Abstract
Background:
Coronavirus disease 2019 (COVID-19) hospitalization definitions do not include a disease severity assessment. Thus, we sought to identify a simple and objective mechanism for identifying hospitalized severe cases and to measure the impact of vaccination on trends.
Methods:
All admissions to a Veterans’ Affairs (VA) hospital, where routine inpatient screening is recommended, between March 1, 2020, and November 22, 2021, with laboratory-confirmed severe acute respiratory coronavirus virus 2 (SARS-CoV-2) were included. Moderate-to-severe COVID-19 was defined as any oxygen supplementation or any oxygen saturation (SpO2) <94% between 1 day before and 2 weeks after the positive SARS-CoV-2 test. Admissions with moderate-to-severe disease were divided by the total number of admissions, and the proportion of admissions with moderate-to-severe COVID-19 was modelled using a penalized spline in a Poisson regression and stratified by vaccination status. Dexamethasone receipt and its correlation with moderate-to-severe cases was also assessed.
Results:
Among 67,025 admissions with SARS-CoV-2, the proportion with hypoxemia or supplemental oxygen fell from 64% prior to vaccine availability to 56% by November 2021, driven in part by lower rates in vaccinated patients (vaccinated, 52% versus unvaccinated, 58%). The proportion of cases of moderate-to-severe disease identified using SpO2 levels and oxygen supplementation was highly correlated with dexamethasone receipt (correlation coefficient, 0.95), and increased after July 1, 2021, concurrent with δ (delta) variant predominance.
Conclusions:
A simple and objective definition of COVID-19 hospitalizations using SpO2 levels and oxygen supplementation can be used to track pandemic severity. This metric could be used to identify risk factors for severe breakthrough infections, to guide clinical treatment algorithms, and to detect trends in changes in vaccine effectiveness over time and against new variants.
Coronavirus disease 2019 (COVID-19) hospitalization rates are used to track the community burden of severe acute respiratory coronavirus virus 2 (SARS-CoV-2). The Centers for Disease Control and Prevention (CDC) COVID-Net defines a COVID-19 hospitalization as any patient admitted to the hospital within 14 days of a laboratory-confirmed diagnosis of SARS-CoV-2 infection, regardless of the reason for the admission. 1
Vaccination impacts the association between SARS-CoV-2 case rates and hospitalization rates because vaccinated patients develop less severe disease and unvaccinated patients tend to be younger and healthier. 2 Thus, reporting metrics designed to determine the level of risk in a community, and to inform policy-making decisions about restrictions and mitigation strategies, should increasingly focus on hospitalizations rather than cases. However, after vaccines became available, 3,4 routine inpatient screening, common or mandated in many facilities, may identify incidental cases; thus, additional data about disease severity are needed to inform policy and practice.
Although many patients are hospitalized for management of severe disease, patients with asymptomatic or mild COVID-19 may be in the hospital for 3 main reasons: (1) admission for reasons unrelated to COVID-19 but infection identified as part of routine admission, inpatient, or predischarge screening (eg, prior to admission to a long-term care facility), (2) admission for reasons unrelated to COVID-19 and subsequently develop symptoms during hospitalization and are therefore tested but never develop severe illness, or (3) admission for mild COVID-19 for observation due to underlying comorbidities or to avoid exposing household contacts. If hospitalizations are to guide policy decisions, patients hospitalized for the management of COVID-19 disease must be distinguished from patients who are hospitalized and are incidentally found to be infected with SARS-CoV-2.
In this retrospective, national cohort study, we measured trends in severity of respiratory disease among inpatients with COVID-19 and how vaccination status has affected these trends. Our results inform the creation of a simple, objective metric comprised of variables easily extracted from the electronic health record (EHR) that could be used to improve the value of the hospitalization metric for reflecting cases of severe disease in the postvaccine era.
