Version Changes
Revised. Amendments from Version 1
This version now also includes the findings and methods for Phase 2 of the Concept Analysis (together with the original findings from Phase 1), and a final definition of ‘trial recruitment’ We have revised the abstract, aim and conclusion to reflect this, and included a section on Phase 2 methods and findings. The background section now includes reference to previous studies that used theoretical frameworks in trying to understand or enhance trial recruitment strategies, and clarification on why variation in how recruitment is reported can be problematic. The methods section has been revised to include further information on why trial protocols were excluded. The conclusion has been revised to reflect on both Phase 1 and 2, to emphasise the importance of clear trial reporting, and to recommend future use of the proposed definition. The conclusion now also includes one further study limitation that the type of randomised trial might also potentially influence the definition of recruitment in trial reports. And finally, a correction in Figure 3: ‘no further novel data captured’ correction from 150 to 91.
Abstract
Background: The International Committee of Medical Journal Editors (ICMJE) requires trials submitted for publication to be registered before recruitment of the first participant; however, there is ambiguity around the definition of recruitment and in anchoring the trial start date, end date, and recruitment, or as often interchangeably referred to, enrolment, temporally to trial processes. There is potential for variation in how recruitment is reported and understood in trial protocols and trial reports. We report on a concept analysis of ‘trial recruitment’ and develop an operational definition of ‘trial recruitment’.
Methods: A concept analysis using the hybrid model. In Phase 1 we examined randomised and non-randomised trial reports (n=150) published between January 2018 and June 2019 to conceptually explore how recruitment was temporally aligned to the four time-points of screening/eligibility, consent, randomisation and allocation. A preliminary operational definition of ‘trial recruitment’ was determined. This definition was further explored, refined and finalised in Phase 2 (field work), through an interactive, discussion-focused workshop with trial recruiters and trial participants.
Results: Of the 150 trial reports analysed, over half did not identify a clear time point of when recruitment took place and varying terminology is used when reporting on trial recruitment. In Phase 2, the workshop attendees agreed that the proposed definition of ‘trial recruitment’ offers an acceptable definition that provides a standardised approach of how trial recruitment may be temporally understood as part of overall trial processes.
Conclusion: There is ambiguity around temporal descriptions of ‘trial recruitment’ in health care journals. Informed by the findings of this concept analysis we propose a temporal operational definition of trial recruitment based on i) trial recruitment of an individual or cluster and ii) the trial recruitment period.
Keywords: Concept analysis, Trial recruitment, Trial report, Trial enrolment
Background
Non-reporting of completed trials and selective outcome reporting in trials can result in a biased assessment of the global body of evidence that inform health care decisions. Insufficient or inaccurate reporting of trials and inconsistency in the interpretation of trial reports, threatens their reliability and integrity; this is problematic for the healthcare community in using the evidence base to make clinical decisions. In addition to this, there is ambiguity around the terminology used to describe trial processes and multiple varying terms are often used to describe similar processes; for instance, enrolment and recruitment are often used interchangeably as can be seen in the International Committee of Medical Journal Editors (ICMJE) and the World Health Organisation’s (WHO) guidance. Since 2004, the International Committee of Medical Journal Editors (ICMJE) has required that trials submitted for publication must be registered before the enrolment of the first trial participant. The ICJME ‘ does not define the timing of first participant enrolment, but best practice dictates registration by the time of first participant consent’ 1 (p. e1). In addition, the World Health Organisation’s (WHO) ‘International Standards for Clinical Trial Registries’ 2 define prospective trial registration as ‘the registration of a trial before the recruitment of the first participant’ (p.8) and date of the first enrolment as the ‘anticipated or actual date of enrolment of the first participant’ (p.28) but the temporal relationship between an invitation to a potential participant, taking consent and randomisation is not defined. For example, the International Standard Randomised Controlled Trial Number (ISRCTN) registry defines a recruitment start date as ‘the date, or planned date, of recruitment of the first participant to the study’ 3 (p. e1). The clincialtrials.gov registry refers to a study start date and defines this as ‘ the estimated date on which the clinical study will be open for recruitment of participants, or the actual date on which the first participant was enrolled’ 4 (p. e1), thus separating recruitment from enrolment whereby a participant is ‘enrolled’ following completion of the informed consent process.
