Abstract
Introduction
Apart from their recognized lipid-lowering effect, Hedan tablets, a mixture of Chinese herbal medicines, have demonstrated a certain weight-loss effect in clinical practice. The aim of this randomized, double-blind, placebo-controlled study was to verify the effect of Hedan tablets on body weight (BW) and insulin resistance (IR) in patients with metabolic syndrome (MetS).
Methods
A total of 62 eligible patients with MetS were divided into two groups: the treatment group (Hedan tablets at 4.38 g/day tid) and the control group (placebo treatment). Both groups attended follow-ups at 8, 16, and 24 weeks during the process. The parameters of the assessment include lipid level, BW, triglyceride (TG) to high-density lipoprotein cholesterol (HDLc) ratio (TG/HDLc), homeostasis model assessment for IR (HOMA-IR) index, and adiponectin.
Results
Patients in the treatment group showed a significant decrease in BW compared to those in the control group (−4.47 vs. 0.06 kg) after 8 weeks of treatment. A significant decrease in body mass index (BMI) was also observed in the treatment group after 16 weeks of treatment (−1.79 vs. −0.03 kg/m2). In the treatment group, 20 out of 31 (64.5%) patients lost 5–10% BW and 4 out of 31 (12.9%) patients lost over 10% BW after 24 weeks of treatment. Although there were no significant changes in the patients' HOMA-IR, the treatment group showed a significant reduction in TG/HDLc (−0.98 vs. −0.19) after 8 weeks of treatment and a significant increase in adiponectin (6.87 vs. −0.43) after 16 weeks of treatment.
Discussion/Conclusion
The Hedan tablets significantly improve BW, BMI, TG/HDLc, and adiponectin in patients with MetS. Thus, Hedan tablets may be used as an adjunct to existing MetS management methods.
Keywords: Hedan tablets, Metabolic syndrome, Body weight, Insulin resistance
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities including obesity, hypertension, hyperglycemia, and dyslipidemia. It is a well-known risk factor for cardiovascular diseases [1, 2]. Obesity, one of these metabolic abnormalities, is an essential MetS component, and insulin resistance (IR) is its vital pathophysiological basis [3]. With the improvement in people's living standards and changes in work habits, MetS has become a global public health problem [4]. As the largest developing country, China is also experiencing the MetS epidemic. In 2010, a national survey showed 33.9% (31.0% in males and 36.8% in females) MetS prevalence in China [5]. The comprehensive MetS intervention mainly includes lifestyle change and pharmacological treatment. In addition to conventional medicine, traditional Chinese medicine (TCM) can also affect MetS [6, 7].
Hedan tablets contain a mixture of Chinese herbal medicines, including Folium Nelumbinis, Radix Salviae Miltiorrhizae, Fructus Crataegi, Folium Sennae, and Fructus Psoraleae. Hedan tablets were originally approved for dyslipidemia treatment in China and demonstrated as a good complement to statins [8]. In fact, apart from their lipid-lowering effect, TCM also believes that certain components of Hedan tablets, such as Folium Nelumbinis and Folium Sennae, have a significant effect on weight loss. However, only a few studies have focused on the effects of Hedan tablets or their main components on obesity and IR [9, 10]. In addition, these studies were not randomized, controlled trials (RCTs). In order to fill this gap, we performed a 24-week RCT study to verify the efficacy of Hedan tablets regarding body weight (BW) and IR in patients with MetS.
Methods
Study Design and Subjects
The study was a randomized, placebo-controlled clinical trial with a 24-week duration. It was conducted at a dosage of 1.46 g 3 times a day with the aim of investigating the efficacy and safety of Hedan tablets (Nanchang Jishun Pharmaceutical Co., Ltd.) on BW and IR in patients with MetS in comparison with the effects of a placebo. This study protocol was reviewed and approved by the Ethics Committee of Shanghai Changhai Hospital, approval number (No. CHEC2014-114). Written informed consent was obtained from all the subjects prior to their participation in the study.
Inclusion Criteria
The subjects included in the study had MetS confirmed by the diagnostic criteria of the Chinese Medical Association Diabetes Association [11]. The basic drugs used for treatment were primarily metformin and statins.
Exclusion Criteria
Patients with severe uncontrolled metabolic disease, hepatic and renal dysfunction, cardiovascular disease, stroke, malignancy, or any other serious disease, as well as patients taking other herbal supplements were excluded. This trial adhered to the Consolidated Standards of Reporting Trials guidelines. A completed Consolidated Standards of Reporting Trials checklist is shown in online suppl. Table 1 (see www.karger.com/doi/10.1159/000520711 for all online suppl. material,).
