Abstract
Background
Sorafenib has consistently served as the control arm in multiple randomized clinical trials (RCTs) evaluating novel therapies for advanced hepatocellular carcinoma (HCC) for more than a decade. Analyzing trends in clinical outcomes of patients treated with sorafenib for the same indication over time offers the opportunity for unique insight into the evolution of clinical trial conduct and potential non-drug factors impacting outcomes.
Methods
We identified RCTs in patients with treatment-naïve advanced HCC where sorafenib was compared to another systemic therapy or placebo. We extracted trial-level demographic, clinicopathologic, and outcome data (overall survival [OS], progression-free survival [PFS], objective response rate [ORR], and duration of therapy). Sample-weighted linear regression was used to identify temporal trends with significance set at p ≤ 0.05.
Results
Sixteen RCTs (9 phase III and 7 phase II) enrolling 4,086 patients treated with sorafenib were included in the analysis. Included trials enrolled patients from 2005 to 2019. OS has significantly improved by 4.5 months from 2005 to 2019 (p = 0.048) over time. Thirteen studies provided data on PFS using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, with no significant change over time (p = 0.69). ORR assessed by RECIST 1.1 has significantly improved by 6.0% over time (p = 0.003). Median duration of therapy with sorafenib has decreased by 53% since the enrollment of the first clinical trial in 2005, from 23.1 weeks to 12.2 weeks (p = 0.0037). There was no significant change in patient demographics were identified over time to explain the OS findings.
Conclusion
The median OS of patients with advanced HCC treated with sorafenib has improved significantly over 15 years. At the same time, the median duration of therapy with sorafenib has decreased. The reason for these findings was not explained by changing demographics of patients enrolled in these trials and has implications for ongoing clinical trials.
Keywords: Cancer outcomes, Sorafenib, Hepatocellular carcinoma, Clinical trials
Introduction
Hepatocellular carcinoma (HCC) is a common cancer globally and is associated with considerable morbidity and mortality [1]. Although early HCC can be managed with potentially curative locoregional therapies such as resection or ablation or with liver transplantation, those who present with advanced disease have limited treatment options and frequently have a poor prognosis [2].
Sorafenib was the first systemic therapy approved by the United States Food and Drug Administration for the treatment of advanced HCC on the basis of clinical trials demonstrating a statistically significant survival benefit compared to placebo [3, 4]. However, sorafenib only has modest antitumor activity and its use is limited by significant toxicity, with side effects driving discontinuation of therapy in nearly 40% of patients [5]. Approximately a decade after the original approval of sorafenib, the REFLECT trial demonstrated that lenvatinib was noninferior to sorafenib with regard to overall survival (OS) and superior to sorafenib with certain secondary outcome measures; lenvatinib became an acceptable initial therapy for advanced HCC [6, 7]. Recently, a trial of the combination of atezolizumab and bevacizumab demonstrated significantly prolonged OS compared to sorafenib in patients with advanced HCC and Child-Pugh (CP) A cirrhosis, and also demonstrated improvements in multiple secondary endpoints [8]. For patients that qualify for this regimen, combination atezolizumab and bevacizumab has supplanted sorafenib as a preferred frontline option in many parts of the world [8]. Despite this, sorafenib remains a standard control arm for most ongoing global clinical trials in frontline advanced HCC, in part because many of these studies were initiated prior to the adoption of combination atezolizumab-bevacizumab as standard frontline therapy.
The use of a single standard systemic therapy arm in multiple global late-phase clinical trials spanning over a decade provides a unique opportunity to develop insights into clinical practices and clinical trials conduct of advanced HCC and potential non-drug factors that impact outcomes. Further, since the initial approval of sorafenib as standard therapy for advanced HCC, multidisciplinary care for all patients with HCC has become more widely accepted and recognized to improve patient outcomes [9, 10]. Additionally, earlier diagnosis, institution of supportive care, recognition and mitigation of side effects of sorafenib, earlier initiation of systemic therapy, and treatment of hepatitis C may also contribute to improved outcomes in patients with advanced HCC [9, 10, 11, 12, 13]. In this study, we explored the sorafenib comparator arms in clinical trials of previously untreated advanced HCC and hypothesized that the OS of patients receiving sorafenib has increased in published comparative trials of systemic treatment-naive HCC.