Ambiguity in anchoring the trial start date, end date, recruitment and enrolment temporally to trial processes (e.g. invitation, consent, and randomisation) has the potential for variation in how recruitment is reported and understood in trial registries, trial protocols and trial reports. In previous studies, theoretical frameworks have been used in an effort to understand or enhance recruitment strategies from the perspective of trial recruiters. For example, in a qualitative study involving nine trial recruiters, Brehaut and colleagues 5 used Shared Decision Making and the Theoretical Domains Framework to explore trial recruiter strategies during recruitment interactions. Six dominant themes are described, namely, coordinating between people, providing guidance to recruiters about challenges, providing resources to recruiters, optimizing study flow, guiding the recruitment decision, and emphasizing the benefits to participation. Developing decision aids for trial participation, underpinned by a theoretical framework, have also been explored (e.g., the Ottawa Decision Support Framework 6 ). No previous studies exist, that the authors can identify, however, which have explicitly analysed the concept of ‘trial recruitment’ or which have sought to explicitly operationalise the concept when planning and undertaking a trial. For this reason, and as part of a wider project that developed an education and training intervention for recruiters to trials (the TRAIN study; full report in progress for publication) we undertook a formal concept analysis of ‘trial recruitment’.
Aim
To report a concept analysis of ‘trial recruitment’ using the hybrid model 7 and provide an operational definition of ‘trial recruitment’.
Methods
Study design
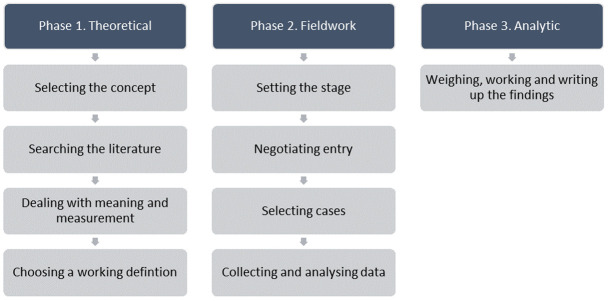
A concept analysis typically involves synthesising evidence on a concept and distinguishing it from other similar/related concepts to help resolve inconsistencies in the knowledge base 8 . Concept analysis offers a means of defining or clarifying concepts, contextually, while also assisting to elucidate patterns of usage which can become a precursor of theory and knowledge development 9 . There are various methods available for formal concept analyses 10 . We chose Schwartz-Barcott and Kim’s hybrid model to analyse the concept of ‘trial recruitment’, because it is considered beneficial in helping resolve ambiguity surrounding a concept and is facilitative of concept expansion and purification 11 . The model consists of three major phases; 1) the theoretical phase, 2) the fieldwork phase, and 3) the analytic phase ( Figure 1).
Figure 1. Phases of the hybrid model of concept analysis 7 .
Phase 1
Searching the literature. Phase 1 of the Concept Analysis aims to comprehensively source and analyse relevant literature to acquire a deep understanding of the concept under study; that is, how the concept has been defined, used, and ways that it has been or might be measured 7 . To gain a contemporary understanding of the concept of ‘trial recruitment,’ we searched randomised (parallel, cluster, and other randomised designs, including pilot and feasibility trials) and non-randomised (i.e. quasi) trial reports published between January 2018 and June 2019. Included studies were sourced from the five top journals in the category of medicine 12 that had the highest impact factor ( Table 1). We excluded trial protocols as, in acknowledging that they provide valuable information this information reflects plans for the trial, rather than providing a reporting of actual trial conduct and recruitment processes that were now complete. We also excluded studies reporting secondary analyses of original/primary trial data, trials not yet started, ongoing studies, meta-analyses/systematic reviews and single-arm studies.
