Abstract
Hilar cholangiocellular carcinoma (CCC) is a malignant neoplasm of epithelial origin occurring at the confluence of the right and left hepatic bile ducts. Typically, these tumors are small, poorly differentiated, exhibit aggressive biologic behavior with non-specific symptoms and tend to obstruct the intrahepatic bile ducts. Surgery is the only available curative option. Unfortunately, in less than half of the patients a complete resection is possible with poor survival rate in unresectable cases. In this report, we present the case of a 58-year-old woman with a history of unresectable hilar cholangiocarcinoma. Initially she was treated with intraductal dilatation of malignancy and placement of a plastic stent and chemotherapy (Gemcitabin® and Platinol®). Two years later she underwent a second-line chemotherapy with Gemcitabin® and Oxyplatin® because of tumor progression. Despite a second line chemotherapy and placement of an uncovered self-expandible metal stent (ucSEMS) that was extended later on by stent-in stent technique, there was tumor progression which led to a complex course with relapsing obstructive cholangiosepsis and cholestasis. Because of tumor ingrowth, endobiliary radiofrequency ablation of the malignant stenosis was performed in repeated sessions. This case illustrates that radiofrequency ablation of solitary malignant biliary obstruction is feasible, safe and allows an improvement of quality of life in non-operable patients.
Keywords: Cholangiocarcinoma, Klatskin tumor, Endobiliary radiofrequency ablation
Introduction
Cholangiocellular carcinoma (CCC) is an epithelial malignant tumor arising from the bile duct around the hepatic fork [1]. With 70%, it represents the most frequent type of bile duct cancer [1]. Surgical resection is feasible in only 20–30% of CCC patients. In more advanced disease stages, palliative care comprising endoscopic plastic stent (PS) placement for biliary drainage and chemotherapy is required [1, 2].
If the initial PS becomes occluded, replacement with an uncovered self-expandable metal stent (ucSEMS) is favored if the estimated survival is expected to be greater than 6 months [2]. However, tumor ingrowth through the stent represents one of the most frequent complications causing cholestatic disease and cholangitis [1, 2, 3]. In this case, different approaches are reported in the literature: percutaneous biliary drainage, endosonography-guided biliary drainage, surgical bypass, local ablative therapy: photodynamic therapy (PDT), intraductal (ID) RFA (ID-RFA), and intraluminal brachytherapy [1].
ID-RFA is a novel endoscopic procedure that involves the use of a biliary catheter during endoscopic retrograde cholangiopancreatography (ERCP) which is positioned in direct contact to the lesion [4]. High-frequency alternating current generates high temperatures (50°C–100°C) that cause coagulation necrosis by thermal energy [5]. A balloon cholangiogram is performed after ablation to confirm the absence of ID-RFA-related complications. Ablated necrotic debris is removed using an extraction balloon [4]. Because ID-RFA can cause post-RFA biliary stricture, biliary drainage should be ensured using a PS or self-expandable metal stent (SEMS) [4]. Currently, two devices are available for ID-RFA that is used over a guidewire during ERCP: HabibTM (EndoHBP) and EndoLuminal Radiofrequency Ablation (ELRATM) [4].
Available studies have shown technical feasibility, safety, and beneficial effects on overall survival and stent patency [4]. We herein report the successful treatment of a nonoperable ID biliary malignancy Bismuth Typ IV.
Case Report
A 58-year-old woman presented to our emergency department (August 2015) with acute cholestasis and colicky pain. There was no history of liver or biliary disease.
A clinical examination revealed a cardiopulmonal compensated afebrile and icteric patient. The main laboratory data were as follows: hemoglobin, 156 g/L; white blood cells, 5.6 × 109/L; platelets, 197 × 109/L; total bilirubin, 24.9 μmol/L; aspartate aminotransferase, 142 U/L; alkaline phosphatase 587 U/L, gamma-glutamyl transferase, 1381 U/L; protein C-reactive 7.5 mg/L, INR 0.92, creatinine 63 μmol/L.