Methods
We searched PubMed to identify relevant randomized clinical trials of sorafenib in patients with advanced HCC naive to systemic therapy. Trials were included if they included patients were naive to systemic therapy, presented outcomes of interest, and had a comparison arm of systemic therapy (active therapy or placebo-controlled). Studies that compared sorafenib to a procedure (e.g., hepatic artery infusion or radiofrequency ablation) were not included. Studies that appeared to meet criteria by abstract review were examined more closely for inclusion. Bibliographies of included studies were then hand-searched for other studies that could potentially meet inclusion criteria. After development of a preliminary list of included studies, we approached content experts (authors T.B.K. and M.Y.) for further identification of studies.
We extracted demographic data and trial-level outcome data from the resulting publications, meeting abstracts, or publicly available protocol information. Outcomes of interest included median OS, median progression-free survival (PFS), objective response rates (ORR), and median duration of therapy of included patients receiving sorafenib. ORRs were separated by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0, 1.1, or modified RECIST (mRECIST). Publication bias was assessed by visual inspection of a funnel plot of sample size versus median OS (the primary outcome of interest). Extracted data were assessed for normality using skewness and kurtosis testing and joint χ2 testing with a p value of less than 0.05 indicating rejection of the null hypothesis of normal distribution. Comparisons of demographics and outcomes were made between phase II and phase III trials using Wilcoxon Rank Sum testing for non-normally distributed factors or 2-sample t-tests if normally distributed.
We performed weighted linear regression analysis to identify temporal trends while accounting for differences in sample sizes. Temporal data from studies were analyzed from the date of first patient enrollment as this was felt to be most representative of contemporary supportive care at the time the respective trials were performed. Data were analyzed in Stata version 17.0. Outliers, when identified, were formally tested with Cook's distance. Analyses were also stratified by trial phase (II vs. III). Significance was set at p ≤ 0.05; no adjustments were made for multiple comparisons due to the exploratory nature of this analysis.
This study did not use any identifiable patient data and only utilized previously published publicly available data. No specific informed consent was sought. This work was supported by the grants from the National Institutes of Health (T32CA009679 to T.J.B., NCI SPORE P50 CA062924, NIH P30 CA006973, and NIH UM1CA186691 all to M.Y.).
Results
A total of 100 studies were included in the initial database screening. Ultimately, 14 studies of systemic therapies in advanced HCC using sorafenib versus placebo or other systemic therapy were identified (6 phase II and 8 phase III); 1 additional phase III study that has only been published in abstract form was identified from discussion with experts (Checkmate-459) [3, 4, 6, 8, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. During data analysis and manuscript preparation, results from 1 additional phase II clinical trial of sorafenib in HCC were published and subsequently included in the analysis [25]. In total, 16 trials were included in at least 1 analysis (Table 1).
Table 1.
Data from included studies
| Trial | Sorafenib arm, n | Date of first enrollment | Region | Phase | Comparison | Included outcomes | Median OS, months | Median PFS, months | ORR, % | Duration of therapy, weeks | Powered OS, months | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHARP | 299 | 10 Mar 2005 | Global | 3 | Placebo | OS, PFS, DOT, ORR | 10.7 | 5.5 | 2 | 13.1 | N/A | [3] |
| Asia-Pacific | 150 | 20 Sep 2005 | China, South Korea, Taiwan | 3 | Placebo | OS, PFS, ORR | 6.5 | 2.8 | 3.3 | NS | N/A | [4] |
| SUN 1170 | 544 | 1 Jul 2008 | Global | 3 | Sunitinib | OS, PFS, ORR | 10.2 | 3 | 6.1 | NS | 10.7 | [16] |