Methods
All inpatient admissions to a Veterans’ Affairs (VA) hospital between March 1, 2020, and November 22, 2021, with a laboratory-confirmed diagnosis of SARS-CoV-2 up to 14 days prior to or during the admission were included, and data were extracted electronically from the VA Corporate Data Warehouse and the VA COVID-19 Shared Data Resource. 5 Within the VA, screening for SARS-CoV-2 infection has been recommended for all inpatient admissions regardless of the presence of symptoms since May 12, 2020, and the recommendation is included in the VA COVID-19 testing manual. 6 Some facilities, particularly those impacted early during the first wave, implemented routine screening of all inpatients earlier than May 12, 2021. At least 1 VA facility tests all inpatients 72 hours after admission, regardless of symptoms. In VA New England Healthcare, genetic sequencing of a sample of cases revealed that the δ (delta) variant was predominant by July 1, 2021, but not for most of June 2021 (unpublished data).
Patients were considered fully vaccinated if ≥14 days had elapsed after receipt of a single dose of an adenovirus vaccine or after receipt of both doses of either mRNA-1273 (Moderna) or BNT162b2 (Pfizer) vaccines. Data on third doses were not available for inclusion in the analysis. Patient-level comorbidities were measured using the CMS Medicare and Medicaid Chronic Conditions Data Warehouse definitions. 7
Moderate-to-severe COVID-19 was defined as receipt of any oxygen supplementation (within the VA, any supplementation ≥0.5 L/min is recorded) or any documented oxygen saturation (SpO2) <94% recorded in the vital signs domain during an inpatient admission at any time between 1 day before and 2 weeks after the positive SARS-CoV-2 test. These criteria were adapted from the most stringent cutoffs for respiratory distress included in the National Institutes of Health (NIH) COVID-19 severity score, and they were selected based on their objective and reproducible nature and ability to reliably capture them from the EHR without the need for manual chart review. 8 A secondary analysis was conducted using a clinical definition of respiratory distress (oxygen supplementation ≥2L NC or any documented SpO2 <90%). In addition, several sensitivity analyses were conducted: one using only SpO2 levels and not oxygen supplementation, another stratified by region, another excluding patients with chronic obstructive pulmonary disease (COPD) or related conditions, and another excluding patients with lung cancer (both defined using Chronic Conditions Data Warehouse definitions).
Admissions meeting criteria for moderate-to-severe disease were divided by the total numbers of admissions in the cohort over different calendar periods. The changing proportion of admissions with moderate-to-severe COVID-19 was modelled as a smooth function of time using a penalized spline in a Poisson regression and stratified by vaccination status. A χ2 test of proportion was completed to assess whether severe breakthrough infections increased during the post–δ-variant period.
Dexamethasone was demonstrated in a clinical trial to improve outcomes among patients receiving supplemental oxygen and is therefore used to treat severe COVID-19 but not mild disease. 9 As an additional assessment of severity of illness, the proportion of cases receiving dexamethasone treatment was also evaluated as a function of time. Dexamethasone use, rather than other therapeutic options, was chosen given its relative consistency for severe disease compared to other therapeutic options, such as remdesivir use, which is variable by facility within the VA health system. 10,11 A Pearson test of correlation was performed between the series of weekly proportions of COVID-19 hospitalizations with moderate-to-severe COVID-19 and the series of weekly proportions of COVID-19 hospitalizations during which dexamethasone was received.
The study was approved by the VA Boston Research and Development Committee with a waiver of informed consent (protocol no. 3328-X).
Results
Among 67,025 admissions (ie, 33,166 before January 24, 2021, when the first patient in the cohort was fully vaccinated, and 33,859 after) representing 53,607 unique patients admitted with laboratory-confirmed SARS-CoV-2, 40,331 admissions had moderate-to-severe disease as measured by SpO2 levels and/or receipt of supplemental oxygen. The median age among all hospitalized patients was 70.9 (mean, 68.5; SD, 14.1); 41,897 (62.5%) were white; and 63,208 (94.3%) were male. Among hospitalized patients, 24% had heart failure, 27.6% percent had COPD, and 29.9% were obese (Table 1).
Table 1.