An abdominal ultrasound in the emergency department showed dilated peripheral left hepatic bile ducts and an irregular hilar situation. ERCP showed an unclear narrowing related to the cystic duct in the cholangiogram and a stone (Fig. 1) that was extracted after papillotomy. An MRCP confirmed the irregular bile duct and a sclerotizing mass forming stricture measured 5 × 6 × 5 cm at the hilum with dilatation of the left intrahepatic ducts consistent with a Klatskin Tumour (Bismuth IV) (Fig. 2, 3). After 2 days, a cytological sampling was performed during ERC, and after dilatation of the malignant stenosis in the ductus hepaticus sinister (DHS), a PS was positioned (Fig. 4). Due to a sampling error with negative cytological result, a laparoscopy with liver biopsy was performed that showed a metastatic cholangiocarcinoma. Our tumor board judged the situation as inoperable and palliative. After extraction of the PS and dilatation of the bifurcation and the left main hepatic duct, a 10 × 100 mm ucSEMS (Biliary-Stent, Boston Scientific, Ort) was inserted in the DHS (Fig. 5).
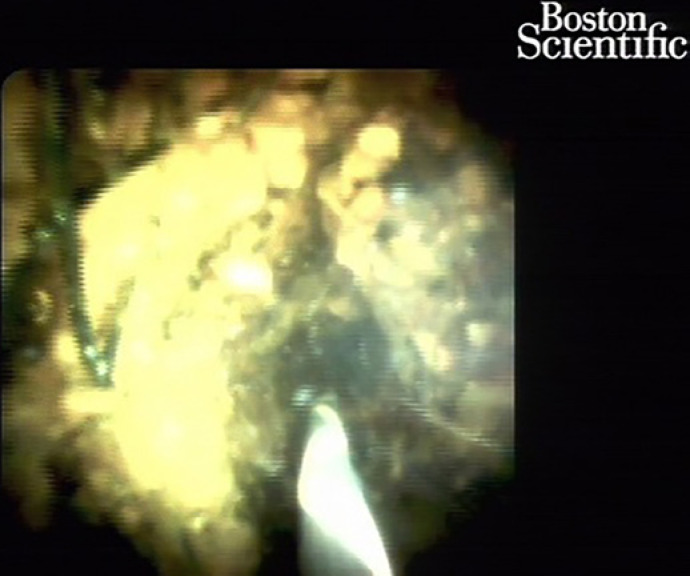
Fig. 1.
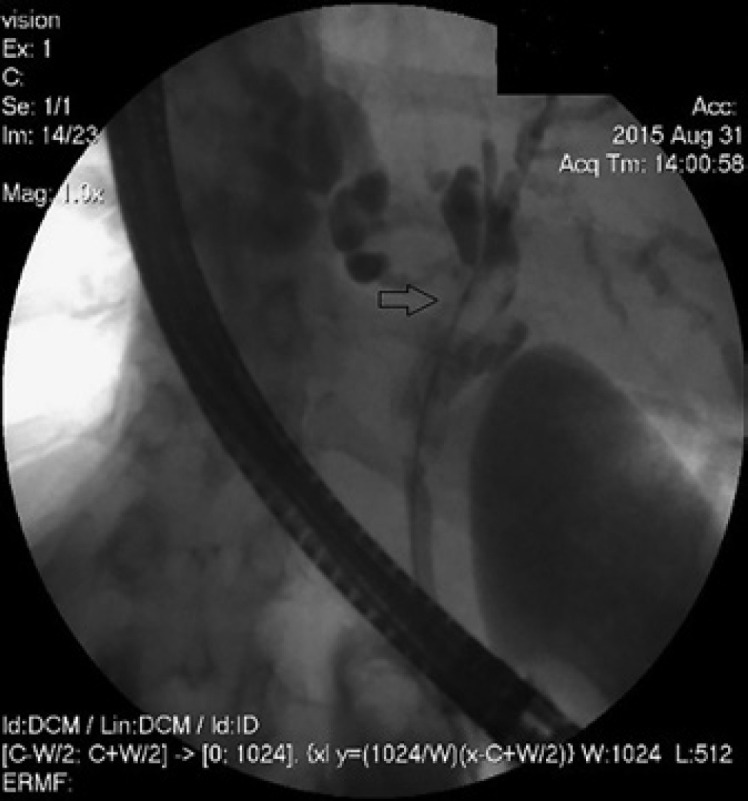
Cholangioscopy showing an unclear anatomy around the cystic duct.