| PRODIGE-10 | 44 | 1 Dec 2008 | France | 2 | Sorafanib + gemcitabine and oxaliplatin | OS, PFS, DOT, ORR | 14.8 | 4.6 | 3.9 | 17 | NS | [18] |
| B + E | 43 | 1 Mar 2009 | USA | 2 | Bevacizumab + erlotinib | OS, PFS, DOT, ORR | 8.55 | 2.76 | 9 | 12 | 8.5 | [17] |
| BRISK-FL | 578 | 1 May 2009 | Global | 3 | Brivanib | OS, DOT | 9.9 | NS | NS | 17.9 | 10 | [14] |
| SEARCH | 358 | 1 May 2009 | Global | 3 | Sorafenib + erlotinib | OS, PFS, DOT | 8.5 | 4 | 6.6 | 17.6 | 10.7 | [22] |
| SAKK7708 | 46 | 1 Dec 2009 | Switzerland | 2 | Sorafenib + everolimus | OS, PFS, DOT, ORR | 10 | 6.6 | 0 | 16.9 | NS | [21] |
| Sorafenib versus | 26 | 1 Sep 2010 | Egypt | 2 | Capecitabine | OS, PFS, DOT, ORR | 7.05 | 6 | 14.5 | 17.4 | NS | [20] |
| capecitabine | ||||||||||||
| Sorafenib-mapatumumab | 51 | 8 Feb 2011 | Germany, Poland, Romania, Russia, USA | 2 | Sorafenib + mapatumumab | OS, DOT | 10.1 | NS | NS | 19.2 | 5.5 | [19] |
| Sorafenib versus dovitinib | 83 | 1 Jul 2011 | Asia-Pacific | 2 | Dovitinib | OS, DOT, ORR | 8.4 | NS | 11 | 13.9 | NS | [24] |
| REFLECT | 476 | 1 Mar 2013 | Global | 3 | Lenvatinib | OS, PFS, DOT, ORR | 12.3 | 3.6 | 6.5 | 16.1 | NS | [6] |
| LIGHT | 521 | 8 Dec 2014 | Global | 3 | Linifanib | OS, PFS, DOT, ORR | 9.8 | 4 | 6.9 | 18.2 | NS | [15] |
| Checkmate-459 | 371 | 5 Nov 2015 | Global | 3 | Sorafenib + nivolumab | OS, PFS, ORR | 14.7 | 3.8 | 7 | NS | 10 | [23] |
| Sorafenib versus donafenib | 331 | 21 Mar 2016 | China | 2 | Donafenib | OS, PFS, DOT | 10 | 4 | 9 | 16.1 | NS | [25] |
| IMBrave 150 | 165 | 15 Mar 2018 | Global | 3 | Atezolizumab + bevacizumab | OS, PFS, DOT, ORR | 13.2 | 4.3 | 11.9 | 12.2 | 12 | [8] |
DOT, duration of therapy; NS, not stated; N/A, not applicable.
A total of 4,086 patients were enrolled from 2005 to 2019 to receive sorafenib in comparison studies of treatment-naive HCC. The median age of patients included was 60.4 years (95% confidence interval 57.5–62.3), 82.4% of participants were male, 55.4% had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, and 43.2% had an ECOG PS 1. Most patients were staged with Barcelona Clinic Liver Cancer (BCLC) stage C HCC and most studies only included patients with CP A cirrhosis.
Phase III trials were larger (p = 0.003) and contained a higher proportion of patients with extrahepatic spread (p = 0.028); however, there were no other significant differences in the proportions of extracted demographics (ECOG0, ECOG 1, CP A cirrhosis, CP B cirrhosis, hepatitis B, hepatitis C, prior locoregional therapies, or vascular invasion) between trial phases. No significant differences were noted in median OS, median PFS by RECIST 1.1, ORR by RECIST 1.1, or median duration of therapy between phase II and phase III studies (see online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000521625).
No linear trends were identified by sample-weighted linear regression between the date of enrollment and proportion of patients with CP A cirrhosis, BCLC B HCC, median age, proportion of ECOG 1 PS, proportion of patients with HBV, or proportion of patients with HCV. There was a trend toward a higher proportion of ECOG0 patients enrolled in more contemporary studies; however, this was not statistically significant (p = 0.084, R2 = 0.17). There was also a positive trend of date of enrollment and proportion of patients who have previously received locoregional therapies; however, this also did not reach statistical significance (p = 0.08, R2 = 0.368). In phase III trials, there was a nonsignificant trend toward an increased proportion of patients with CP A cirrhosis (p = 0.08, R2 0.098); however, no other demographic trend approached significance by weighted linear regression.
The sample-weighted median OS of all patients receiving sorafenib was 10.1 months (95% CI 9.0–11.6 months, 16 studies), and the sample-weighted median PFS by RECIST 1.1 was 3.8 months (95% CI 3.5–4.9 months, 13 studies) and 4.1 months (95% CI 3.1–4.9 months, 3 studies) by mRECIST. The median of reported median OS weighted by participants was 10.0 months for phase II studies (95% CI 7.6–12.1 months) and 10.2 months (95% CI 8.7–12.5 months) for phase III studies. No linear trend was noted for sample size by year of enrollment (p = 0.89, R2 = 0.002).