Table 1. Top five impact factor medical journals 2019 12 .
| Journal Title | Impact factor
(2019) |
|---|---|
| New England Journal of Medicine (N Engl
J Med) |
55.873 |
| Lancet | 45.217 |
| Journal of the American Medical
Association (JAMA) |
35.289 |
| Annals of Internal Medicine (Ann Intern
Med) |
17.81 |
| British Medical Journal (BMJ) | 17.445 |
The search strategy (available as Extended data 13 ) was executed in June 2019, using the Cochrane Collaboration’s EMBASE ‘trial’ search string 14 combined with the respective journal titles, and limited by year 2018-2019 and ‘article’ publication type.
Dealing with meaning and measurement. The following data were extracted and used to analyse the concept of ‘trial recruitment’; study characteristics (data source, the aim of the study, location of study, and health condition); implicit or explicit temporal descriptions and definitions of the trial start date, end date, trial duration, gaining consent, recruitment, enrolment, and randomisation. Once data were extracted, significant points of contrast and similarity were explored. This type of comparison gives the researcher an insight into the degree of consensus among users of the concept of ‘trial recruitment’ and can help ascertain the degree of intersubjectivity of meaning 7 . Anticipating that few explicit definitions of trial recruitment might exist, Schwartz-Barcott and Kim recommend analysis of the authors’ writings to determine implied definitions of the concept under study, using the format given in Table 2 as a guide 7 .
Table 2. Sample format for organising and analysing definitions 7 .
| Reference | Explicit | Implicit | Examples | Comments |
|---|---|---|---|---|
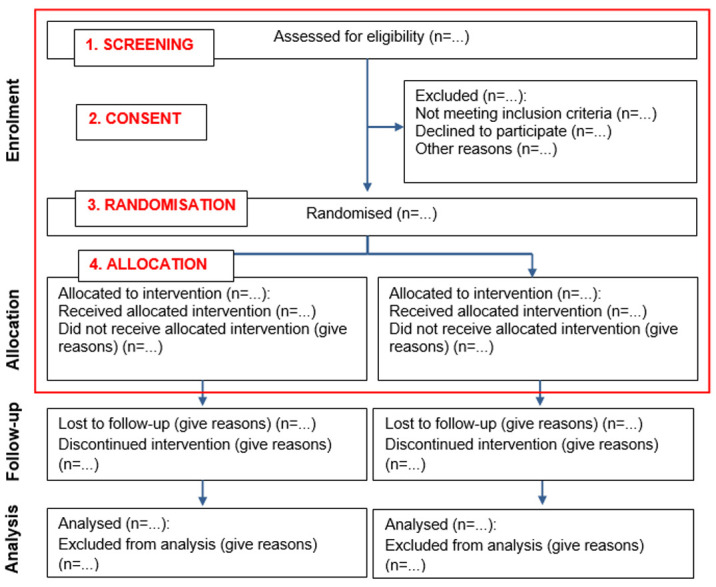
Data analysis. The CONSORT flow diagram 15 recommends that the following main time points should be reported when presenting the progress of participants through a trial: screening, consent, randomisation, allocation, follow-up and analysis. As we were concerned explicitly with recruitment in this analysis, we focused on screening, consent randomisation and allocation. For our analysis, we examined how recruitment was defined temporally to these four time points. (see Figure 2). We then developed a preliminary operational definition of ‘trial recruitment’ in concluding Phase 1 of the concept analysis.
Figure 2. CONSORT flow diagram 15 - edited to include the four time points analysed for this study.