Interventions and Outcomes
A 4.38-g dose of Hedan tablets 3 times a day was used in the treatment group. Similarly, placebo tablets (Nanchang Jishun Pharmaceutical Co.), which had the same appearance as Hedan tablets and were composed of starch, were used for the control group. The study comprised a total of 5 visits: visit 1 − screening (day −7 ± 3); visit 2 − randomization (day 0); visit 3 (week 8 ± 3 days); visit 4 (week 16 ± 3 days); and visit 5 (week 24 ± 3 days). The weight and body mass index (BMI) was used to assess the Hedan tablets' weight-loss effect. Both a non-insulin-dependent indicator, the triglyceride (TG) to high-density lipoprotein cholesterol (HDLc) ratio (TG/HDLc), and an insulin-dependent indicator, homeostasis model assessment of the IR (HOMA–IR) index, were selected for IR assessment.
Sample Size
The sample size was determined by the two-means comparison formula. Because there is currently no study on the effects of Hedan tablets on weight loss and IR, the sample size was calculated based on a previous study conducted by Xu et al. [8], which focuses on the effects of Hedan tablets on lipid parameters. In Xu et al.'s [8] study, there was a mean low-density lipoprotein cholesterol difference of 0.67 mmol/L between the treatment group (18 cases) and the control group (19 cases) [8]. To get 90% of power using a two-tailed hypothesis, both groups would require 25 patients each. Considering a 15% dropout rate, a sample of 60 patients divided into 2 groups would be necessary in order to detect a difference.
Randomization and Blinding
A total of 62 eligible subjects were enrolled and randomized by the investigator between January 2013 and January 2014. The stratified randomization method was used to match age, sex, BMI, and dyslipidemia. Intervention allocation blinding was carried out for both the participants and investigators. Placebo tablets were created by the Nanchang Jishun Pharmaceutical Co. based on the appearance of the Hedan tablets.
Data Collection
Data from the study were collected at baseline and during follow-ups. Height and weight were measured by trained healthcare personnel. During this process, the participants wore light clothing and no shoes. Systolic blood pressure, diastolic blood pressure, and heart rate were measured using an electronic sphygmomanometer.
Blood samples from all subjects were drawn after an overnight fast. Fasting plasma glucose (FPG) was measured by the glucose oxidase-peroxidase method; total cholesterol, TG, and HDLc were measured by routine enzymatic methods; and low-density lipoprotein cholesterol was calculated using the Friedwald formula. Uric acid level was detected by uricase-based spectrophotometry, serum creatinine and blood urea nitrogen levels were measured using Jaffe's kinetic method, and levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma glutamyltranspeptidase were measured using a coupled-enzyme assay. The biochemical parameters were measured using Bayer reagent packs (Bayer Diagnostics, Leverkusen, Germany) on an automated chemistry analyzer (Advia 1650 Autoanalyzer; Bayer Diagnostics).
Adiponectin level was measured by a commercial, enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA), and insulin level was measured using an enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden). Homeostasis model assessment for IR (HOMA-IR) was calculated as fasting insulin (μIU/mL) × FPG/22.5. BMI was calculated as weight divided by the square of height.
Statistical Analysis
Quantitative variables are expressed as the mean ± standard deviation and qualitative variables as frequency and percentage. Student's t test was used to compare parameters in different time periods within both groups, respectively, and changes in parameters between the two groups. The χ2 test was used to compare categorical variables between the groups. The statistical analysis was performed using the Statistical Package for Social Sciences 20.0 (Statistical Package for Social Sciences Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
Of the 62 eligible subjects, 31 (13 males and 18 females) received Hedan tablets, and 31 (15 males and 16 females) received a placebo version of the treatment. One participant in the control group withdrew from the study, while all participants in the treatment group completed the study. The baseline characteristics of the subjects, which indicate that the subjects in both groups were homogenous, are shown in Table 1.
Table 1.