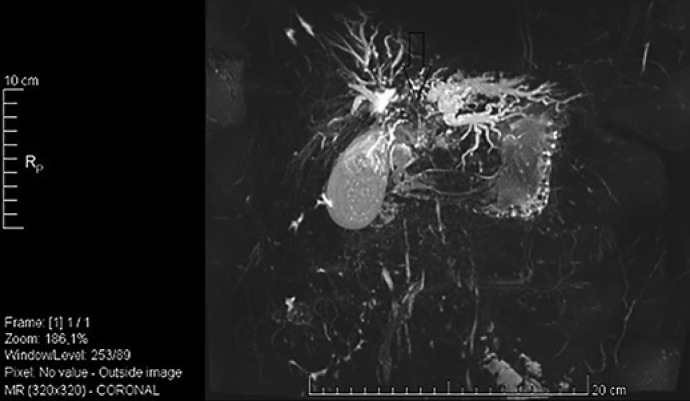
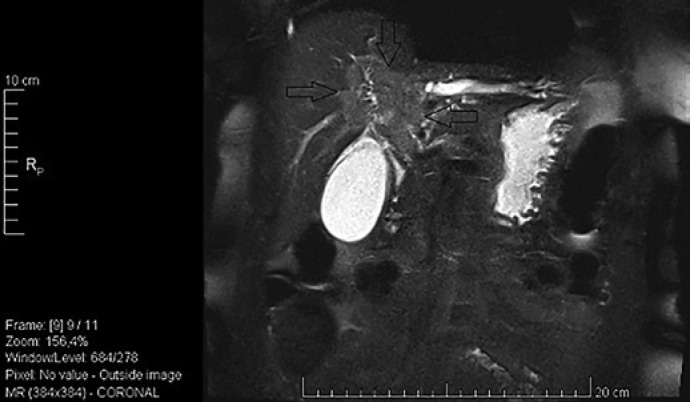
Fig. 2.
MRCP revealing hilar dilatation of the left intrahepatic ducts.
Fig. 3.
MRCP showing a mass forming stricture at the hilum.
Fig. 4.
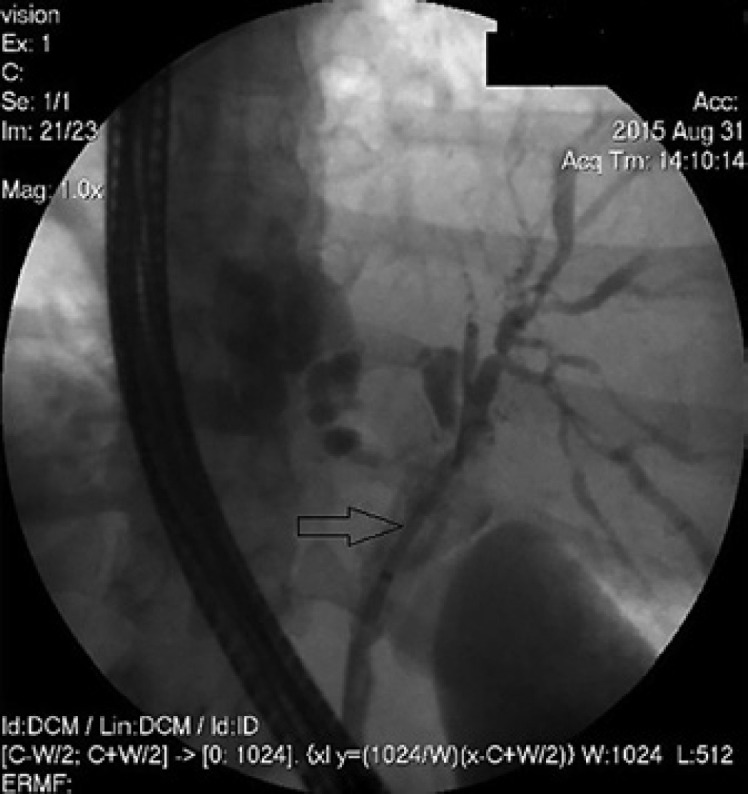
ERCP showing the insertion of a PS after dilatation of the malignancy in the DHS.
Fig. 5.