Phase 2 and 3
Phases 2 of the Hybrid Model 7 involves ‘field’ data collection to test and further refine the preliminary Phase 1 definition of trial recruitment, followed by an analytic Phase (Phase 3) which merges the phases in finalising the operational definition of the concept and writing up the findings ( Figure 1). As part of the wider TRAIN study, an intervention co-design workshop involving neonatal trial recruiters and parents of infants previously invited to take part in a trial was held. A section of this workshop was dedicated to Phase 2 of the concept analysis.
Setting and sample. Phase 2 focused on refining the preliminary definition, within the setting of neonatal trials. Phase 2, ‘setting the stage’ involved defining the setting and sample for data collection. Aligned with the TRAIN study, the setting was neonatal trials and the sample were workshop attendees. A purposeful sampling approach was taken when recruiting workshop attendees, with support from the Irish Neonatal Health Alliance (INHA) and the NPEU Clinical Trials Unit in advertising and inviting relevant representatives. Workshop attendees included parents previously invited (with their infant) to take part in a neonatal trial, and all individuals involved in designing, implementing and/or reporting on the recruitment methods or processes for neonatal trials across Ireland and the UK, including:
-
-
Researchers involved in frontline recruitment to trials (e.g., research assistant, research associate, research nurse/midwife)
-
-
Clinicians involved in frontline recruitment to trials (e.g., doctor, nurse, midwife, other allied health care professional)
-
-
Principal investigators
-
-
Trial managers and co-ordinators/clinical research co-ordinators
-
-
Trial methodologists
Collecting and analyzing data. The workshop, facilitated by AH and HD, was conducted online over the Zoom platform, in April 2021, and lasted approximately 2 hours. The discussions were audio recorded for recall and write up purposes only, and subsequently erased. Ethical approval for the TRAIN study, inclusive of the workshop, was granted by the School of Nursing and Midwifery, Trinity College Dublin, and workshop attendees gave consent for the audio recording.
Workshop attendees were asked to share their perceptions, interpretations and experiences of trial recruitment; this part of the discussion was left open for attendees to share their opinions without being influenced by the preliminary definition. Attendees were then asked for their specific feedback on the preliminary definition of ‘trial recruitment,’ based on their perceptions, experiences and how they interpret ‘trial recruitment’ in everyday practice as trial recruiters, and as individuals previously invited to take part in a trial. The recruiter attendees were also invited to specifically discuss how ‘trial recruitment’ is reported on, and the use of varying terminology in reports (informed by Phase 1 findings, see Section 4.0). As per the Hybrid Model 7 , information gathered during the ‘field’ phase were thematically analysed and the findings from Phases 1 and 2 collectively analysed to develop an operational definition of ‘trial recruitment’.
Findings of Phase 1
Results of the search
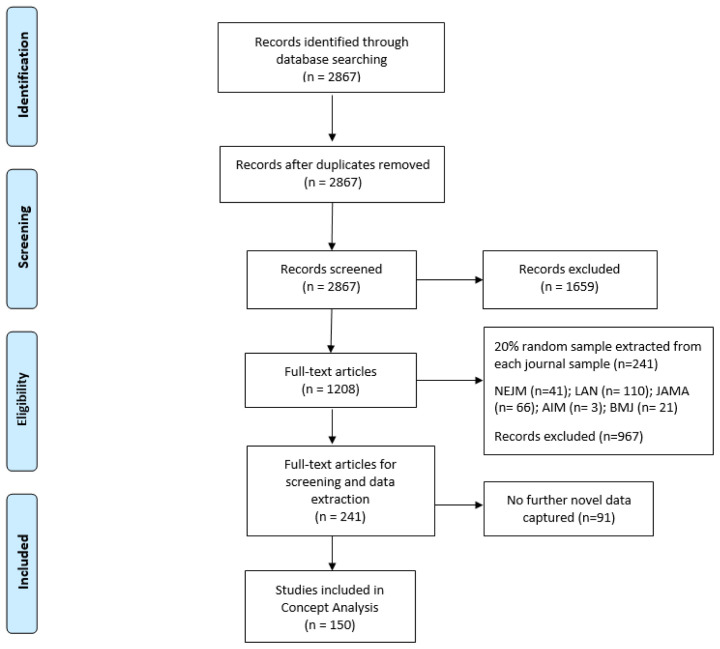