Comorbidities of Veteran Patients Hospitalized with SARS-CoV-2 Infection a
| Comorbidity | All Hospitalizations, No. (%) |
Moderate-to-Severe COVID-19, No. (%) |
Cases Without Low SpO2 or Oxygen Supplementation, No. (%) |
|---|---|---|---|
| Age, median y (IQR) | 70.7 (60.7–76.6) | 71.6 (62.4–77.4) | 68.3 (57.3–75.1) |
| Hypertension | 42,410 (79.1%) | 28,348 (81.3) | 14,062 (75.1) |
| Hyperlipidemia | 36,321 (67.8) | 24,265 (69.6) | 12,056 (64.4) |
| Chronic kidney disease | 29,455 (54.9) | 20,034 (57.4) | 8,279 (44.2) |
| Diabetes | 25,70 (48.1) | 17,491 (50.1) | 8,279 (44.2) |
| Ischemic heart disease | 19,177 (35.8) | 12,965 (37.2) | 6,212 (33.2) |
| Benign prostatic hypertrophy | 16,865 (31.5) | 11,594 (33.2) | 5,271 (28.2) |
| Obesity | 16,019 (29.9) | 11,292 (32.4) | 4,727 (25.2) |
| COPD and related conditions | 14,772 (27.6) | 10,682 (30.6) | 4,090 (21.8) |
| Arthritis (rheumatoid or osteoarthritis) | 13,960 (26.0) | 9,323 (26.7) | 4,637 (24.8) |
| Heart failure | 12,856 (24.0) | 8,866 (25.4) | 3,990 (21.3) |
| Peripheral neuropathy | 12,467 (23.3) | 8,291 (23.8) | 4,176 (22.3) |
| Atrial fibrillation | 10,940 (20.4) | 7,490 (21.5) | 3,450 (18.4) |
| Alzheimer’s disease and related conditions | 9,397 (17.5) | 6,665 (19.1) | 2,732 (14.6) |
| Sensory deafness and hearing loss | 7,954 (14.8) | 5,465 (15.7) | 2,489 (13.3) |
| Acquired hypothyroidism | 6,705 (12.5) | 4,678 (13.4) | 2,027 (10.8) |
| Peripheral vascular disease | 6,605 (12.3) | 4,442 (12.7) | 2,163 (11.6) |
| Pressure and chronic ulcers | 5,257 (9.8) | 3,556 (10.2) | 1,701 (9.1) |
| Lung cancer | 1,469 (2.7) | 1,052 (3.0) | 417 (2.2) |
| Leukemias and lymphomas | 1,347 (2.5) | 947 (2.7) | 400 (2.1) |
| Alzheimer’s disease | 1,208 (2.3) | 8,47 (2.4) | 361 (1.9) |
| Sensory blindness and visual impairment | 1,110 (2.1) | 784 (2.2) | 326 (1.7) |
Note. SpO2, oxygen saturation; COPD, chronic obstructive pulmonary disease.
Comorbidities calculated using the CMS Chronic Conditions Warehouse definitions and are reported on a per-person rather than per-hospitalization basis. Conditions that are not associated at a level of P < .001 with increased risk of hospitalization with hypoxemia are not listed.
