; received OAC 3–6 months without recurrence | (78, 79) |
| Italiy2*, AESOPUS investigators | January 1999 to July 2003 | Italy | 9 university or hospital centers in Italy | First proximal DVT; age ≥ 18; received OAC 3 months without recurrence | (80) |
| MEGA follow-up study: Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis | March 1999 to September 2004 | Netherlands | 6 anticoagulation clinics | First DVT/PE; age <70 | (53, 54, 81) |
| Florence | January 1999 to January 2007 | Italy | Thrombosis center at University hospital Careggi Florence | First VTE | (82) |
| Jordan* | January 2005 to December 2007 | Jordan | Jordan University Hospital | Acute PE | (83) |
| REVERSE I | 2001 to 2007 | Canada, France, Switzerland, USA | 12 tertiary care centers | First unprovoked proximal DVT/PE; age ≥ 18; received OAC 5–7 month without recurrence | (84, 85) |
| AUREC: Austrian Study on Recurrent Venous Thromboembolism | July 1992 to August 2008 | Austria | 4 thrombosis centers in Vienna; secondary care/tertiary care | First unprovoked DVT/PE; age ≥ 18; received OAC ≥ 3 months | (11, 16, 42, 43, 86–96) |
| MATS: Malmö Thrombophilia Study | March 1998 to December 2008 | Sweden | Skane University Hospital (emergency department) | VTE; age ≥ 18 | (10, 14, 37, 97–100) |
| TEHS-follow up study: Thromboembolism Hormone Study | 2003 to 2009 | Sweden | 43 secondary/tertiary hospitals | First DVT/PE; age > 18 and <64 | (39) |
| FARIVE study: Facteurs de risqué et de récidives de la maladie thromboembolique veineuse | 2003 to 2009 | France | 11 centers | First unprovoked DVT/PE; age ≥ 18 | (12, 36) |
| MAISTHRO: Main-Isar-Thrombosis registry | March 2000 to February 2010 | Germany | University hospital's outpatient department, Goethe University Hospital Frankfurt/Main | Acute or documented history of DVT/PE; age ≥ 18 | (38) |
| France* | January 1992 to June 2011 | France | Brest University Hospital | First DVT/PE; age ≥ 18 and <50; Women | (101, 102) |
| Madrid* | March 2004 to August 2013 | Spain | 2 University hospitals in Madrid | First unprovoked DVT/PE; age ≥ 18; received OAC ≥ 3 months | (103) |
| SWITCO65+: Swiss Venous Thromboembolism Cohort | September 2009 to December 2013 | Switzerland | 9 tertiary hospitals in Switzerland | First unprovoked DVT/PE; age ≥ 65 | (48) |
| Germany* | December 2008 to December 2018 | Germany | Multicenter | First VTE; age adolescents to 60 years | (40) |
| Egypt* | January 2015 to December 2020 | Egypt* | Tanta University Hospital | First VTE; age ≥ 18 | (41) |
| Conference Abstract I* | – | France | – | First proximal DVT/PE | (104) |
No cohort name available.
Cohort Characteristics
Thirty-one prospective cohort studies conducted in Europe (n = 23), North America (n = 3), Europe and North America (n = 2), and other areas (n = 3) were identified. The number of publications per cohort ranged from 1 (17, 38–41, 45–48, 54, 63, 64, 69, 70, 73, 80, 82, 83, 103, 104) to 15 (86). Twenty-three cohorts included patients with a first VTE (36, 39–41, 44–46, 48, 54, 64, 65, 67, 69, 70, 75, 78, 80, 82, 84, 86, 101, 103, 104), and eight cohorts included patients with any VTE (17, 37, 38, 47, 63, 71, 73, 83). Detailed cohort characteristics are reported in Table 1.
Studies Characteristics and Patients
Details of the primary studies included in the meta-analysis are reported in Table 2, summarizing data of 13,571 patients, including 2,840 patients with FVL mutation (21%). The number of patients varied between 72 (83) and 1,267 (37). The prevalence of FVL mutation ranged between 8.4% (36) and 28% (86). The mean or median age varied between 37 years (40) and 76 years (48). The observation periods varied from six (83) to 88 (63, 65) months. VKA were used in most studies (36, 37, 41, 46, 48, 64, 65, 67, 69, 71, 73, 75, 78, 82, 84, 86, 103), summarizing 8,654 patients (64%). DOAC were used as anticoagulant in one study (17), and the type of anticoagulant was not specified in six studies (38–40, 63, 70, 83). The inclusion criteria and the type and location of the primary event is reported in Table 1. Eight studies included patients with a first unprovoked VTE only (36, 41, 48, 69, 73, 84, 86, 103) and one study provided separate data (provoked/unprovoked) (67). Both provoked and unprovoked VTE were included in 15 primary studies (17, 37–40, 46, 63–65, 70, 71, 75, 78, 82, 83). Patients with cancer were excluded in 16 studies (36, 39–41, 46, 48, 64, 65, 67, 69, 73, 78, 82, 84, 86, 103) and not reported in two studies (63, 83). Overall, 341 cancer patients were reported in six studies (17, 37, 38, 70, 71, 75). A funnel plot is given in Supplementary Figure S1.
Table 2.