Phase 1 literature searches yielded 2867 records, and no duplicates were found. Following title and abstract screening, 1659 records were excluded based on our predefined inclusion and exclusion criteria. Given the scope of our inclusion criteria, we were confident that the majority of the 1208 records would be included following full-text screening. For this reason, we decided to do full-text screening and data extraction concurrently, dividing the papers equally between three authors (HD, VS and AH). After piloting the data extraction form with a subset of 10 of the 1208 records, and considering the similar reporting format across the five included journals, we selected a 20% random sample of records from each of the five journals, resulting in the inclusion of 241 studies on which to base the concept analysis (see Extended data 11 ). Although we anticipated extracting data from all 241 included studies, at 150 records we had reached a point where no further novel data were being captured (see Figure 3 for further details). For this reason, we concluded data extraction with these 150 trial reports and based the theoretical analysis on the data extracted from these 150 records as we believed this offered data sufficiency in meeting the aim of Phase 1 of this concept analysis. We recognise, however, in omitting the additional 91 records, that the proportions reported in our findings may have been impacted on but not necessarily on the overall conclusions derived from the analysis.
Figure 3. PRISMA flow diagram 16 .
Characteristics of included studies
One hundred and forty-eight of the records reported on randomised trials and two reported on non-randomised trials. Twenty-one of the included trials reported on trials in oncology, with the remaining trials reporting in the medical areas of: cardiology (n=6), psychiatry (n=6), diabetes (n=5), stroke (n=5), dermatology (n=5), HIV (n=5), paediatrics (n=4), ophthalmology (n=4), and other (n=89) (see Extended data 13 ). The majority of trials were carried out in multiple countries (n=55). Twenty-nine were based in America and 15 in the United Kingdom. The remaining trials were conducted in: Asia (n=9), Australia (n=7), Africa (n=5), Netherlands (n=5), France (n=5), Germany (n=4), Switzerland (n=3), Norway (n=2), Canada (n=2), not stated (n=3), and one in each of Hong Kong, Ireland, Poland, Portugal, Russia, South Africa.
Temporal descriptions of ‘recruitment’
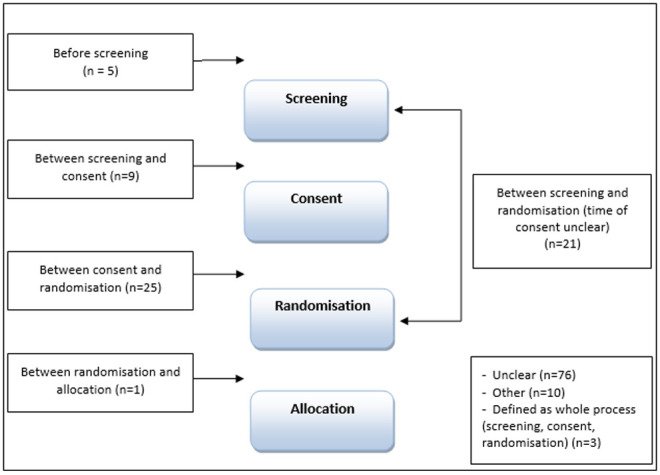
Of the 150 trials analysed, over half (n=76) did not clearly identify when recruitment took place in relation to any of screening, consent, randomisation, allocation (see Figure 4). Twenty-five of the trial reports referred to recruitment as taking place after consent and before randomisation (explicit n=15, implicit n=10); 21 as the point between screening and randomisation (explicit n=10, implicit n=11) with the timing of consent unspecified; and nine referred to recruitment as the point between screening and consent (explicit n=3, implicit n=6). The remaining trials defined recruitment at the time-point before screening (n=5, 3 explicit and 2 implicit); between randomisation and allocation (n=1, explicit). Three studies referred to recruitment generally as including screening, consent and randomisation (explicit n=1, implicit n=2), 10 were categorised as ‘other’: in seven of these trial reports the order of trial processes differed to the order identified in the CONSORT flow diagram and three trials referred to recruitment taking place at randomisation, but the timing of randomisation was unclear.