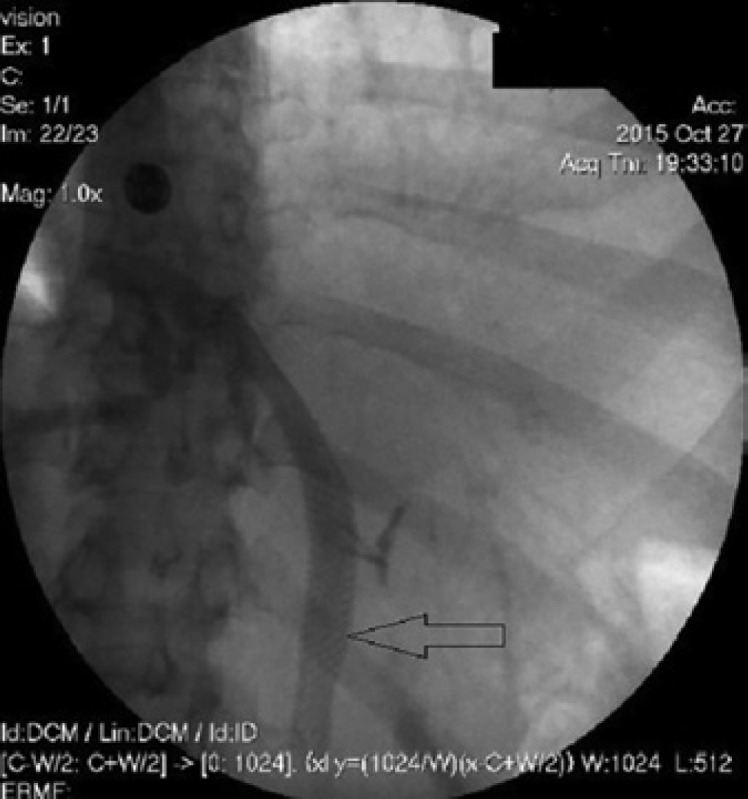
ERCP showing the insertion of a noncovered self-expanding metal stents.
Nine months later, tumor progression resulted in obstructive jaundice and cholangiosepsis. The stenosis was dilated and a second ucSEMS (Biliary-Stent 8 × 80 mm, Boston Scientific) was placed through the first stent in the DHS (Fig. 6). After progredient tumor ingrowth and subsequent episodes of cholangiosepsis despite a first-line chemotherapy with Gemcitabine® and Cisplatin® and a second-line chemotherapy with Gentamicine® and Platinol®, we managed to pass and dilate the stenosis under cholangioscopic guidance (Fig. 7). To stabilize stent patency and improve our patient's quality of life, informed consent was obtained for ELRA (Taewoong Medical, Gimpo-si, South Korea) (Fig. 8). The ELRA-catheter (7 Fr, ablation length 18 mm) was placed under radiologic guidance in the tumor stenosis. We used the standard setting of 7-W, 70°C for 2 min. The procedure was repeated to cover the whole length of the stricture. Necrotic debris was removed with a balloon the same day and again 2 days later while patency was confirmed by contrast. Depending on the patient's clinic and cholestatic parameters this was repeated every 2–4 months for a total amount of 11 sessions from May 2017 to January 2020. No immediate or late adverse events were recorded.
Fig. 6.
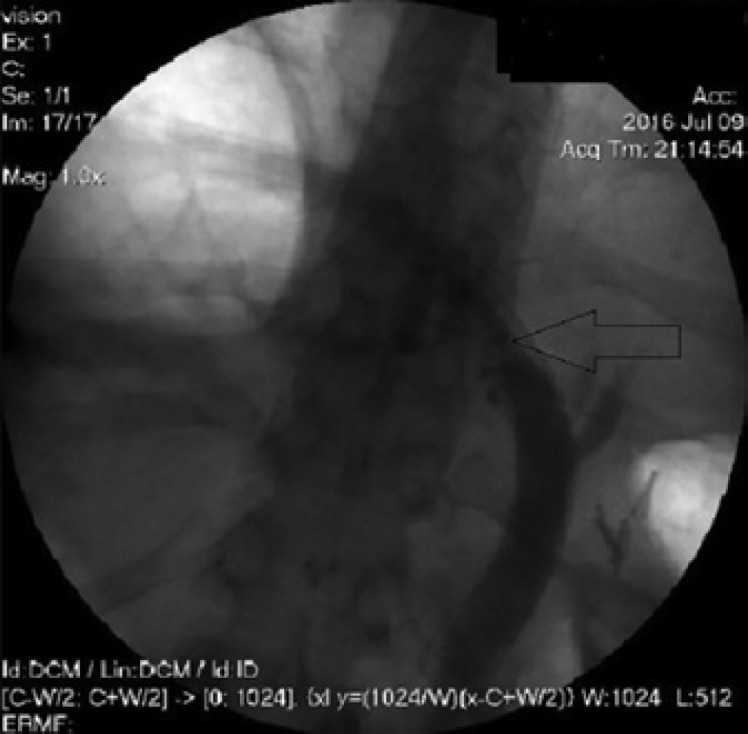
ERCP showing the insertion of a second stent through the first stent in the DHS.