Characteristics of studies included in meta-analysis.
| Author/year | Age | Anticoagulant used | Patients, total | Patients, FVL mutation | Patients with unprovoked VTE | Observation period | Recurrences, total | Recurrences, FVL mutation |
|---|---|---|---|---|---|---|---|---|
| Years (mean or median) | Numbers | Numbers | Numbers | Months (mean/median) | Numbers (%) | Numbers (%) | ||
| Simioni et al. (67)+ (provoked VTE) | 63 | VKA | 106 | 13 | 0 | 47 | 10 (9.4) | 4 (30.1) |
| Simioni et al. (67)+ (unprovoked VTE) | 63 | VKA | 145 | 28 | 145 | 47 | 39 (26.9) | 10 (35.7) |
| Kearon et al. (69)# (placebo group) | 58 | VKA | 83 | 19 | 83 | 9 | 17 (20.5) | 2 (10.5) |
| Kearon et al. (69)# (intervention group) | 59 | VKA | 79 | 15 | 79 | 12 | 1 (1.7) | 0 (0.0) |
| Lindmarker et al. (64) | 58 | VKA | 467 | 118 | 267 | 48 | 65 (13.9) | 19 (16.1) |
| Miles et al. (63) | 40–84$ | – | 218 | 26 | 101 | 88 | 29 (13.3) | 5 (19.2) |
| Palareti et al. (75) | 67 | VKA | 599 | 68 | 282 | 17 | 58 (9.7) | 15 (22.1) |
| Christiansen et al. (65) | 45 | VKA | 474 | 84 | 259 | 88 | 90 (19) | 19 (22.6) |
| Vossen et al. (70) | 40 | – | 304 | 76 | 167 | 67 | 51 (16.8) | 12 (15.8) |
| Wahlander et al. (17)# (placebo group) | 58 | VKA | 531 | 121 | – | 18 | 57 (10.7) | 16 (13.2) |
| Wahlander et al. (17)# (intervention group) | 56 | DOAC | 549 | 100 | – | 18 | 9 (1.6) | 2 (2) |
| Gonzalez-Porras et al. (46) | 47 | VKA | 181 | 29 | 117 | 56 | 27 (14.9) | 5 (17.2) |
| Prandoni et al. (78) | 66 | VKA | 953 | 111 | – | 50 | 208 (21.8) | 38 (34.2) |
| Poli et al. (82) | 64 | VKA | 169 | 22 | 107 | 30 | 27 (15.9) | 5 (22.7) |
| Eichinger et al. (86) | 49 | VKA | 1,107 | 307 | 1,107 | 44 | 168 (15.2) | 60 (19.5) |
| Rodger et al. (84) | 53 | VKA | 646 | 100 | 646 | 18 | 91 (14.1) | 19 (19) |
| Kearon et al. (73) | 57 | VKA | 661 | 161 | 661 | 28 | 14 (2.1) | 3 (1.9) |
| Chaireti et al. (71) | 61 | VKA | 158 | 39 | – | 84 | 42 (26.5) | 17 (43.6) |
| Obeidat et al. (83) | 50 | – | 72 | 17 | 23 | 6 | 7 (9.7) | 2 (11.8) |
| Sveinsdottir et al. (37) | 63 | VKA | 1,267 | 339 | 511 | 58 | 131 (10.3) | 49 (14.5) |
| Olie et al. (36) | 49 | VKA | 583 | 49 | 583 | 27 | 74 (12.6) | 9 (18.4) |
| Weingarz et al. (38) | 43 | – | 1,221 | 287 | 299 | 77 | 261 (21.4) | 63 (22) |
| Franco Moreno et al. (103) | 61 | VKA | 398 | 106 | 398 | 21 | 65 (16.3) | 45 (42.5) |
| Bruzelius et al. (39) | 46 | – | 1,010 | 238 | 367 | 60 | 101 (10) | 33 (13.9) |
| Mean et al. (48) | 76 | VKA | 354 | 32 | 354 | 30 | 54 (15.3) | 4 (12.5) |
| Limperger et al. (40) | 37 | – | 1,012 | 275 | 223 | 51 | 178 (17.6) | 68 (24.7) |
| Hodeib et al. (41) | 52 | VKA | 224 | 60 | 224 | 50 | 58 (25.9) | 22 (36.7) |
Provoked and unprovoked VTE patients were reported separately;
intervention and placebo group of a randomized controlled trial were reported separately; –data not reported;
range.
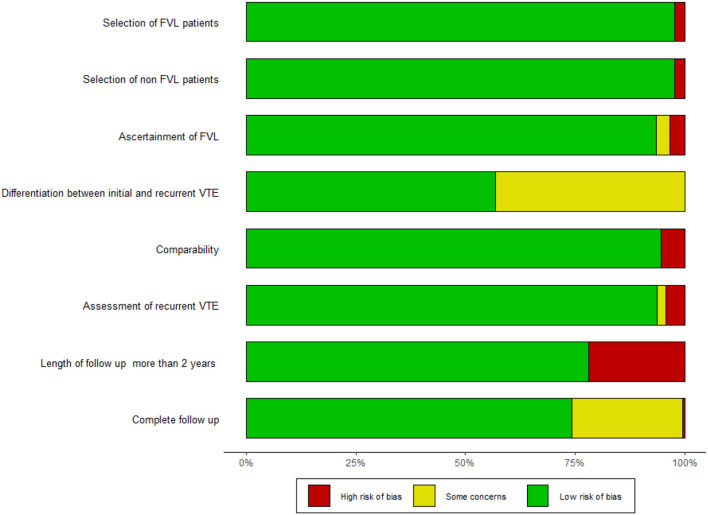
Methodological Quality
A summary of the methodological quality according to the NOS tool is given in Figure 2; detailed results for all studies are reported in the Supplementary Table S1. With at least six NOS criteria fulfilled in twenty studies, the overall methodological quality was high (17, 36–41, 48, 63–65, 67, 69, 71, 73, 75, 78, 82, 84, 86). Three to five criteria were fulfilled by four studies (46, 70, 83, 103). The three domains most frequently not met were (1) method reported for distinguishing the initial and recurrent VTE (37, 39–41, 46, 48, 63, 65, 67, 70, 71, 83, 103), (2) follow-up longer than 2 years (17, 69, 75, 83, 84, 103), and (3) follow-up rate ≥90% of patients (17, 40, 41, 46, 48, 67, 83, 103).
Figure 2.
Summary of methodological quality. Rating according to the NOS questionnaire. The detailed questionnaire is shown in the Supplementary List 2.