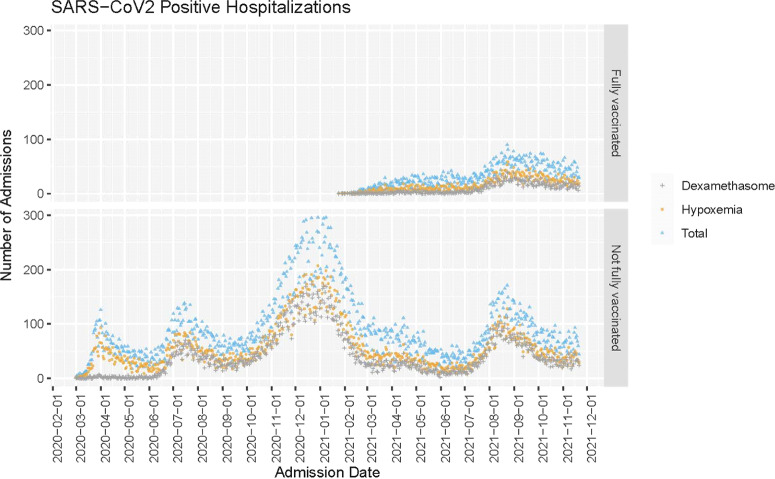
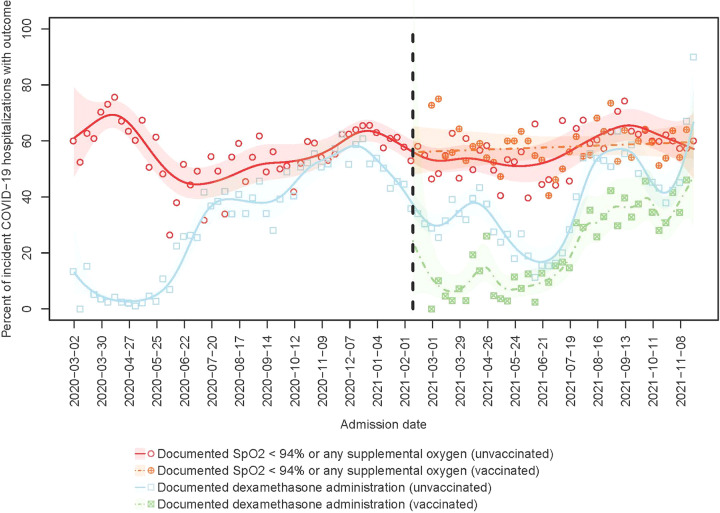
The total number of COVID-19 hospitalizations in the VA tracks with the national data from CDC COVID-Net (Fig. 1). 1 The proportion of inpatients with any documented SpO2 <94% or oxygen supplementation changed over time (P < .001) (Fig. 2). From March 1, 2020, through January 23, 2021, the proportion of hospitalized patients with moderate-to-severe disease was 64.0% (95% CI, 63.1%–64.9%) and from January 21, 2021, through November 21, 2021, this proportion was 56.4% (95% CI, 55.6%–57.2%).
Fig. 1.
Raw count of hospitalizations with COVID-19 diagnosis in the National VA Healthcare System from March 1, 2020, to November 22, 2021. Total cases are indicated in blue, cases with dexamethasone receipt are indicated in grey, and cases with documented oxygen saturation (SpO2) <94% or any oxygen supplementation are indicated in orange.
Fig. 2.
Percent of incident COVID-19 hospitalizations meeting criteria for moderate-to-severe disease from March 1, 2020, to November 22, 2021. Points represent observed percentages of incident hospitalizations with documented oxygen saturation (SpO2) <94% or any supplemental oxygen usage (circles) or use of dexamethasone (squares) in each week of the study period, among unvaccinated (blank) or vaccinated (cross) patients. Lines (respectively, shaded areas) represent smoothed estimates (respectively, their 95% confidence intervals) over time based on Poisson regression with a penalized spline term, among unvaccinated (solid) and vaccinated (dashed) patients.
Among 33,859 admissions on or after January 24, 2021, (24,524 unvaccinated and 9,335 vaccinated), 19,097 met the case definition for moderate-to-severe disease (14,277 unvaccinated and 4,820 vaccinated). Among unvaccinated inpatients during this period, 58.2% (95% CI, 57.3%–59.2%) had at least 1 documented SpO2 <94%, compared with 51.6% (95% CI, 50.2%–53.1%) of vaccinated inpatients (Fig. 2). Proportions of COVID-19 admissions receiving treatment with dexamethasone dropped in parallel with the reduction in proportion of cases with moderate-to-severe disease (Pearson correlation coefficient, 0.95). After July 1, 2021, when the δ (delta) variant became predominant, the proportion of breakthrough infections with moderate-to- severe disease increased, as measured by either SpO2 and/or oxygen supplementation or receipt of dexamethasone (P < .05). The increase was evident in all US Census regions, although it peaked earlier in the Southeast (Supplementary Fig. 1 online).