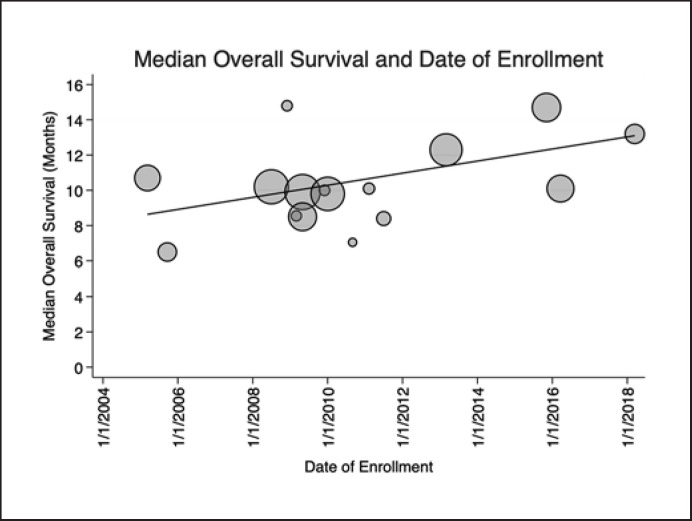
All 16 included studies comprising 4,086 patients contributed data on median OS. A significant positive linear trend was noted between date of first enrollment and OS (p = 0.048, R2 = 0.38) indicating improvement in median OS of 4.5 months over time favoring the more contemporary studies after accounting for differences in sample sizes. This amounted to a median increase of 0.34 months improvement in OS each year since the first patient was enrolled, or 10.5 days improvement in OS per year (Table 1; Fig. 1). When analyzed by trial phase, significant linear trends were again noted. Phase III studies (9 studies, 3,462 patients) had a positive linear trend (p = 0.02) with a slope of 0.47 months per year enrolled (R2 = 0.64). No significant trend of OS and date of enrollment was identified when this analysis was limited to phase II trials (p = 0.92, R2 = 0.002, 7 studies, 624 patients).
Fig. 1.
Median OS and year of enrollment. Scatterplot showing reported median OS in each included trial in months and the first date of enrollment. Since the publication of the first trial to demonstrate an improvement in OS with treatment with sorafenib (SHARP), a significant positive trend in improving OS has emerged in subsequent publications. OS has been improving by 0.34 months per year since the initial study (p = 0.048, R2 = 0.38). Markers are weighted by sample size and trendline represents the weighted linear regression fit.
Thirteen studies (3,374 patients) reported PFS by RECIST 1.1. No linear relationship was noted between median PFS by RECIST 1.1 with sorafenib by year of enrollment weighted by enrolled patients (Fig. 2, p = 0.69, R2 0.021). When this analysis was stratified by phase, again no significant linear relationship with year of enrollment was identified. However, visual inspection of the scatterplot of median PFS by RECIST 1.1 and date of enrollment in phase III trials revealed the median PFS reported by SHARP (5.5 months with first date of enrollment of March 10, 2005) to likely be an outlier, which was confirmed by Cook's distance. An exploratory analysis that dropped SHARP from the weighted linear regression model resulted in improved R2 from 0.0135 to 0.3153; however, the resulting positive trend was not statistically significant (p = 0.11). There were too few studies reporting PFS by mRECIST to perform an analogous analysis.
Fig. 2.
Median PFS and year of enrollment. Scatterplot showing reported median PFS by RECIST 1.1 and the first date of enrollment. No significant linear trend was noted between PFS by RECIST 1.1 and date of first enrollment (p = 0.69, R2 = 0.021). Markers are weighted by sample size and trendline represents the weighted linear regression line of best fit.
Fourteen studies of 3,457 patients reported on ORR by RECIST 1.1. A weighted linear regression showed ORR has significantly increased by 6.0% over time (slope = 0.46% improvement in ORR per year of enrollment, p = 0.003, R2 = 0.50; Fig. 3). This finding was preserved in the phase III trials (slope = 0.46% increased ORR/year, p = 0.027, R2 = 0.654, 8 studies, 2,084 patients) whereas no linear trend was identified when the analysis was limited to phase II studies (p = 0.72, R2 = 0.023, 6 studies, 573 patients).