Risk of Recurrent VTE Among FVL Patients
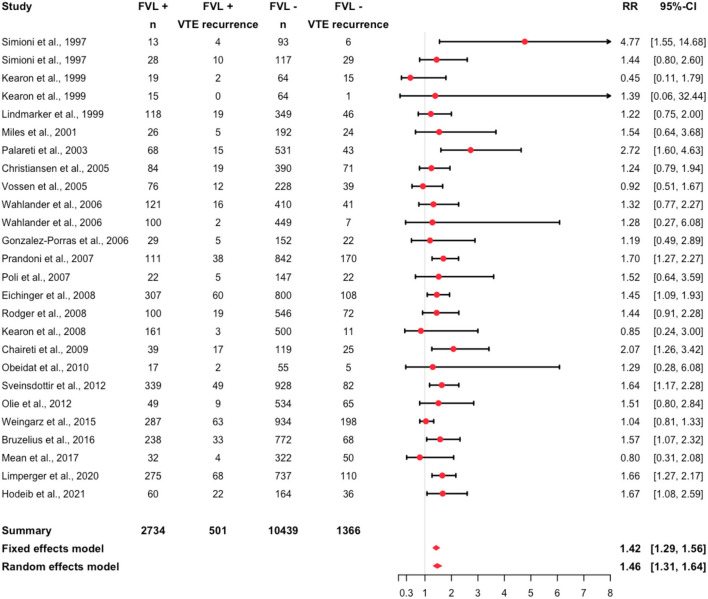
Assessing all studies for potential influential outliers using statistical criteria (105), we identified the study from Franco Moreno et al. (103) (Supplementary Table S2; Supplementary Figure S2). Thus, this study was excluded for the purpose of the overall analysis. A recurrent event was recorded in 1,867 individuals (14%). Recurrent events were observed in 18% of the FVL mutation patients and in 13% of the non-FVL mutation patients. Details are reported in Table 2. The relative risk was 1.46 (95% CI: 1.31, 1.64, I2 = 0.17; 95% prediction interval 1.10, 1.94) (Figure 3).
Figure 3.
Forest plot summarizing the relative risk of recurrent VTE among heterozygous FVL mutation patients (I2 = 0.17).
In several sensitivity analyses, we assessed the risk in specific subgroups. Among the primary studies, the RR varied between 0.45 (95% CI: 0.11, 1.79) (69) and 4.77 (95% CI: 1.55, 14.68) (67). A RR smaller than one was calculated in four primary studies (48, 69, 70, 73). Focusing on different anticoagulants, the RR was 1.65 (95% CI: 1.33, 2.04) in patients treated with VKA, and 1.28 (95% CI: 0.27, 6.08) in patients treated with DOAC (Supplementary Figure S3). Pooling studies with unprovoked VTE only, the RR was 1.53 (95% CI: 0.99, 2.35) (Supplementary Figure S4). It was 1.47 (95% CI: 1.27, 1.71) in studies including both, patients with provoked and unprovoked VTE. In one study group (67), patients with a first provoked VTE only were analyzed, resulting in a RR of 4.77 (95% CI: 1.55, 14.68). Considering different localizations of the initial event, the RR was 1.29 (95% CI: 0.28, 6.08) in patients with PE (Supplementary Figure S5), and 1.52 (95% CI: 1.2, 1.93) in patients with proximal DVT or PE. It was 1.6 (95% CI: 1.32, 1.95) in patients with proximal DVT/PE or distal DVT. Excluding patients with cancer, the RR was 1.59 (95% CI: 1.27, 1.99) (Supplementary Figure S6). The RR was 1.69 (95% CI: 1.14, 2.51) in studies published after 2011, 1.52 (95% CI: 1.33, 1.75) in studies published between 2001 and 2011, and 1.44 (95% CI: 0.77, 2.68) in studies published before 2001 (Supplementary Figure S7).
FVL Mutation Testing
Analysis of Helsana health care claims data in Switzerland showed that 46,522 APCR tests and 49,625 polymerase-chain reaction (PCR) for FVL mutation were recorded between 2014 and 2020 (Supplementary Table S3; Supplementary Figure S8). For APCR, the frequency of testing varied between 6,206 (0.1% of the population, 2014) and 7,206 (0.1%, 2016). For PCR, the frequency ranged between 6,793 (0.1%, 2017) and 7,614 (0.1%, 2019). Considering patients having any test, the total number of patients with APCR and/or PCR varied between 9,661 (0.2%, 2017) and 10,614 (0.2%, 2016). The frequency of testing was stable between 2014 and 2020.
Discussion
We conducted a comprehensive systematic review retrieving all high-quality epidemiological data investigating the association of heterozygous FVL mutation and recurrent VTE. Thirty-one prospective cohort studies were identified and 24 publications summarizing 13,571 patients were included in the meta-analysis. Overall, a 42% increased risk of recurrence was found in patients with heterozygous FVL mutation. Various subgroup analyses did not identify a population with a significantly modified risk. However, a significant proportion of the analyzed Swiss population was tested for FVL mutation each year.
The present work is the most comprehensive systematic review to date. Considering all currently available data, we were able to analyze various subgroups of patients. However, our results are essentially consistent with previous investigations (24–26). Segal et al. (26) included 13 prospective studies summarizing 4,730 patients, reporting an overall odds ratio of 1.56. Marchiori et al. (25) included 10 prospective studies with 3,203 patients concluding on a relative risk of 1.39. Ho et al. (24) summarized two retrospective studies and eight prospective studies, reporting an odds ratio of 1.41. We analyzed a number of patient subgroups (type of anticoagulation, triggering risk factors, VTE localization, presence of cancer, and year of publication) and none of these analyses revealed statistically significant differences in the recurrence risk (Supplementary Figures S3–S7). However, a remarkable higher recurrence risk was reported in the only study including patients with provoked VTE (67). However, this was a small study published in 1997 and the results were never confirmed in other settings.