Fig. 7.
Spyglass cholangioscopy showing malignant hilar restenosis.
Fig. 8.
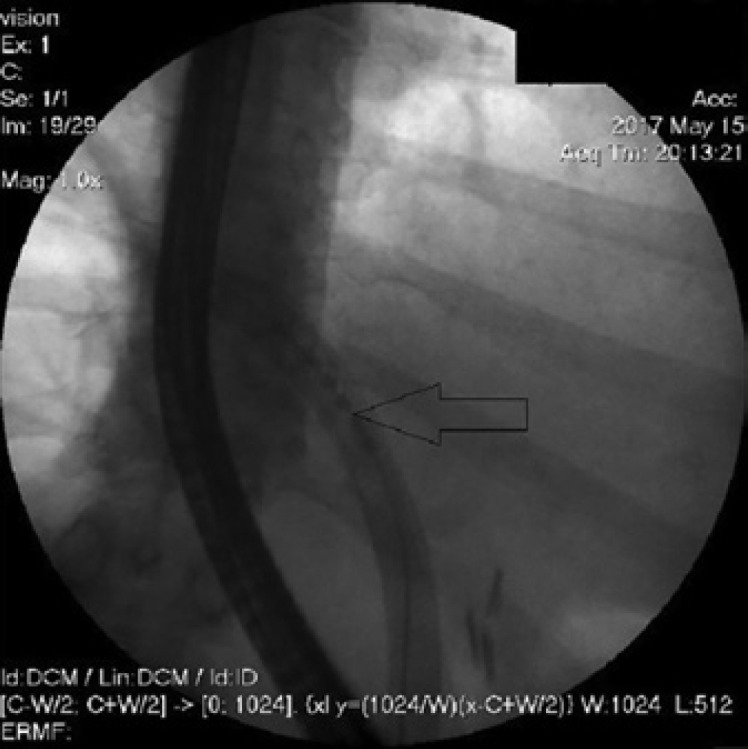
ELRA.
Results
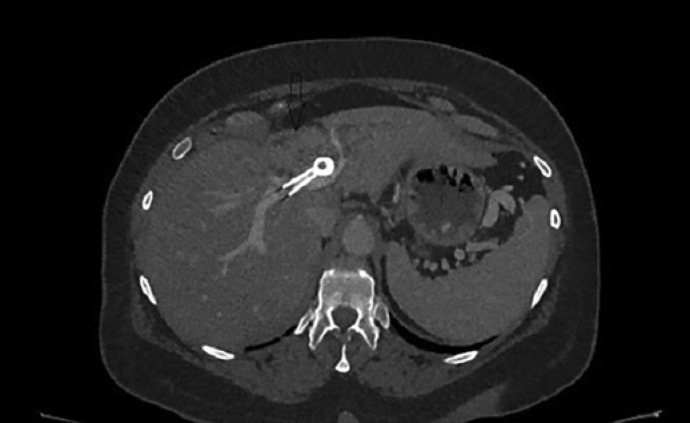
On the patients' last visit on January 2020, she remained asymptomatic at 32-month follow-up after 11 sessions of ELRA. She was not jaundiced and regained her initial body weight, while CA 19-9 decreased from 149 U/mL to 28.9 U/mL. The patient's functional status was stable at 1 Eastern Cooperative Oncology Group. Surveillance CT showed presence of the unilateral SEMS in the common and main left biliary duct with slight left intrahepatic duct dilatation and decreased tumor mass, consistent with radiological improvement (Fig. 9).
Fig. 9.
Abdominal CT showing in situ SEMS and decreased hilar tumor mass.