Baseline characteristics of the two groups
| Parameters | Hedan (n = 31) | Placebo (n = 31) | p value |
|---|---|---|---|
| Age, years | 54.9+11.8 | 51.7+14.1 | 0.331 |
| Female, n (%) | 18 (58.1) | 16 (51.6) | 0.543 |
| Weight, kg | 77.5±10.1 | 77.0±15.7 | 0.799 |
| BMI, kg/m2 | 28.6±4.7 | 28.2±7.0 | 0.073 |
| SBP, mm Hg | 138.8±12.6 | 134.8±11.7 | 0.236 |
| DBP, mm Hg | 82.8±5.8 | 78.6±7.9 | 0.050 |
| Heart rate, beats/min | 71.3±9.3 | 74.2±4.0 | 0.214 |
| FPG, mmol/L | 7.83±3.09 | 7.43±1.45 | 0.689 |
| FINS, µU/mL | 10.1±2.4 | 11.8±6.0 | 0.137 |
| UA, μmol/L | 358.2±73.4 | 377.9±100.2 | 0.451 |
| TC, mmol/L | 5.52±1.00 | 5.48±1.09 | 0.803 |
| TG, mmol/L | 3.13±1.80 | 3.61±4.04 | 0.857 |
| HDLc, mmol/L | 1.16±0.37 | 1.06±0.25 | 0.232 |
| LDLc, mmol/L | 3.21±1.03 | 3.23±1.18 | 0.927 |
| Overweight, n (%) | 15 (48.4) | 15 (48.4) | - |
| Obesity, n (%) | 16 (51.6) | 16 (51.6) | - |
| Diabetes, n (%) | 25 (80.6) | 25 (80.6) | - |
| Dyslipidemia, n (%) | 26 (83.9) | 27 (87.1) | - |
| Hypertension, n (%) | 6 (19.4) | 4 (12.9) | - |
| Hypoglycemic agents, n (%) | 25 (80.6) | 25 (80.6) | - |
| Antihypertensive agents, n (%) 4 (12.9) | 3 (9.7) | - | |
| Lipid-lowering agents, n (%) | 0 (0) | 1 (3.2) | - |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FINS, fasting insulin; UA, plasma uric acid; TC, total cholesterol; LDLc, low-density lipoprotein cholesterol.
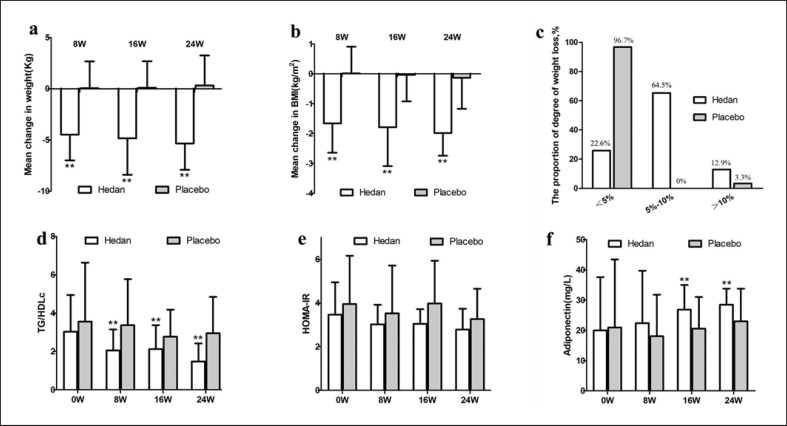
Not surprisingly, treatment with Hedan tablets resulted in a significant reduction in BW and BMI (Fig. 1a, b). At 8 weeks, the reduction in BW in the treatment group was significantly greater than in the control group. At 16 weeks, the reductions in BW and BMI in the treatment group were significantly greater than in the control group. Moreover, in the treatment group, 20 out of 31 (64.5%) patients lost 5–10% BW and 4 out of 31 (12.9%) patients lost over 10% BW after 24 weeks of treatment, compared with 0% and 3.3% in the control group (Fig. 1c).
Fig. 1.
a–f Changes in BW, TG/HDLc, HOMA-IR, and adiponectin in the Hedan tablet and placebo groups. The two asterisks represent not only the statistical difference between the Hedan table group and its baseline, the change is also a significant difference between the two groups.
There were no significant changes in HOMA-IR with either treatment, but treatment with Hedan tablets resulted in a significant reduction in TG/HDLc after 8 weeks (Fig. 1d, e). We also tested changes in adiponectin, as it is closely related to IR. After 16 weeks of treatment, the increase in adiponectin in the treatment group was significantly greater than that in the control group (Fig. 1f).
There were no significant changes in hepatic or renal functions in either the treatment group or the control group (online suppl. Fig. 1). All subjects tolerated the therapy well, and no serious adverse events were recorded during the study. No patients in either group terminated the study due to serious adverse events.
Discussion
This trial is the first to explore the effects of Hedan tablets on BW and IR among patients with MetS. During the study, we found that Hedan tablets significantly reduced BW, BMI, and TG/HDLc and increased the level of adiponectin in patients with MetS. However, they had no impact on the patients' HOMA-IR.