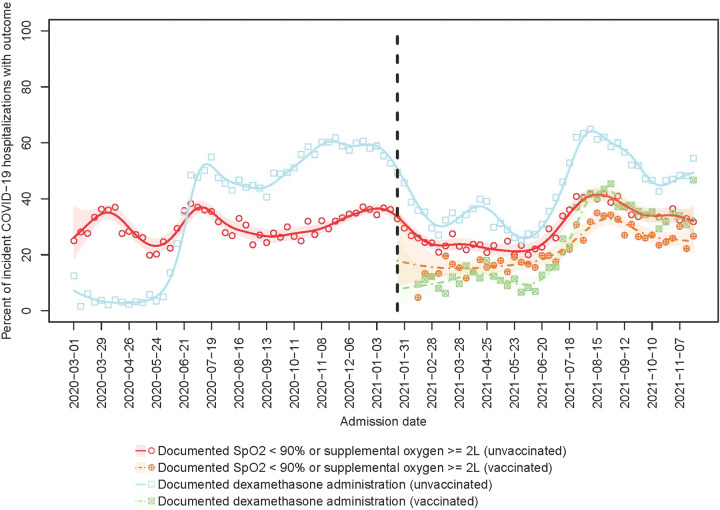
Findings from the secondary analysis using a clinical definition of respiratory distress (SpO2 <90 and/or ≥2 L oxygen supplementation) were similar. Overall, 20,737 admissions met the stricter case definition, including 32.4% (95% CI, 31.8%–33.0%) of COVID-19 admissions prior to January 24, 2021, and 29.5% (95% CI, 29.0%–30.1%) afterward. Among them, 31.2% (95% CI, 30.5%–31.9%) were unvaccinated patients compared with 25.0% (95% CI, 24.0%–26.1%) who were vaccinated (Fig. 3).
Fig. 3.
Percent of incident COVID-19 hospitalizations with severe disease (SpO2 <90% and/or oxygen supplementation ≥2L) from March 1, 2020, to November 22, 2021. Points represent observed percentages of incident hospitalizations with documented oxygen saturation (SpO2) <90% or receipt of ≥2 L oxygen supplementation (circles) or use of dexamethasone (squares) in each week of the study period, among unvaccinated (blank) or vaccinated (cross) patients. Lines (respectively, shaded areas) represent smoothed estimates (respectively, their 95% confidence intervals) over time based on Poisson regression with a penalized spline term, among unvaccinated (solid) and vaccinated (dashed) patients.
In the sensitivity analyses, the metric performed similarly with or without inclusion of the oxygen supplementation criterion in addition to SpO2 cutoffs (Supplementary Fig. 2 online). When patients with lung cancer or COPD were excluded, the proportion of cases with moderate-to-severe disease decreased, but overall trends were similar (Supplementary Figs. 3 and 4 online).
Discussion
Early in the pandemic, SARS-COV-2 cases were strongly correlated with hospitalizations, and both reflected community risk. Because vaccine availability is widespread, these metrics have become uncoupled, as reflected by the falling proportion of SARS-CoV-2 admissions with moderate-to-severe COVID-19. 2 The uncoupling is more pronounced in fully vaccinated individuals, who continue to be strongly protected against severe disease. However, during the period after June 2021, when the δ (delta) variant became predominant, there appeared to be an increasing proportion of severe breakthrough cases. 12,13 The changes in frequency of admissions with moderate-to-severe disease as defined using SpO2 levels and/or oxygen supplementation are strongly correlated with the changes in proportions of cases receiving dexamethasone, which is recommended for patients with severe but not mild disease, 9 supporting an objective and reproducible approach that uses SpO2 values and supplemental oxygenation receipt to identify patients hospitalized for COVID-19.
For the purposes of monitoring hospital capacity and estimating resource needs, such as personal protective equipment, reporting all COVID-19 hospitalizations will continue to be important for the foreseeable future. Our findings suggest that reporting definitions should be revised to also include disease severity metrics to reflect the changing nature of the pandemic, particularly among vaccinated patients and in regions with high levels of vaccine uptake to identify severe cases. Definitions that include a measure of disease severity are also important for identifying patients at high risk of severe disease despite vaccination. They could be used as part of an early monitoring system to identify waning vaccine effectiveness for preventing critical illness 14 or to alert to the possibility of viral evolution, such as the emergence of new variants, that cause clinically severe disease despite vaccination. Early identification of an increase in the proportion of cases with severe disease would allow for rapid response, such as deployment of viral genetic testing and sequencing, recommendations for booster vaccinations, or need for new vaccine formulations. Therefore, early identification of the most clinically significant problems is likely to translate into improved pandemic responses and better clinical outcomes.