Discussion
In the current case, the patient was diagnosed as having a nonoperable hilar cholangiocarcinoma. The mass forming stricture was located at the hilum with dilatation of the left intrahepatic ducts consistent with a Klatskin Tumor (Bismuth IV). The definitive diagnosis was obtained by diagnostic laparoscopy, nonetheless retrospectively, a diagnostic approach with ERCP in conjunction with spyglass cholangioscopy should have been done which was not available as standard at that time. Because our patient's life expectancy was more than 6-month, we initially treated her with an ucSEMS and chemotherapy. The course was characterized by progredient tumor ingrowth with cholestatic disease and episodes of cholangiosepsis despite a second-line chemotherapy and insertion of a second ucSEMS. After interdisciplinary discussion, we decided to start a local therapy with ELRA. We performed 11 sessions of ELRA (1st session May 2017, 11th session January 2020) without any postprocedural complications or adverse events. We were able to achieve a long-term (32 months) stent patency without relapse of cholestasis and cholangitis. Furthermore, we observed a decreasing Ca 19-9 as well as a reduction in tumor mass. The course of disease was characterized by regaining body weight combined with a good functional status and quality of life. Most hilar cholangiocarcinoma is not suitable for surgery because of advanced disease stages at diagnosis. Palliative chemotherapy and placement of a stent for biliary drainage is a well-established and widely accepted treatment in patients with unresectable CCC [1, 2, 4, 6]. There is still no common consensus on the relative merits of PS versus metal stents and single versus multiple stents. In patients with complex hilar lesions, retrospective case-control studies suggest that bilateral versus unilateral endoscopic biliary drainage may result in improved jaundice, postprocedural cholangitis, and overall survival [7], although this was not confirmed in a recent randomized trial [8]. Therefore, the choice of stent for endoscopic palliation of unresectable hilar CCC should consider several conditions, such as the affected biliary ducts, life expectancy, endoscopists expertise, and material expenses [9, 10, 11]. PS is characterized by a smaller diameter (10–12 Fr), resulting in faster occlusion with a median patency time of only 1.4–3 months [12]. On the other hand, SEMS has a wider diameter (8–10 mm), resulting in longer patency of 6–10 months [12]. SEMS used in palliative hilar CCC are uncovered with an open mesh, allowing the drainage of side branches [1, 12]. ucSEMS seems to be cost-effectiveness compared to PS if the expected survival exceeds 4–6 months and should be considered as initial treatment after diagnosis of inoperable CCC [1, 2, 6, 12]. In case more than one stent (PS or SEMS) has to be placed, the stents are usually placed side by side. However, a novel dual stent design called “stent-in-stent” has been developed for ucSEMS [13]. “Stent-in stent” technique is characterized by a first stent with an open-cell design which allows a second stent to pass easily through the first one [13]. Stent patency is an important factor in improving quality of life, symptoms, and decreasing interventional costs [14]. Antibiotic prophylaxis is mandatory in patients with anticipated incomplete biliary drainage by any technique; antibiotics should be continued in cases of incomplete biliary drainage [15]. However, stent patency is difficult to preserve due to sludge (in PSs) or tissue ingrowth/overgrowth (in SEMSs) [6]. PS should be exchanged with a single/multiple PSs or a ucSEMS [6]. Mechanical SEMS cleansing is poorly effective for restoring biliary patency if occluded [6]. In this case, a second SEMS should be inserted within the occlusion (a covered model should be selected if the first SEMS was uncovered). In case of a life expectancy ≤3 months, occluded SEMSs should be treated by inserting a PS instead of a second SEMS [6]. Local ablative techniques have been used to improve the results of biliary stenting with the aim of delaying stent obstruction and prolonging patient survival. These techniques include PDT, radiotherapy, and RFA. PDT uses a photosensitive agent that concentrates preferentially in malignant tumors. Subsequent photoactivation with red laser lights of a specific wavelength creates reactive oxygen, leading to selective tumor cell death [16]. Radiotherapy has a very limited role in HC. Intraluminal brachytherapy showed good short-term effects in term of prolonged stent patency and improved survival [17]. However, several studies from Japan have shown better SEMS patency (10–18 months vs. 4–12 months) after external beam radiotherapy [18, 19]. RFA is a well-established ablative procedure originally used for small solid liver tumors [20]. Since 2011, an endoscopic catheter is available that allows ID-RFA in biliary or pancreatic ducts.