Figure 4. At what time point is recruitment defined?
The majority of the assessed trials (n=138) provided a time frame in relation to the trial (i.e. start and end date); however, the process that this time frame referred to differed between studies (see Table 3). For instance, 24 studies included the start and end date of the duration of the trial such as 17 ; '...multicentre phase 3 trial was conducted from August 4, 2011, to June 20, 2017’ (p.599). Twenty-two studies stated the start and end date of the randomisation period, such as 18 ; 'Between Oct 1, 2012, and June 20, 2014, we randomly assigned 155 participants...' (p.41). Others included dates between which ‘enrolment’ (n=18), ‘recruitment’ (n=15), and ‘screening’ (n=13) took place. Forty of the trials reported on the start and end date of multiple processes, for instance:
Table 3. Reported start and end date.
| Reported on the start and end date for: | Total
studies |
|---|---|
| Multiple recruitment processes (i.e. the time frame provided referred to more than one process, such as
‘enrolment and randomisation’) |
40 |
| Trial duration (i.e. providing a start and end date for the ‘study’ or ‘trial’ period) | 24 |
| Period of randomisation (i.e. reporting the start and end date for when ‘randomisation’ took place) | 22 |
| Period of enrolment (i.e. reporting the start and end date for when ‘enrolment’ took place) | 18 |
| Period of recruitment (i.e. reporting the start and end date for when ‘recruitment’ took place) | 15 |
| No start/end date reported | 12 |
| Screening period (i.e. reporting the start and end date for when ‘screening’ took place) | 13 |
| Other (i.e. reporting a time frame for trial processes not related to ‘recruitment’ such as data collection and
rounds of treatment) |
6 |
| Total | 150 |
‘During the study period (August 2015 and May 2017), 151 patients were screened, 117 underwent randomization’ 19 (p.2301)
‘Between July 2, 2013, and May 10, 2016, 80 patients were enrolled, randomly assigned, and started their allocated treatment' 20 (p.328)
The studies categorised as ‘other’ (n=6) reported on the start and end date of other processes such as data collection (n=1), rounds of treatment (n=2), and the use of the same start and end date with differing terminology (n=3); for instance, enrolment and recruitment were used interchangeably. Further findings on the variation in language are presented in the next section.
Variation in terminology
There was variation across the studies in the terminology used to describe entry (the point at which a participant was considered to have ‘joined’ a trial) to the trial (see Table 4). Of the 150 analysed trials, just over a third (n=52) used the term ‘enrolment’, and just over one fifth (n=34) did not use a specific term to describe entry to the trial. Thirty studies used multiple terms; this was mostly in the form of ‘recruitment’ used interchangeably with another term such as ‘enrolment’ (n=19), ‘randomisation’ (n=3), randomisation and enrolment (n=2), screening (n=1), screening and randomisation (n=1). Other studies used the term ‘randomisation’ interchangeably with ‘accrued’ (n=1) and ‘enrolment’ (n=1). One study used the terms ‘included’ and ‘enrolment’ interchangeably. Table 4 and Table 5 illustrate the variation across the studies in the terminology used to describe the entry of participants to the trial.