Our definition was adapted from the NIH criteria but includes some adjustments. Specifically, our definition did not include patients who may have severe COVID-19 symptoms that are nonrespiratory in nature, such as severe gastrointestinal distress. In addition, we did not include imaging findings, which are included in NIH criteria, in our electronic surveillance definition. Imaging findings were not included for 2 reasons. First, imaging results are not structured or standardized and are thus not ideal for inclusion in a simple electronic surveillance tool that is easy to program and reproduce. Second, during the conduct of a multicenter COVID-19 therapeutics trial in which we screened hundreds of inpatients for severe disease, we found no cases that met imaging criteria for severe disease that did not also have other objective criteria of clinical respiratory illness. 15 Thus, while it is possible that including imaging results would change severe hospitalization estimates somewhat, the impact is likely to be extremely small, and including imaging results would substantially impact the feasibility of the simple approach for measuring severe cases.
In the adapted NIH definition, we included any oxygen supplementation to ensure all cases with any degree of respiratory discomfort were captured. Depending upon the goals of the surveillance, the more typical clinical cutoffs, which appear to track closely with prescribed treatment for severe disease, may be preferred over the cutoffs using the adapted NIH criteria, which may overestimate cases of severe disease. This is particularly true of the oxygen supplementation metric, which may include individuals for whom very low levels of oxygen support are provided for comfort rather than therapeutic effect.
The current definition of COVID-19 hospitalizations includes progressively more mild or incidental diagnoses for example, cases identified due to institutional screening practices prior to admission or discharge, rather than hospitalizations due to severe COVID-19, particularly in vaccinated patients. 16 Other recent studies similarly found that current definitions of “COVID-19 hospitalizations” combined with routine, and often mandatory, screening of all inpatient admissions may substantially overestimate the number of hospitalizations caused by SARS-CoV-2 infection. 17,18 In a pediatric population, 41% of reported admissions associated with SARS-CoV-2 infection were for reasons other than COVID-19, and we found rates similar to those when the simple definition of moderate-to-severe disease was applied in our cohort. 18
Our study had several limitations. The VA population is not representative of the US population as a whole, having few women and no children. Screening at the time of admission is recommended throughout the VA healthcare system, and findings may be different in hospitals that do not test all admissions. We did not control for baseline hypoxemia or oxygen requirement (relatively common among VA patients) or for altitude; thus, some mild cases may have been misclassified as moderate to severe. However, to ensure that severe cases are captured, a sensitive definition is preferable to one that favors specificity; we found similar results when patients with lung cancer and COPD were excluded. Measured changes in overall incidence of severe hospitalizations may be due to several factors independent of vaccination, such as the increasing availability of different therapeutic options, like monoclonal antibody treatments, during the study period. 19 Variable clinical practices, common in the VA and elsewhere, may also contribute to observed differences in rates of hospitalization severity. 10 Severe COVID-19 cases may have been admitted nondifferentially to non-VA hospitals. Some veterans may have been vaccinated outside the VA, however, vaccines were typically available earliest within the VA, thus mitigating the impact of this limitation somewhat.
In this large, national cohort, the proportion of hospitalizations due to moderate-to-severe COVID-19 decreased following vaccine availability, particularly among vaccinated patients. Consideration should be given to updating definition of COVID-19 hospitalizations for tracking pandemic burden to improve differentiation between hospitalization caused by COVID-19 and those associated with detection of SARS-CoV-2 through the addition of straightforward and objective disease severity measures.
Acknowledgment
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript, or the decision to submit the manuscript for publication.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.13.
click here to view supplementary material
Financial support
This work was supported by the VA Office of Research and Development, Cooperative Studies Program.