Biliary RFA is performed after biliary tract cannulation with ERCP [21]. A sphincterotomy is generally performed but not mandatory [4]. After cholangiographic localization of stricture extent and width that can be combined with cholangioscopy and other imaging modalities, dilation can be performed if needed. The probe is then inserted over the guidewire across the stricture and energy is applied for the desired period, according to the different RFA probe manufacturer's indications. By applying thermal energy to the tissue through high-frequency alternating current, RFA induces coagulative necrosis and causes local destruction of the tumor [20, 21]. Usually, multiple radiofrequency applications are completed during the same session [4, 20, 21]. Before withdrawing the probe, a pause of about 60 s is necessary to prevent tissue from adhering to the electrodes. After removing the probe, coagulated tissue debris is extracted with an extraction balloon, and a PS or metal stent is positioned to guarantee biliary drainage [22]. To date, available retrospective comparative studies demonstrated the superiority of RFA over PDT in terms of efficacy, safety, stent patency, and adverse events with comparable survival rate [23, 24]. Benefits of RFA over PDT are notable and include: cost-effectiveness, easier to perform (catheter can be inserted over a guidewire), and more practical for the patient (procedure done in 1 day and no need to avoid sunlight exposure), [23]. Absolute contraindications for RFA include cardiac pacemakers, pregnancy, coagulation disorders, and massive ascites. The incidence of adverse events has been reported to range from approximately 5–27% [25]. Common RFA-related adverse events include cholangitis and pancreatitis; however, more serious events have also been reported, including portal vein thrombosis, hemobilia, hepatic infarction, sepsis, liver abscess, and death [26, 27]. Bokemeyer et al. [28] demonstrated that in patients with unresectable Bismuth type III and IV hilar cholangiocarcinoma, ID-RFA with biliary stenting significantly prolonged survival time compared to controls receiving standard treatment with stenting alone (342 days vs. 221 days; p = 0.046). These results were confirmed by a meta-analysis by Sofi et al. [29] that demonstrated that biliary RFA had the advantage of prolonging stent patency (mean difference, 50.6 days; 95% CI, 32.83–68.48) and improving patient survival (hazard ratio, 1.395; 95% CI, 1.145–0.7; p < 0.001) without causing serious adverse events. The improved median survival after ID-RFA could be related to activation of anticancer immunity after ablation [30]. An interesting study regarding the feasibility of RFA for occluded SEMS was conducted by Kadayifci et al. [31]. His group matched 25 patients with ucSEMS occlusion treated with endobiliary RFA or PSs across the ucSEMS. Biliary drainage was restored in all patients. Stent patency was confirmed at day 90 in 56% (RFA) and 24% (control), respectively. Additionally, the stent was patent significantly longer in the RFA group compared to the control group (119.5 days vs. 65.3 days, p = 0.03) [32]. Moreover, in extrahepatic distal cholangiocarcinoma Wu et al. [32] achieved a better functional status (Eastern Cooperative Oncology Group Performance Status 1) and quality of life in ID-RFA patients. In the present case, we achieved a 32-month stent patency, without tumor ingrowth and serious adverse events. The choice of inserting an ucSEMS was justified by a life expectancy >6 months and combined RFA therapy achieved a significant improvement in quality of life, weight stabilization, stent patency as well as reduction of tumor size and Ca 19-9. The reduction of tumor size is most probably a combined result of RFA and chemotherapy but comparative studies of RFA, RFA, and chemotherapy versus chemotherapy alone are lacking.
We can only speculate that RFA therapy might not be only a palliative therapy by improving stent patency but also can lead to tumor mass reduction. In our case, we showed that RFA is a valuable, minimal invasive and easy-to-use tool that can ensure long-term stent patency and significantly improve the quality of life in unresectable Bismuth type IV hilar CCC. In conclusion, despite limited survival in nonresectable hilar CCC, ID-RFA seems to be a useful tool in well-selected patients to preserve and prolong stent patency and increase life expectancy.
Statement of Ethics
We authors hereby declare that patient has given their written informed consent to publish her case (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
We authors declare that this work was not supported by any grant or funding.
Author Contributions
Davide Lanza: first-author, he contributed substantially to the design, structuring, data acquisition, and drafting of the work. He critically reviewed the available literature and structured the conclusion. Adrian Casty: co-author (responsible for the oncology section of the manuscript). Stefan H. Schlosser: senior-author (endoscopist who performed ELRA. He contributed substantially in revising the final version of the work and structuring in the patterns required for publication).
Data Availibility Statement
The data that support the findings of this study are available on request from the corresponding author (D.L.).