Our investigation has several strengths. First, we conducted a comprehensive literature search and applied strict inclusion criteria to include high-quality data only. Secondly, we pooled three times more patients compared to the latest systematic review. Thirdly, most of the studies had a low risk of bias and the between-study heterogeneity is low. Fourthly, we were able to conduct several subgroup analyses, thus strengthening the interpretation. Of course, our study has limitations as well. First, inherent with any meta-analytic approach, our investigation relies on data retrieved from primary studies. However, only four studies were estimated to have a high risk of bias. One of those studies was classified as a potentially influential outlier and thus excluded for overall analysis. The remaining three studies affected only 4% of the patients. Thus, we do not believe that this might have influenced our results. Secondly, the number of patients were limited in certain subgroups; patients with provoked VTE, cancer, DOAC, and PE were underrepresented. Thirdly, it was impossible to retrieve separate data for hetero- and homozygous patients in few studies. However, we do not believe that this might have influenced our results because only few patients are included in the large number of patients. Fourthly, one might argue that the proportion of patients with unprovoked VTE varies considerably among studies. However, as long as the between-study heterogeneity was low, this might be regarded as a strength of our study, increasing external validity.
Our data confirm that the presence of FVL mutation represents a minor risk factor only. Compared to the much stronger risk factors unprovoked VTE, proximal DVT/PE, male sex, elevated D-Dimers, high factor VIII plays the presence of FVL mutation only a marginal role (9, 11, 78, 106–109). Consistently, several prediction models for recurrent VTE were developed and FVL mutation was not identified as a relevant predictor in any of the models including clinical characteristics (11, 84, 107, 110, 111). Thus, an important task is to translate this evidence into clinical practice. Determination of FVL mutation shall be challenged and the reimbursement of these analyses might be questioned. However, some authors argue that the presence of FVL mutation might contribute to a significantly elevated risk if combined with other (high risk) thrombophilia. To date, the data supporting this hypothesis are not sufficient. Individual patient-data meta-analyses are a promising tool to study this research question.
Conclusions
Summarizing all currently available high-quality epidemiological data, the risk of recurrent VTE was only moderately increased. This observation was consistent among various subgroups. Our data confirm that the presence of FVL mutation plays only a marginal role in the risk assessment for recurrent VTE. Efforts should be made to reduce the still very frequent determination in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
DE developed the search strategy, conducted the literature search, retrieved the data, interpreted the results, wrote the manuscript, and contributed to the study protocol. HN wrote the analysis plan, conducted the analysis, and interpreted the data. BA developed the search strategy and contributed to the literature search. CH collected the data (health care claims) and contributed to the study protocol and the interpretation of the data. MN developed the study protocol, conducted the literature search, contributed to the analysis plan, interpreted the results, and wrote the manuscript. All authors contributed to and approved the final manuscript.
Funding
MN was supported by a research grant of the Swiss National Science Foundation (#179334).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.883986/full#supplementary-material
References
- 1.Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med. (2017) 377:1177–87. 10.1056/NEJMra1700365 [DOI] [PubMed] [Google Scholar]
- 2.Middeldorp S. Inherited thrombophilia: a double-edged sword. Hematology Am Soc Hematol Educ Program. (2016) 2016:1–9. 10.1182/asheducation-2016.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilius H, Mertins T, Boss R, Knuchel M, Blozik E, Kremer Hovinga JA, et al. Long-term survival after venous thromboembolism: a prospective cohort study. Front Cardiovasc Med. (2021) 8:749342. 10.3389/fcvm.2021.749342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ISTH Steering Committee for World Thrombosis Day . Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. (2014) 12:1580–90. 10.1111/jth.12698 [DOI] [PubMed] [Google Scholar]
- 5.Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. (2013) 126:832.e13–21. 10.1016/j.amjmed.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 6.Verso M, Agnelli G, Ageno W, Imberti D, Moia M, Palareti G, et al. Long-term death and recurrence in patients with acute venous thromboembolism: the MASTER registry. Thromb Res. (2012) 130:369–73. 10.1016/j.thromres.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 7.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. (2012) 379:1835–46. 10.1016/S0140-6736(11)61904-1 [DOI] [PubMed] [Google Scholar]
- 8.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. (2016) 41:3–14. 10.1007/s11239-015-1311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. (2010) 376:2032–9. 10.1016/S0140-6736(10)60962-2 [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A, Sundquist K, Palmer K, Svensson PJ, Sundquist J, Memon AA. Risk prediction of recurrent venous thromboembolism: a multiple genetic risk model. J Thromb Thrombolysis. (2019) 47:216–26. 10.1007/s11239-018-1762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. (2010) 121:1630–6. 10.1161/CIRCULATIONAHA.109.925214 [DOI] [PubMed] [Google Scholar]