Folium Nelumbinis is the monarch drug of Hedan tablets. It has the abilities to remove phlegm and dehumidify. Folium Nelumbinis is also a major component of many TCM drugs for the treatment of dyslipidemia [12, 13]. However, there are few studies on weight loss associated with drugs containing Folium Nelumbinis. Modern medicine considers obesity to be the accumulation of excess ectopic fat as a result of excess calories [14]. TCM believes that obesity is mainly due to excessive endogenous stagnation of phlegm and dampness [15]. Therefore, patients with obesity often show shortness of breath and fatigability. Compendium of Materia Medica (compiled by Li Shizhen in 1552–1578), the most established TCM book, also contains records of Folium Nelumbinis' weight-loss effect. In the present study, we observed that Hedan tablets began to show a significant weight-loss effect during the 8th week. Therefore, the weight loss effect of Folium Nelumbinis, which was proposed almost 500 years ago, has been further confirmed by modern RCT research methods.
IR is probably the pivotal pathophysiological stage in the course of MetS development. Another interesting finding of this study is that Hedan tablets could significantly reduce TG/HDLc but not HOMA-IR. This may be related to the advantages and disadvantages of different IR indicators, as the performance of HOMA-IR is highly susceptible to insulin measurement. Insulin is typically measured by immunoassays that differ in their capture and detection antibodies. This may produce a range of values in the same samples [16]. This study's small sample size may magnify this HOMA-IR limitation. The application of TG/HDLc, a non-insulin-dependent surrogate marker of IR, can make up for the shortage of HOMA-IR to a certain extent [17].
We further detected changes in adiponectin, which has been well demonstrated to be negatively correlated with the IR degree [18, 19], and found that Hedan tablets can significantly increase adiponectin concentrations. Therefore, the results of this study suggest that Hedan tablets may have the potential to improve IR. It is possible that this improvement is due to the improvement in blood lipids and weight loss. More importantly, this effect may be related to some of the activity of Folium Nelumbinis itself.
The main, well-known, phytochemical Folium Nelumbinis compound is nuciferine [20]. Numerous studies have demonstrated its anti-inflammatory effect and impact on metabolic improvement [21, 22, 23]. Nguyen et al. [24] found that nuciferine can stimulate insulin secretion by closing potassium-adenosine triphosphate channels. The above-described pharmacological effects of nuciferine may partially explain the potential role of Hedan tablets in IR improvement. In addition to nuciferine, other bioactive Folium Nelumbinis ingredients, such as flavonoids and phenolic compounds, have also been shown to have anti-inflammatory, antidiabetic, and anti-obesity activities.
The strengths of the present study include the placebo-controlled, randomized design and relatively long follow-up time. However, several limitations should also be acknowledged. First, the sample size of 62 patients is relatively small. Second, although the Hedan and placebo groups were randomized regarding the use of medications, the potential effects of metformin on reducing BW and increasing insulin sensitivity [25] may affect the results to some extent.
In conclusion, our study demonstrates that Hedan tablets show a significant beneficial effect on BW and a potential effect on IR in patients with MetS. This finding suggests that Hedan tablets may be a safe supplement to existing MetS drug treatments.
Statement of Ethics
This study protocol was reviewed and approved by the Ethics Committee of Shanghai Changhai Hospital, approval number (No. CHEC2014-114). Written informed consent was obtained from all the subjects prior to their participation in the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by the Outstanding Leaders Training Program of the Pudong Health Bureau of Shanghai (Grant No. PWRI2018-02), and Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZzk2017-29).