Conflicts of interest
W.B.E. and P.M. are site investigators for a study funded by Gilead Pharmaceuticals with funds to the institution. W.B.E. is the recipient of VA HSRD funding. N.R.F. is supported by the American Heart Association (grant no. 857078). All other authors report no competing interests.
References
- 1.Coronavirus disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html#:∼:text=About%20COVID%2DNET-,Coronavirus%20Disease%202019%20(COVID%2D19)%2DAssociated%20Hospitalization%20Surveillance%20Network,care%20hospitals%20in%2014%20states. Published 2020. Accessed March 18, 2022.
- 2.Vaccine Breakthrough Case Investigations Team. COVID-19 vaccine breakthrough infections reported to CDC—United States, January. Centers for Disease Control and Prevention website. https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.html. Accessed March 18, 2022.
- 3. Oliver SE, Gargano JW, Marin M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. Morb Mortal Wkly Rep 2020;69:1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen H, Vusirikala A, Flannagan J, et al. Increased household transmission of COVID-19 cases associated with SARS-CoV-2 variant of concern B. 1.617. 2: a national case–control study. Lancet Reg Health Eur 2022;12:100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. COVID-19 Shared Data Resource (2020). VA Healthcare System website. https://www.va.gov. Published 2020. Accessed December 7, 2021.
- 6. Gelman, M. VA Guidebook on COVID-19 Testing v 1.2 27 (2021). Veterans’ Affairs Health website. https://dvagov.sharepoint.com/sites/VACOVHAPublicHealth/HCI/Screening%20Diagnosis%20%20Treatment/Forms/AllItems.aspx?RootFolder=%2Fsites%2FVACOVHAPublicHealth%2FHCI%2FScreening%20Diagnosis%20%20Treatment%2FTesting&FolderCTID=0x012000EE9E64DFF6DF2E4C97D13B1AE9C60CCA. Updated January 21, 2022. Accessed March 18, 2022.
- 7. Chronic Conditions Data Warehouse. Centers for Medicare and Medicaid Services website. https://www2.ccwdata.org/web/guest/condition-categories. Published 2021. Accessed November 30, 2021.
- 8.Clinical spectrum of SARS-CoV-2 infection. National Institutes of Health website. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Updated September 3, 2021. Accessed September 6, 2021.
- 9.Therapeutic management of hospitalized adults with COVID-19. National Institutes of Health website. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/. Accessed September 17, 2021.
- 10. Asundi A, Resnik J, Benedict PA, Shin M, Rani Elwy A, Branch-Elliman W. How are emerging data translated into clinical practice? A mixed methods investigation of coronavirus disease 2019 institutional treatment protocols. Open Forum Infect Dis 2021;8(4):ofab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohl ME, Miller DR, Lund BC, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open 2021;4:e2114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 vaccine breakthrough case investigation and reporting. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/php/hd-breakthrough.html. Published 2021. Accessed March 18, 2022.
- 13. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med 2021;385:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Branch-Elliman W, Ferguson R, Doros G, et al. Subcutaneous sarilumab for the treatment of hospitalized patients with severe COVID-19 disease: a pragmatic, embedded, randomized controlled trial. PLoS One 2022;17:e0263591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calderwood MS, Deloney VM, Anderson DJ, et al. Policies and practices of SHEA Research Network hospitals during the COVID-19 pandemic. Infect Control Hosp Epidemiol 2020;41:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Webb NE, Osburn TS. Characteristics of hospitalized children positive for SARS-CoV-2: experience of a large center. Hosp Pediatr 2021;11:e133–e141. [DOI] [PubMed] [Google Scholar]
- 18. Kushner LE, Schroeder AR, Kim J, Mathew R. “For COVID” or “With COVID”: classification of SARS-CoV-2 hospitalizations in children. Hosp Pediatr 2021;11:e151–e156. [DOI] [PubMed] [Google Scholar]
- 19.COVID-19 Treatment Guidelines. National Institutes of Health website. https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/. Updated October 27, 2021. Accessed November 30, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.13.
click here to view supplementary material