- 12.Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. (2009) 29:298–310. 10.1161/ATVBAHA.108.182428 [DOI] [PubMed] [Google Scholar]
- 13.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. (2007) 98:756–64. 10.1160/TH07-03-0212 [DOI] [PubMed] [Google Scholar]
- 14.Sundquist K, Sundquist J, Svensson PJ, Zoller B, Memon AA. Role of family history of venous thromboembolism and thrombophilia as predictors of recurrence: a prospective follow-up study. J Thromb Haemost. (2015) 13:2180–6. 10.1111/jth.13154 [DOI] [PubMed] [Google Scholar]
- 15.Zöller B, Ohlsson H, Sundquist J, Sundquist K. Family history of venous thromboembolism (VTE) and risk of recurrent hospitalization for VTE: a nationwide family study in Sweden. J Thromb Haemost. (2014) 12:306–12. 10.1111/jth.12499 [DOI] [PubMed] [Google Scholar]
- 16.Hron G, Eichinger S, Weltermann A, Minar E, Bialonczyk C, Hirschl M, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. (2006) 119:50–3. 10.1016/j.amjmed.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 17.Wahlander K, Eriksson H, Lundstrom T, Clason SB, Wall U, Nystrom P, et al. Risk of recurrent venous thromboembolism or bleeding in relation to thrombophilic risk factors in patients receiving ximelagatran or placebo for long-term secondary prevention of venous thromboembolism. Br J Haematol. (2006) 133:68–77. 10.1111/j.1365-2141.2006.05960.x [DOI] [PubMed] [Google Scholar]
- 18.Anderson FA, Jr. Spencer FA. Risk factors for venous thromboembolism. Circulation. (2003) 107(23 Suppl. 1):I9–I16. 10.1161/01.CIR.0000078469.07362.E6 [DOI] [PubMed] [Google Scholar]
- 19.Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. (2016) 41:154–64. 10.1007/s11239-015-1316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives: a review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. (2013) 110:697–705. 10.1160/TH13-01-0011 [DOI] [PubMed] [Google Scholar]
- 21.Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. (2010) 149:209–20. 10.1111/j.1365-2141.2009.08022.x [DOI] [PubMed] [Google Scholar]
- 22.NICE . Venous Thromboembolic Diseases: Diagnosis, Management and Thrombophilia Testing [NG158]. National Institute for Health and Care Excellence; (2012). [PubMed] [Google Scholar]
- 23.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. (2005) 365:1163–74. 10.1016/S0140-6736(05)71880-8 [DOI] [PubMed] [Google Scholar]
- 24.Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. (2006) 166:729–36. 10.1001/archinte.166.7.729 [DOI] [PubMed] [Google Scholar]
- 25.Marchiori A, Mosena L, Prins MH, Prandoni P. The risk of recurrent venous thromboembolism among heterozygous carriers of factor V Leiden or prothrombin G20210A mutation. A systematic review of prospective studies. Haematologica. (2007) 92:1107–14. 10.3324/haematol.10234 [DOI] [PubMed] [Google Scholar]
- 26.Segal JB, Brotman DJ, Necochea AJ, Emadi A, Samal L, Wilson LM, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. (2009) 301:2472–85. 10.1001/jama.2009.853 [DOI] [PubMed] [Google Scholar]
- 27.Legnani C, Palareti G, Antonucci E, Poli D, Cosmi B, Falanga A, et al. Thrombophilia testing in the real-world clinical setting of thrombosis centres taking part in the Italian Start 2-Register. Blood Transfus. (2021) 19:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M, Lee HL, Yang IA, Masel PJ. Utility of thrombophilia testing in patients with venous thrombo-embolism. J Thorac Dis. (2016) 8:3697–703. 10.21037/jtd.2016.12.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Favaloro EJ, McDonald D. Futility of testing for factor V Leiden. Blood Transfus. (2012) 10:260–3. 10.2450/2012.0097-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tientadakul P, Chinthammitr Y, Sanpakit K, Wongwanit C, Nilanont Y. Inappropriate use of protein C, protein S, and antithrombin testing for hereditary thrombophilia screening: an experience from a large university hospital. Int J Lab Hematol. (2011) 33:593–600. 10.1111/j.1751-553X.2011.01332.x [DOI] [PubMed] [Google Scholar]
- 31.Blinkenberg EØ, Kristoffersen A-H, Sandberg S, Steen VM, Houge G. Usefulness of factor V Leiden mutation testing in clinical practice. Eur J Hum Genet. (2010) 18:862–6. 10.1038/ejhg.2010.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laberge AM, Psaty BM, Hindorff LA, Burke W. Use of Factor V Leiden genetic testing in practice and impact on management. Genet Med. (2009) 11:750–6. 10.1097/GIM.0b013e3181b3a697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middeldorp S, Van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. (2008) 143:321–35. 10.1111/j.1365-2141.2008.07339.x [DOI] [PubMed] [Google Scholar]
- 34.Zöller B, Svensson PJ, Dahlbäck B, Lind-Hallden C, Hallden C, Elf J. Genetic risk factors for venous thromboembolism. Expert Rev Hematol. (2020) 13:971–81. 10.1080/17474086.2020.1804354 [DOI] [PubMed] [Google Scholar]
- 35.Hotoleanu C. Genetic risk factors in venous thromboembolism. In: Islam MS, editor. Thrombosis and Embolism: From Research to Clinical Practice, Vol. 1. Cham: Springer International Publishing; (2017). p. 253–72. [DOI] [PubMed] [Google Scholar]