Fig. 3.
ORRs (by RECIST 1.1) and year of enrollment. Scatterplot showing reported ORRs by RECIST 1.1 and the first date of trial enrollment. ORRs have been significantly increasing over time, at a rate of 0.46% per year (p = 0.003, R2 = 0.50). This trend was driven by results seen on phase III trials (p = 0.027, R2 = 0.654), no significant linear trend was identified on a subset analysis of phase II trials only. Markers are weighted by sample size and trendline represents the weighted linear regression fit.
Thirteen studies (3,021 total patients) contributed data for the median duration of therapy with sorafenib. The median duration of therapy with sorafenib has significantly decreased over time by 53%, from 23.1 weeks to 12.2 weeks since the first clinical trial enrollment in 2005 (p = 0.0037, R2 = 0.669) (Fig. 4). When stratified by phase, phase III trials have a significant negative linear trend, with an average reduction of 0.77 weeks of therapy per year of enrollment (p < 0.001, R2 = 0.91, 6 studies, 2,397 patients). However, no significant linear trend was noted when this analysis was restricted to phase II trials (p = 0.77, R2 = 0.012, 7 studies, 624 patients).
Fig. 4.
Median duration of therapy and year of enrollment. Scatterplot showing the median reported durations of therapy with sorafenib and the first date of enrollment. Thirteen studies including 3,021 patients reported duration of therapy. Since the initial study, the median duration of therapy has decreased by 53% (p = 0.0037, R2 = 0.669). Markers are weighted by sample size and trendline represents the weighted linear regression fit.
Review of a funnel plot of sample size and median reported OS shows near symmetry around a median OS of 9.9 months with a peak sample size of 578 patients (correlating to the BRISK-FL study) [14]. There is a paucity of smaller studies with prolonged OS (n = 0–300 and median OS 16–20 months). It is unlikely that if these studies have been completed that they would remain unpublished given the potential impact on clinical management of prolonged survivals compared to historical outcomes with sorafenib; however, publication bias cannot be completely ruled out (online suppl. Fig. 1).
Discussion
To our knowledge, this is the first rigorous review of the clinical outcomes associated with the use of sorafenib in clinical trials of advanced HCC. Several trends are noteworthy. First, the median OS of patients with advanced HCC requiring systemic therapy enrolled on a clinical trial and treated with sorafenib has increased from 2005 to 2019. ORRs by RECIST 1.1 have also significantly increased. During this time, the median duration of treatment with sorafenib has also significantly decreased. Despite this, PFS as measured by RECIST 1.1 has not significantly changed over time. On subgroup analyses, the trends in outcomes appear to be driven mostly by the robust phase III trials, with no discernible trends noted when the analysis was limited to phase II trials. Although the combination of bevacizumab and atezolizumab has since replaced sorafenib as a default frontline therapy for patients with adequate liver function in many parts of the world, these findings have implications for multiple ongoing global phase III studies that continue to use sorafenib as a comparator, and for the clinical management of advanced HCC. For example, the improvement in OS with sorafenib may make the demonstration of superiority over sorafenib more challenging in ongoing clinical studies that are using sorafenib as a control arm. This is exemplified by the failure of nivolumab to demonstrate improved OS versus sorafenib in the CheckMate-459 frontline study, despite producing a median OS of 16.4 months with nivolumab, which compares favorably to the OS of historical sorafenib trials [23]. Currently, there are at least 3 more phase III trials that are ongoing using sorafenib as a control arm − HIMALAYA (NCT03298451) comparing the combination of durvalumab and tremelimumab versus durvalumab versus sorafenib, CheckMate-9DW (NCT04039607) comparing the combination of nivolumab and ipilimumab versus lenvatinib or sorafenib, and COSMIC-312 (NCT03755791) comparing the combination of cabozantanib and atezolizumab versus sorafenib. The trend of increasing median OS of patients receiving sorafenib threatens to affect power calculations and trial accrual and may ultimately affect the outcome and interpretation of these studies. For example, preliminary results were recently announced via press release for COSMIC-312 indicating improved PFS with combination cabozantanib and atezolizumab compared to sorafenib control, but it is unlikely to result in improved OS [26]. It is possible that the failure to demonstrate superior OS in this study was due to better-than-anticipated outcomes with the sorafenib control arm [26]. These preliminary, unpublished results were not included in the present analysis.