Table 4. Terminology.
| Term used to describe entry to the trial | Total studies |
|---|---|
| Enrolment | 52 |
| No specific term used | 34 |
| Multiple terms used | 30 |
| Recruitment | 29 |
| Other | 5 |
| Total | 150 |
Table 5. Variation in terminology.
| Journal [reference] | Healthcare area | Description |
|---|---|---|
| N Engl J Med 21 | Lung Disease | ‘863 infants were enrolled during the period from April 2010 through August 2013’(p.149) |
| Lancet 22 | Osteoperosis |
‘...we
enrolled post-menopausal women with at least two moderate or one severe
vertebral fracture and a bone mineral density’ (p.30) ‘We enrolled 680 patients in each group…’ (p.30) |
| Lancet 23 | Oncology |
‘Of 601 patients assessed for eligibility, a total of
452 patients… were recruited and
randomly assigned’ (p.233) ‘...601 patients assessed for eligibility, of whom 452 patients were enrolled and 226 were randomly assigned ’ (p.229) |
| JAMA 24 | Anaesthesia |
'Patients undergoing anaesthesia with RSI were
enrolled from February 2014 until
February 2017...' (p.E1) '... Recruitment began in February 2014 and ended in February 2017’ (p.E2) |
| Lancet 25 | Adolescent health |
‘Of the 112 eligible schools, 75 were
randomly selected to participate in the trial...' (p.2471)
'...we recruited 75 schools' (p.2471) |
| JAMA 26 | Retinopathy of prematurity |
‘Patients were
recruited between September 2014 and August 2016. 20 infants were
screened and 19 were randomized’…(p.278) ‘20 patients were screened and 19 were enrolled' (p.279) |
| Lancet 27 | Inflammatory diseases |
‘Between Oct 6, 2015, and Nov 30, 2016, 166 patients were
screened, of whom 102 were
randomly assigned ...’ (p.1330) ‘...Patients were recruited between Oct 6, 2015, and Nov 30, 2016’ (p.1335) |
| BMJ Open 28 | Critical care |
‘...an
enrolment of 114 patients was planned....’ (p.3)
‘One hundred fourteen patients were included in this study with 57 patients randomised in each group’ (p.3) |
Preliminary operational definition
From the findings, a preliminary temporal operational definition of ‘trial recruitment’ emerged based on the contexts of whom and when as follows:
1. Trial recruitment of participants (an individual or cluster) - ‘the time point after screening and consent, and before randomisation’
2. The trial recruitment period - ‘the time point after screening and consent of the first participant, and before randomisation of the last participant’.
Findings of Phase 2
Nine individuals took part in the workshop. These included four parents of neonates previously invited to take part in a trial, and one Trial Manager, two Clinicians and two Research Nurses who were involved in reporting on and implementing recruitment to neonatal trials across Ireland and the UK. The workshop attendees were of the opinion that defining ‘trial recruitment’ is a challenging task, however, they agreed in principle that an individual should not be considered as recruited to a trial unless an individual has given consent. Furthermore, although some differences of opinion were evident in discussions (e.g., recruited after giving consent, but randomisation was required before an individual could be considered as taking part in a trial), the attendees collectively suggested that an individual should only be considered ‘recruited’ if they are officially reported on and counted in the trial participant flow diagram. It was agreed that this takes place after consenting only, regardless of whether they withdraw their consent or not. In this regard, the field data indicated that an individual should only be considered ‘recruited’ after the time-point of consent.
Workshop attendees also reported that they viewed ‘recruitment’ as “a whole process of communicating” from when an individual is first approached and informed about a trial, until the point at which they give consent. Attendees also discussed their experience of reporting and implementing trials where consent took place after randomisation and after the individual had taken part in the trial. Attendees identified that a variety of recruitment scenarios such as deferred consent and perspective assent exist. However, when considering the standard approach they agreed that the preliminary definitions which emerged in Phase 1, were optimally suitable and acceptable as operational definitions for trial recruitment without further refinement.