- 36.Olie V, Zhu T, Martinez I, Scarabin PY, Emmerich J. Sex-specific risk factors for recurrent venous thromboembolism. Thromb Res. (2012) 130:16–20. 10.1016/j.thromres.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 37.Sveinsdottir SV, Saemundsson Y, Isma N, Gottsater A, Svensson PJ. Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thromb Res. (2012) 130:467–71. 10.1016/j.thromres.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 38.Weingarz L, Schindewolf M, Schwonberg J, Hecking C, Wolf Z, Erbe M, et al. Thrombophilia and risk of VTE recurrence according to the age at the time of first VTE manifestation. VASA. (2015) 44:313–23. 10.1024/0301-1526/a000447 [DOI] [PubMed] [Google Scholar]
- 39.Bruzelius M, Ljungqvist M, Bottai M, Bergendal A, Strawbridge RJ, Holmstrom M, et al. F11 is associated with recurrent VTE in women. A prospective cohort study. Thromb Haemost. (2016) 115:406–14. 10.1160/th15-06-0459 [DOI] [PubMed] [Google Scholar]
- 40.Limperger V, Kenet G, Kiesau B, Kother M, Schmeiser M, Langer F, et al. Role of prothrombin 19911 A>G polymorphism, blood group and male gender in patients with venous thromboembolism: Results of a German cohort study. J Thromb Thrombolysis. (2020) 51:494–501. 10.1007/s11239-020-02169-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodeib H, Youssef A, Allam AA, Selim A, Tawfik MA, Abosamak MF, et al. Genetic risk profiling associated with recurrent unprovoked venous thromboembolism. Genes. (2021) 12:874. 10.3390/genes12060874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichinger S, Pabinger I, Stumpflen A, Hirschl M, Bialonczyk C, Schneider B, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. (1997) 77:624–8. 10.1055/s-0038-1656023 [DOI] [PubMed] [Google Scholar]
- 43.Eichinger S, Weltermann A, Mannhalter C, Minar E, Bialonczyk C, Hirschl M, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. (2002) 162:2357–60. 10.1001/archinte.162.20.2357 [DOI] [PubMed] [Google Scholar]
- 44.Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. (2003) 362:523–6. 10.1016/S0140-6736(03)14111-6 [DOI] [PubMed] [Google Scholar]
- 45.Mansilha A, Araujo F, Severo M, Sampaio SM, Toledo T, Albuquerque R. Genetic polymorphisms and risk of recurrent deep venous thrombosis in young people: prospective cohort study. Eur J Vasc Endovasc Surg. (2005) 30:545–9. 10.1016/j.ejvs.2005.05.038 [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Porras JR, Garcia-Sanz R, Alberca I, Lopez ML, Balanzategui A, Gutierrez O, et al. Risk of recurrent venous thrombosis in patients with G20210A mutation in the prothrombin gene or factor V Leiden mutation. Blood Coagul Fibrinolysis. (2006) 17:23–8. 10.1097/01.mbc.0000201488.33143.09 [DOI] [PubMed] [Google Scholar]
- 47.Zee RY, Bubes V, Shrivastava S, Ridker PM, Glynn RJ. Genetic risk factors in recurrent venous thromboembolism: a multilocus, population-based, prospective approach. Clin Chim Acta. (2009) 402:189–92. 10.1016/j.cca.2009.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mean M, Limacher A, Stalder O, Angelillo-Scherrer A, Alberio L, Fontana P, et al. Do Factor V Leiden and Prothrombin G20210A mutations predict recurrent venous thromboembolism in older patients? Am J Med. (2017) 130:1220.e17–e22. 10.1016/j.amjmed.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 49.Kapoor S, Opneja A, Gollamudi J, Nayak LV. Prior history of venous thromboembolism is a significant risk factor for recurrence of thrombosis after cancer diagnosis. Blood. (2020) 136(Suppl. 1):32–3. 10.1182/blood-2020-141961 [DOI] [Google Scholar]
- 50.Pabinger I, Ay C, Dunkler D, Thaler J, Reitter EM, Marosi C, et al. Factor V Leiden mutation increases the risk for venous thromboembolism in cancer patients - results from the Vienna Cancer And Thrombosis Study (CATS). J Thromb Haemost. (2015) 13:17–22. 10.1111/jth.12778 [DOI] [PubMed] [Google Scholar]
- 51.Mohammed AI, Abdulqader AMR, Jalal SD, Mahmood SN. ABO blood groups and thrombophilia markers in patients with unstimulated thrombosis in Kurdistan Region of Iraq. Clin Appl Thromb Hemost. (2020) 26:1076029620922913. 10.1177/1076029620922913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hylckama Vlieg A, Baglin CA, Bare LA, Rosendaal FR, Baglin TP. Proof of principle of potential clinical utility of multiple SNP analysis for prediction of recurrent venous thrombosis. J Thromb Haemost. (2008) 6:751–4. 10.1111/j.1538-7836.2008.02920.x [DOI] [PubMed] [Google Scholar]
- 53.Timp JF, Braekkan SK, Lijfering WM, Van Hylckama Vlieg A, Hansen JB, Rosendaal FR, et al. Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP). PLoS Medicine. (2019) 16:e1002883. 10.1371/journal.pmed.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Hylckama Vlieg A, Flinterman LE, Bare LA, Cannegieter SC, Reitsma PH, Arellano AR, et al. Genetic variations associated with recurrent venous thrombosis. Circ Cardiovasc Genet. (2014) 7:806–13. 10.1161/CIRCGENETICS.114.000682 [DOI] [PubMed] [Google Scholar]
- 55.Moher D Liberati A Tetzlaff J Altman DG The The PG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. (2018) 2:3226–56. 10.1182/bloodadvances.2018024828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2012) 141(2 Suppl.):e351S–e418S. 10.1378/chest.11-2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. (2010) 15:399–406. 10.1177/1358863X10378788 [DOI] [PubMed] [Google Scholar]
- 59.Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; (2014). [Google Scholar]
- 60.Huber CA, Schwenkglenks M, Rapold R, Reich O. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord. (2014) 14:44. 10.1186/1472-6823-14-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haller E, Watzke B, Blozik E, Rosemann T, Reich O, Huber CA, et al. Antidepressant prescription practice and related factors in Switzerland: a cross-sectional analysis of health claims data. BMC Psychiatry. (2019) 19:196. 10.1186/s12888-019-2178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huber CA, Nagler M, Rosemann T, Blozik E, Näpflin M, Markun S. Trends in micronutrient laboratory testing in Switzerland: a 7-year retrospective analysis of healthcare claims data. Int J Gen Med. (2020) 13:1341–8. 10.2147/IJGM.S275406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. (2001) 37:215–8. 10.1016/S0735-1097(00)01080-9 [DOI] [PubMed] [Google Scholar]