It is unclear why median survival with frontline sorafenib has increased over time. The changes do not appear to be driven by quantified patient demographic data such as ECOG PS, age, BCLC stage, prior locoregional therapies, HBV or HCV infection, or CP cirrhosis status, although we do note a positive trend in the proportion of patients with CP A cirrhosis in phase III trials over time. Trial design by phase also does not seem to be a factor, with a significant difference in demographics only detected in a higher proportion of patients with extrahepatic spread in phase III trials compared to phase II trials (online suppl. Table 1). Survival improvements with frontline sorafenib could reflect changes in the use of subsequent therapies, but no second-line therapy was approved for HCC outside of clinical trials until regorafenib's approval in 2017, whereas the trend of increased survival is evident prior to 2017. Alternatively, these changes in sorafenib treatment outcomes could reflect unquantified changes in patient selection in the context of clinical trials in the 15 years of observations that are reported in this study, such as improvement in multidisciplinary care coordination and supportive care, and innovations in viral hepatitis treatment [9, 10, 11]. Such contributing factors to patient outcomes would not be easily captured in the resulting publications [27]. The observation of decreasing duration of therapy with sorafenib in patients on trials in tandem with improving median survival with sorafenib is particularly intriguing and an analysis of post-progression therapies for patients on these studies would be enlightening to determine if patients are discontinuing therapy with sorafenib earlier than anticipated in order to pursue novel second-line treatment options. Lastly, the finding of improvement in ORRs by RECIST 1.1 reinforces the importance of standardized and blinded assessment methods, although the association between blinding and ORR was not formally tested in this analysis.
This analysis has several limitations. The associations we describe here are correlative, determining true causality would require a review of patient level data from each trial. Similarly, it is not known how the observations of this study of improved OS over time correlate with the “real-world” clinical experience over time. Patient selection in oncology trials is a known contributor to the efficacy-effectiveness gap bridging clinical trials to real-world patients, and the outcomes of this efficacy-effectiveness gap have historically been wide in HCC treated with sorafenib [28, 29]. Lastly, we only reviewed published trials of first-line sorafenib in advanced HCC. Although many of the trials are deemed “negative” in that the experimental arm failed to improve the OS compared to the OS with treatment with sorafenib, we cannot rule out the existence of publication bias. However, a review of a funnel plot graphing sample size and median OS revealed an absence of studies of small sample sizes with long median OSs. Given the implication of these hypothetical studies, it is unlikely these studies would remain unpublished.
Although the combination of atezolizumab and bevacizumab is now the standard of care for patients with advanced HCC and preserved liver function, sorafenib remains an option for frontline therapy for patients that do not qualify for atezolizumab and bevacizumab [3, 8]. Here, we demonstrate that the median OS and ORRs of treatment-naive patients with advanced HCC on clinical trials has clearly improved over the last 15 years. Meanwhile, the median duration of therapy has significantly decreased over the same period. The available data do not provide an immediate rationale for these changes and are not accounted for by quantified or published patient demographics. It is possible that better supportive care and multidisciplinary care are contributing to improved survival and the availability of second-line therapies is contributing to decreased durations of therapy with sorafenib. These observations should be considered when designing future clinical trials in advanced HCC.
Statement of Ethics
No human subjects were involved in this study. All included studies required written informed consent and were approved by the relevant Ethics Committees. Please see source studies for more information. This study is exempt from specific Ethical Committee approval as it used only aggregated, publicly available, de-identified data, and posed no risk to human subjects.
Conflict of Interest Statement
M.Y. reports receiving research grants from Incyte, Bristol Myers Squibb, and Exelixis and is a consultant for AstraZeneca, Eisai, Exelixis, and Genentech. The other authors declare no competing interests.
Funding Sources
This study was supported by NIH T32CA009679 (T.J.B.), NIH NCI SPORE P50 CA062924 (M.Y.), NIH P30 CA006973 (M.Y.), and NIH UM1CA186691 (M.Y.).
Author Contributions
Conception, design, analysis, and interpretation of data were contributed by all authors. Drafting, revision, and final approval were contributed by all authors.