Author Contributions
Lian-Yong Liu: conceived and designed the experiments; Lian-Yong Liu and Lin Zhou: performed the experiments; Xing-Zhen Liu: analyzed and interpreted the data; Da-Jin Zou: contributed reagents, materials, analysis tools, or data; Da-Jin Zou: wrote the paper. All authors read and approved the final draft.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
References
- 1.Polovina M, Hindricks G, Maggioni A, Piepoli M, Vardas P, Ašanin M, et al. Association of metabolic syndrome with non-thromboembolic adverse cardiac outcomes in patients with atrial fibrillation. Eur Heart J. 2018;39((45)):4030–4039. doi: 10.1093/eurheartj/ehy446. [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24((4)):683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 3.Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. 2017;15((1)):30–9. doi: 10.2174/1570161114666161007164510. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome: a new worldwide definition. Lancet. 2005;366((9491)):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Wang L, Li M, Xu Y, Jiang Y, Wang W, et al. Metabolic syndrome among adults in China: the 2010 China noncommunicable disease surveillance. J Clin Endocrinol Metab. 2017;102((2)):507–515. doi: 10.1210/jc.2016-2477. [DOI] [PubMed] [Google Scholar]
- 6.Feng YL, Zheng GY, Ling CQ. The investigation of the correlation between metabolic syndrome and Chinese medicine constitution types in senior retired military personnel of the People's Liberation Army. Chin J Integr Med. 2012;18((7)):485–9. doi: 10.1007/s11655-011-0948-z. [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Kong LD. High fructose diet-induced metabolic syndrome: pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol Res. 2018;130:438–450. doi: 10.1016/j.phrs.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Xu RX, Wu NQ, Li S, Zhang Y, Li XL, Guo YL, et al. Effects of Hedan Tablet () on lipid profile, proprotein convertase subtilisin/kexin type 9 and high-density lipoprotein subfractions in patients with hyperlipidemia: a primary study. Chin J Integr Med. 2016;22((9)):660–5. doi: 10.1007/s11655-015-2140-3. [DOI] [PubMed] [Google Scholar]
- 9.Tang H, Ge FF, Song XL. Clinical study of the effect of Hedan tablets on body weight and blood lipid of overweight or obese patients. Liaoning J Tradit Chin Med. 2011;38((7)):1391. [Google Scholar]
- 10.Ren C, Gao YH, Geng FT, Zhang RZ. Effect of Hedan tablets on insulin resistance, blood lipids and inflammatory factors in patients with non-diabetic metabolic syndrome. Mod J Integr Tradit Chin West Med. 2010;19((12)):1434–1438. [Google Scholar]
- 11.Chinese Medical Association Diabetes Association Metabolic Syndrome Research Group Recommendations of Chinese Medical Association Diabetes Association (CDS) on metabolic syndrome. Chin J Diab. 2004;12((3)):156–161. [Google Scholar]
- 12.Xie W, Zhao Y, Du L. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J Ethnopharmacol. 2012;140((2)):345–367. doi: 10.1016/j.jep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zhao H, Yang Y, Liu B, Ni J, Wang W. Lipid-lowering and antioxidant activities of Jiang-Zhi-Ning in Traditional Chinese Medicine. J Ethnopharmacol. 2011;134((3)):919–930. doi: 10.1016/j.jep.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 14.Wilding JPH, Mooney V, Pile R. Should obesity be recognised as a disease? BMJ. 2019;366:l4258. doi: 10.1136/bmj.l4258. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Mo S, Lv Y, Tang Z, Dong J. A study of traditional Chinese medicine body constitution associated with overweight, obesity, and underweight. Evid Based Complement Alternat Med. 2017;2017:7361896. doi: 10.1155/2017/7361896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53((5)):922–932. doi: 10.1373/clinchem.2006.077784. [DOI] [PubMed] [Google Scholar]
- 17.Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59((2)):299–304. doi: 10.1016/j.metabol.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Haluzík M, Parízková J, Haluzík MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res. 2004;53((2)):123–9. [PubMed] [Google Scholar]
- 19.Acharya SD, Evans RW, Brooks MM, Linkov F, Burke LE. Total and high-molecular-weight adiponectin levels in relation to insulin resistance among overweight/obese adults. Cent Asian J Glob Health. 2013;2((2)):55. doi: 10.5195/cajgh.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Cao J, Hou X, Li Z, Qu X. Pharmacokinetics, tissue distribution, bioavailability, and excretion of nuciferine, an alkaloid from lotus, in rats by LC/MS/MS. Drug Dev Ind Pharm. 2018;44((9)):1557–1562. doi: 10.1080/03639045.2018.1483399. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Deng J, Liu D, Tuo X, Yu Y, Yang H, et al. Nuciferine inhibits proinflammatory cytokines via the PPARs in LPS-induced RAW264.7 cells. Molecules. 2018;23((10)):2723. doi: 10.3390/molecules23102723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Deng J, Liu D, Tuo X, Xiao L, Lai B, et al. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br J Pharmacol. 2018;175((22)):4218–4228. doi: 10.1111/bph.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Yang Y, Guo S, Yang J, Jiang K, Zhao G, et al. Nuciferine ameliorates inflammatory responses by inhibiting the TLR4-mediated pathway in lipopolysaccharide-induced acute lung injury. Front Pharmacol. 2017;8:939. doi: 10.3389/fphar.2017.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen KH, Ta TN, Pham TH, Nguyen QT, Pham HD, Mishra S, et al. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J Ethnopharmacol. 2012;142((2)):488–495. doi: 10.1016/j.jep.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Lentferink YE, Knibbe CAJ, van der Vorst MMJ. Efficacy of metformin treatment with respect to weight reduction in children and adults with obesity: a systematic review. Drugs. 2018;78((18)):1887–901. doi: 10.1007/s40265-018-1025-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.