- 64.Lindmarker P, Schulman S, Sten-Linder M, Wiman B, Egberg N, Johnsson H. The risk of recurrent venous thromboembolism in carriers and non-carriers of the G1691A allele in the coagulation factor V gene and the G20210A allele in the prothrombin gene. Thromb Haemost. (1999) 81:684–9. 10.1055/s-0037-1614554 [DOI] [PubMed] [Google Scholar]
- 65.Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. (2005) 293:2352–61. 10.1001/jama.293.19.2352 [DOI] [PubMed] [Google Scholar]
- 66.Lijfering WM, Christiansen SC, Rosendaal FR, Cannegieter SC. Contribution of high factor VIII, IX and XI to the risk of recurrent venous thrombosis in factor V Leiden carriers. J Thromb Haemost. (2009) 7:1944–6. 10.1111/j.1538-7836.2009.03580.x [DOI] [PubMed] [Google Scholar]
- 67.Simioni P, Prandoni P, Lensing AW, Scudeller A, Sardella C, Prins MH, et al. The risk of recurrent venous thromboembolism in patients with an Arg506–>Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med. (1997) 336:399–403. 10.1056/NEJM199702063360602 [DOI] [PubMed] [Google Scholar]
- 68.Simioni P, Prandoni P, Lensing AW, Manfrin D, Tormene D, Gavasso S, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. (2000) 96:3329–33. 10.1182/blood.V96.10.3329.h8003329a_3329_3333 [DOI] [PubMed] [Google Scholar]
- 69.Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. (1999) 340:901–7. 10.1056/NEJM199903253401201 [DOI] [PubMed] [Google Scholar]
- 70.Vossen CY, Walker ID, Svensson P, Souto JC, Scharrer I, Preston FE, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. (2005) 25:1992–7. 10.1161/01.ATV.0000174806.76629.7b [DOI] [PubMed] [Google Scholar]
- 71.Chaireti R, Jennersjö C, Lindahl TL. Thrombin generation and D-dimer concentrations in a patient cohort investigated for venous thromboembolism. Relations to venous thrombosis, factor V Leiden and prothrombin G20210A: The LIST study. Thromb Res. (2009) 124:178–84. 10.1016/j.thromres.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 72.Chaireti R, Jennersjo C, Lindahl TL. Is thrombin generation at the time of an acute thromboembolic episode a predictor of recurrence? The LInkoping Study on Thrombosis (LIST)–a 7-year follow-up. Thromb Res. (2013) 131:135–9. 10.1016/j.thromres.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 73.Kearon C, Julian JA, Kovacs MJ, Anderson DR, Wells P, MacKinnon B, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. (2008) 112:4432–6. 10.1182/blood-2008-06-163279 [DOI] [PubMed] [Google Scholar]
- 74.Baglin T, Luddington R, Brown K, Baglin C. High risk of recurrent venous thromboembolism in men. J Thromb Haemost. (2004) 2:2152–5. 10.1111/j.1538-7836.2004.01050.x [DOI] [PubMed] [Google Scholar]
- 75.Palareti G, Legnani C, Cosmi B, Valdre L, Lunghi B, Bernardi F, et al. Predictive value of D-dimer test for recurrent venous thromboembolism after anticoagulation withdrawal in subjects with a previous idiopathic event and in carriers of congenital thrombophilia. Circulation. (2003) 108:313–8. 10.1161/01.CIR.0000079162.69615.0F [DOI] [PubMed] [Google Scholar]
- 76.Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. (2004) 124:504–10. 10.1046/j.1365-2141.2003.04795.x [DOI] [PubMed] [Google Scholar]
- 77.Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. (2005) 94:969–74. 10.1160/TH05-02-0095 [DOI] [PubMed] [Google Scholar]
- 78.Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. (2007) 92:199–205+III–IV. 10.3324/haematol.10516 [DOI] [PubMed] [Google Scholar]
- 79.Prandoni P, Tormene D, Spiezia L, Pesavento R, Simioni P. Duration of anticoagulation and risk of recurrent thromboembolism in carriers of factor V Leiden or prothrombin mutation. J Thromb Haemost. (2008) 6:2223–4. 10.1111/j.1538-7836.2008.03173.x [DOI] [PubMed] [Google Scholar]
- 80.Prandoni P, Prins MH, Lensing AW, Ghirarduzzi A, Ageno W, Imberti D, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. (2009) 150:577–85. 10.7326/0003-4819-150-9-200905050-00003 [DOI] [PubMed] [Google Scholar]
- 81.Nemeth B, Lijfering WM, Nelissen RGHH, Schipper IB, Rosendaal FR, Le Cessie S, et al. Risk and risk factors associated with recurrent venous thromboembolism following surgery in patients with history of venous Thromboembolism. JAMA Netw Open. (2019) 2:e193690. 10.1001/jamanetworkopen.2019.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poli D, Antonucci E, Ciuti G, Abbate R, Prisco D. Anticoagulation quality and the risk of recurrence of venous thromboembolism. Thromb Haemost. (2007) 98:1148–50. 10.1160/TH07-05-0348 [DOI] [PubMed] [Google Scholar]
- 83.Obeidat NM. The effect of genetically related risk factors on the recurrence rate of acute pulmonary embolism in a Tertiary Teaching Hospital in Jordan. Jordan Med J. (2010) 44:398–403. [Google Scholar]
- 84.Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. (2008) 179:417–26. 10.1503/cmaj.080493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gandara E, Kovacs MJ, Kahn SR, Wells PS, Anderson DA, Chagnon I, et al. Non-OO blood type influences the risk of recurrent venous thromboembolism: a cohort study. Thromb Haemost. (2013) 110:1172–9. 10.1160/TH13-06-0488 [DOI] [PubMed] [Google Scholar]
- 86.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. (2008) 168:1678–83. 10.1001/archinte.168.15.1678 [DOI] [PubMed] [Google Scholar]