References
- 1.Goenka MK, Goenka U. Palliation: hilar cholangiocarcinoma. World J Hepatol. 2014;6:559–569. doi: 10.4254/wjh.v6.i8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6((Suppl 6)):VI1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do MY, Cho JH, Jang SI, Lee DK. A review of the recent advances in endoscopic retrograde cholangiography-guided intraductal radiofrequency ablation for malignant biliary strictures. Int J Gastrointest Interv. 2021;10:90–5. doi: 10.4166/kjg.2021.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriemma F, De Luca L, Bianchetti M, Repici A, Mangiavillano B. Radiofrequency and malignant biliary strictures: an update. World J Gastrointest Endosc. 2019 Feb 16;11((2)):95–102. doi: 10.4253/wjge.v11.i2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, et al. Biliary stenting: indications, choice of stents and results − European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–298. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 7.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 8.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 9.Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013 Feb;20((2)):214–222. doi: 10.1007/s00534-012-0508-8. [DOI] [PubMed] [Google Scholar]
- 10.Gao DJ, Hu B, Ye X, Wang TT, Wu J. Metal versus plastic stents for unresectable gallbladder cancer with hilar duct obstruction. Dig Endosc. 2017 Jan;29((1)):97–103. doi: 10.1111/den.12700. [DOI] [PubMed] [Google Scholar]
- 11.Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010 Oct;72((4)):728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 13.Chahal P, Baron TH. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointest Endosc. 2010;71:195–9. doi: 10.1016/j.gie.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Jha AK, Jha P, Jha SK, Keshari R. Plastic versus metal stents for inoperable gallbladder cancer with hilar biliary obstruction: the jury is still out. Ann Gastroenterol. 2021;34((1)):12–9. doi: 10.20524/aog.2020.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, et al. Asge Standards of Practice Committee Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791–8. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458–473. doi: 10.1111/j.1572-0241.2007.01645.x. [DOI] [PubMed] [Google Scholar]
- 18.Isayama H, Tsujino T, Nakai Y, Sasaki T, Nakagawa K, Yamashita H, et al. Clinical benefit of radiation therapy and metallic stenting for unresectable hilar cholangiocarcinoma. World J Gastroenterol. 2012;18:2364–2370. doi: 10.3748/wjg.v18.i19.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinchi H, Takao S, Nishida H, Aikou T. Length and quality of survival following external beam radiotherapy combined with expandable metallic stent for unresectable hilar cholangiocarcinoma. J Surg Oncol. 2000;75:89–94. doi: 10.1002/1096-9098(200010)75:2<89::aid-jso3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.McCarty TR, Rustagi T. New indications for endoscopic radiofrequency ablation. Clin Gastroenterol Hepatol. 2018 Jul;16((7)):1007–1017. doi: 10.1016/j.cgh.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality. Gastrointest Endosc. 2011;74:935–7. doi: 10.1016/j.gie.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Rustagi T, Jamidar PA. Intraductal radiofrequency ablation for management of malignant biliary obstruction. Dig Dis Sci. 2014 Nov;59((11)):2635–2641. doi: 10.1007/s10620-014-3237-9. [DOI] [PubMed] [Google Scholar]
- 23.Uppal DS, Wang AY. Advances in endoscopic retrograde cholangiopancreatography for the treatment of cholangiocarcinoma. World J Gastrointest Endosc. 2015 Jun 25;7((7)):675–687. doi: 10.4253/wjge.v7.i7.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt A, Bloechinger M, Weber A, Siveke J, von Delius S, Prinz C, et al. Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma. United European Gastroenterol J. 2016;4:570–9. doi: 10.1177/2050640615621235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751–760. doi: 10.1055/s-0043-124870. [DOI] [PubMed] [Google Scholar]
- 26.Kasugai H, Osaki Y, Oka H, Kudo M, Seki T. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72((Suppl 1)):72–5. doi: 10.1159/000111710. [DOI] [PubMed] [Google Scholar]
- 27.Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, et al. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014;6:13–9. doi: 10.4253/wjge.v6.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokemeyer A, Matern P, Bettenworth D, Cordes F, Nowacki TM, Heinzow H, et al. Endoscopic radiofrequency ablation prolongs survival of patients with unresectable hilar cholangiocellular carcinoma: a case-control study. Sci Rep. 2019;9:13685. doi: 10.1038/s41598-019-50132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofi AA, Khan MA, Das A, Sachdev M, Khuder S, Nawras A, et al. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:944–51.e1. doi: 10.1016/j.gie.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations: boosting the anticancer immune response. J Immunother Cancer. 2017;5:78. doi: 10.1186/s40425-017-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadayifci, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy. 2016 Dec;48((12)):1096–101. doi: 10.1055/s-0042-115938. [DOI] [PubMed] [Google Scholar]
- 32.Wu TT, Li WM, Li HC, Ao GK, Zheng F, Lin H. Percutaneous intraductal radiofrequency ablation for extrahepatic distal cholangiocarcinoma: a method for prolonging stent patency and achieving better functional status and quality of life. Cardiovasc Intervent Radiol. 2017 Feb;40((2)):260–9. doi: 10.1007/s00270-016-1483-2. [DOI] [PubMed] [Google Scholar]