Data Availability Statement
All relevant data are available in this manuscript. Stata source code can be made available on request to corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Previous Presentation: Presented in abstract form at the 2021 ASCO Gastrointestinal Cancers Symposium, January 15–17, 2021, Virtual.
Twitter Handles: @TimothyJBrownMD @guptaarjun90 @ramsedhom @ShaalanBeg @MarkYarchoan.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156((2)):477–91.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Keating GM. Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 2017;12((2)):243–253. doi: 10.1007/s11523-017-0484-7. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16((10)):617–630. doi: 10.1038/s41575-019-0179-x. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 9.Chang TA, Sawhney R, Monto A, Ben Davoren J, Kirkland JA, Stewart L, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB. 2008;10((6)):405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, Reddick M, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21((4)):1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71((2)):265–273. doi: 10.1016/j.jhep.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Rich NE, Yopp AC, Singal AG. Medical management of hepatocellular carcinoma. J Oncol Pract. 2017;13((6)):356–364. doi: 10.1200/JOP.2017.022996. [DOI] [PubMed] [Google Scholar]
- 13.Mudumbi SK, Bourgeois CE, Hoppman NA, Smith CH, Verma M, Bakitas MA, et al. Palliative care and hospice interventions in decompensated cirrhosis and hepatocellular carcinoma: a rapid review of literature. J Palliat Med. 2018;21((8)):1177–1184. doi: 10.1089/jpm.2017.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PJ, Qin S, Park JW, Poon RTP, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31((28)):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 15.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33((2)):172–9. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31((32)):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 17.Thomas MB, Garrett-Mayer E, Anis M, Anderton K, Bentz T, Edwards A, et al. A randomized phase II open-label multi-institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the first-line treatment of patients with advanced hepatocellular carcinoma. Oncology. 2018;94((6)):329–339. doi: 10.1159/000485384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assenat E, Pageaux GP, Thézenas S, Peron JM, Bécouarn Y, Seitz JF, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1 st -line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120((9)):896–902. doi: 10.1038/s41416-019-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciuleanu T, Bazin I, Lungulescu D, Miron L, Bondarenko I, Deptala A, et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann Oncol. 2016;27((4)):680–7. doi: 10.1093/annonc/mdw004. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Rahman O, Abdel-Wahab M, Shaker M, Abdel-Wahab S, Elbassiony M, Ellithy M. Sorafenib versus capecitabine in the management of advanced hepatocellular carcinoma. Med Oncol. 2013;30((3)):655. doi: 10.1007/s12032-013-0655-z. [DOI] [PubMed] [Google Scholar]
- 21.Koeberle D, Dufour J-F, Demeter G, Li Q, Ribi K, Samaras P, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29) Ann Oncol. 2016 May;27((5)):856–861. doi: 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- 22.Zhu AX, Rosmorduc O, Evans TRJ, Ross PJ, Santoro A, Carrilho FJ, et al. Search: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33((6)):559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 23.Yau T, Park JW, Finn RS, Cheng A-L, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30((Suppl 5)):v851–934. [Google Scholar]
- 24.Cheng AL, Thongprasert S, Lim HY, Sukeepaisarnjaroen W, Yang TS, Wu CC, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016;64((3)):774–784. doi: 10.1002/hep.28600. [DOI] [PubMed] [Google Scholar]
- 25.Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J Clin Oncol. 2021;39((27)):3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exelixis and Ipsen announce cabozantinib in combination with an immune checkpoint inhibitor significantly improved progression-free survival in phase 3 COSMIC-312 pivotal trial in patients with previously untreated advanced liver cancer [Internet] 2021. [cited 2021 Aug 19]. Available from: https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/2021/06/09120111/Ipsen-Exelixis-COSMIC-312-28-June-2021.pdf.
- 27.Yeh ML, Kuo HT, Huang CI, Huang CF, Hsieh MY, Liang PC, et al. Eradication of hepatitis C virus preserve liver function and prolong survival in advanced hepatocellular carcinoma patients with limited life expectancy. Kaohsiung J Med Sci. 2021;37((2)):145–153. doi: 10.1002/kjm2.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanoff HK, Chang Y, Lund JL, O'Neil BH, Dusetzina SB. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21((9)):1113–1120. doi: 10.1634/theoncologist.2015-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence: what is it and what can it tell us? N Engl J Med. 2016;375((23)):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
All relevant data are available in this manuscript. Stata source code can be made available on request to corresponding author.