- 87.Hron G, Eichinger S, Weltermann A, Quehenberger P, Halbmayer WM, Kyrle PA. Prediction of recurrent venous thromboembolism by the activated partial thromboplastin time. J Thromb Haemost. (2006) 4:752–6. 10.1111/j.1538-7836.2006.01868.x [DOI] [PubMed] [Google Scholar]
- 88.Lechner D, Wiener C, Weltermann A, Eischer L, Eichinger S, Kyrle PA. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost. (2008) 6:1269–74. 10.1111/j.1538-7836.2008.02998.x [DOI] [PubMed] [Google Scholar]
- 89.Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. (2008) 54:2042–8. 10.1373/clinchem.2008.112243 [DOI] [PubMed] [Google Scholar]
- 90.Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. (2004) 350:2558–63. 10.1056/NEJMoa032959 [DOI] [PubMed] [Google Scholar]
- 91.Eichinger S, Pecheniuk NM, Hron G, Deguchi H, Schemper M, Kyrle PA, et al. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. (2007) 115:1609–14. 10.1161/CIRCULATIONAHA.106.649954 [DOI] [PubMed] [Google Scholar]
- 92.Schonauer V, Kyrle PA, Weltermann A, Minar E, Bialonczyk C, Hirschl M, et al. Superficial thrombophlebitis and risk for recurrent venous thromboembolism. J Vasc Surg. (2003) 37:834–8. 10.1067/mva.2003.157 [DOI] [PubMed] [Google Scholar]
- 93.Hoke M, Kyrle PA, Minar E, Bialonzcyk C, Hirschl M, Schneider B, et al. Tissue factor pathway inhibitor and the risk of recurrent venous thromboembolism. Thromb Haemost. (2005) 94:787–90. 10.1160/TH05-06-0412 [DOI] [PubMed] [Google Scholar]
- 94.Eichinger S, Weltermann A, Minar E, Stain M, Schonauer V, Schneider B, et al. Symptomatic pulmonary embolism and the risk of recurrent venous thromboembolism. Arch Intern Med. (2004) 164:92–6. 10.1001/archinte.164.1.92 [DOI] [PubMed] [Google Scholar]
- 95.Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. (2000) 343:457–62. 10.1056/NEJM200008173430702 [DOI] [PubMed] [Google Scholar]
- 96.Kyrle PA, Eichinger S, Pabinger I, Stumpflen A, Hirschl M, Bialonczyk C, et al. Prothrombin fragment F1+2 is not predictive for recurrent venous thromboembolism. Thromb Haemost. (1997) 77:829–33. 10.1055/s-0038-1656062 [DOI] [PubMed] [Google Scholar]
- 97.Ahmad A, Sundquist K, Zoller B, Dahlback B, Svensson PJ, Sundquist J, et al. Identification of polymorphisms in Apolipoprotein M gene and their relationship with risk of recurrent venous thromboembolism. Thromb Haemost. (2016) 116:432–41. 10.1160/TH16-03-0178 [DOI] [PubMed] [Google Scholar]
- 98.Ahmad A, Sundquist K, Zoller B, Svensson PJ, Sundquist J, Memon AA. Thrombomodulin gene c.1418C>T polymorphism and risk of recurrent venous thromboembolism. J Thromb Thrombolysis. (2016) 42:135–41. 10.1007/s11239-015-1328-x [DOI] [PubMed] [Google Scholar]
- 99.Sundquist K, Wang X, Svensson PJ, Sundquist J, Hedelius A, Larsson Lonn S, et al. Plasminogen activator inhibitor-1 4G/5G polymorphism, factor V Leiden, prothrombin mutations and the risk of VTE recurrence. Thromb Haemost. (2015) 114:1156–64. 10.1160/TH15-01-0031 [DOI] [PubMed] [Google Scholar]
- 100.Strandberg K, Svensson PJ, Ohlin AK. Venous thromboembolism in carriers of the Factor V Leiden mutation and in patients without known thrombophilic risk factor; prediction of recurrence and APC-PCI complex concentration and/or soluble thrombomodulin antigen and activity. Thromb Res. (2007) 121:145–51. 10.1016/j.thromres.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 101.Le Moigne E, Delluc A, Tromeur C, Nowak E, Mottier D, Lacut K, et al. Risk of recurrent venous thromboembolism among young women after a first event while exposed to combined oral contraception versus not exposed to: a cohort study. Thromb Res. (2013) 132:51–5. 10.1016/j.thromres.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 102.Moigne EL, Delluc A, Novak E, Mottier D, Gal GL. Risk of recurrence after contraception-related venous thrombosis: Cohort study. J Thromb Haemost. (2011) 2:174. 10.1016/S0049-3848(11)70136-0 [DOI] [Google Scholar]
- 103.Franco Moreno AI, Garcia Navarro MJ, Ortiz Sanchez J, Martin Diaz RM, Madronal Cerezo E, de Ancos Aracil CL, et al. A risk score for prediction of recurrence in patients with unprovoked venous thromboembolism (DAMOVES). Eur J Intern Med. (2016) 29:59–64. 10.1016/j.ejim.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 104.Bizien N, Noel-Savina E, Tromeur C, Delluc A, Mottier D, Leroyer C, et al. Age is a major risk factor of venous thromboembolism (VTE). Eur Respir J. (2011) 38(Suppl. 55):p3936. [Google Scholar]
- 105.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis With R: A Hands-On Guide, 1st Edn. Boca Raton, FL; London: Chapman & Hall/CRC Press; (2021). [Google Scholar]
- 106.Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. (2011) 342:d813. 10.1136/bmj.d813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagler M, Van Kuijk SMJ, Ten Cate H, Prins MH, Ten Cate-Hoek AJ. Predicting recurrent venous thromboembolism in patients with deep-vein thrombosis: development and internal validation of a potential new prediction model (Continu-8). Front Cardiovasc Med. (2021) 8:655226. 10.3389/fcvm.2021.655226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 109.Bruinstroop E, Klok FA, Van de Ree MA, Oosterwuk FL, Huisman MV. Elevated d-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost. (2009) 7:611–8. 10.1111/j.1538-7836.2009.03293.x [DOI] [PubMed] [Google Scholar]
- 110.Ensor J, Riley RD, Moore D, Snell KI, Bayliss S, Fitzmaurice D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open. (2016) 6:e011190. 10.1136/bmjopen-2016-011190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. (2012) 10:1019–25. 10.1111/j.1538-7836.2012.04735.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.