Conclusion and final definition
This concept analysis has identified that defining ‘trial recruitment’ is a complex task. Phase 1 revealed that there is ambiguity around temporal descriptions of ‘trial recruitment’ in health care journals, and varying terminology is used when reporting on trial recruitment. Sixty-one of the analysed trials identified a time point, in relation to the four main trial processes (screening, consent, randomisation, allocation), at which trial recruitment took place. The majority of these studies identified trial recruitment as being between consent and randomisation or between screening and randomisation (with time of consent unclear) as the time point of actual recruitment. Over half of the trials analysed (n=76) did not identify a clear time point of when trial recruitment took place. Our analysis also revealed variation in terminology used to describe entry to the trial, and often multiple terms were used interchangeably. Enrolment (n=52) and recruitment (n=29) were the most common terms used, but the use of numerous terms was frequent in the trial reports (n=30).
There are some limitations to this concept analysis that should be noted. We acknowledge that trial design could potentially impact on the variation and type of terminology used when reporting trials, for instance whether or not a trial is randomised and whether the trial includes a run-in period. We did not extract data relating to trial run-in periods and, as most of the trials analysed were randomised trials (n=148/150) we could not compare descriptions in randomised compared to non-randomised trial reports. However, we included both randomised and non-randomised trials in our search strategy and the selection of trials for inclusion in analysis was based on a random sample of records from each of the five journals. Type of randomised trial (e.g., cluster, parallel, feasibility or pilot trials) might also potentially influence the definition of recruitment in trial reports. As our focus was on description and definition of recruitment, irrespective of type of trial, we did not specifically seek or extract this information. We acknowledge, however, that this could present as a potential limitation in providing a complete picture of the dataset which informed the concept analysis.
This concept analysis provided a preliminary temporal operational definitions of ‘trial recruitment’, based on Phase 1 and tested these with healthcare professionals and previous trial participants in a workshop session in Phase 2. Based on their practical experience, the workshop attendees concluded that while there may be exceptions to the preliminary definitions, the definitions proposed offer acceptable definitions without refinement. Furthermore, the definitions standardise how trial recruitment may be temporally understood as part of overall trial processes. In this regard, we conclude by proposing the following temporal operational definitions of ‘trial recruitment’ in the context of whom and when: 1) Trial recruitment of participants (an individual or cluster) is defined as ‘the time point after screening and consent, and before randomisation’ and 2) the trial recruitment period is ‘the time point after screening and consent of the first participant, and before randomisation of the last participant’. Having clear and consistent terminology across trial reports is crucial for consistent, accurate and reliable trial reports. Having standardised definitions of trial recruitment will help optimise homogenous understandings and interpretations by those who will ultimately use these trial reports to inform clinical decision making, practice or further research. To overcome ambiguity and potential misinterpretations which can adversely impact on the reliability and integrity of trial reports we offer, based on this concept analysis, a standard definition of recruitment to trials. We recommend that those involved in reporting on trials, and in setting trial reporting guidance, should implement/apply this definition to ensure that trial reporting is optimally standardised into the future.
Data availability
Underlying data
Figshare: https://doi.org/10.6084/m9.figshare.13109870.v1 13
This project contains the following underlying data:
Delaney et al. 2020_Concept Analysis_Extracted Data.xlsx
Extended data
Figshare: https://doi.org/10.6084/m9.figshare.13109870.v1 13
This project contains the following extended data:
Delaney et al. 2020_search strategy.pdf
Delaney et al. 2020_records per journal.pdf
Delaney et al. 2020_characteristics of included studies.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
Health Research Board [TMRN-2017-2].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. International Committee of Medical Journal Editors: Publishing and Editorial Issues Related to Publication in Medical Journals – Clinical Trials. Reference Source [Google Scholar]
- 2. World Health Organisation: International standards for clinical trial registries 2012. Reference Source [Google Scholar]
- 3.International Standard Randomised Controlled Trial Number (ISRCTN) registry: Definitions. Reference Source [Google Scholar]
- 4. Clinicaltrials.gov Registry: Protocol Registration Data Element Definitions for Interventional and Observational Studies. Reference Source [Google Scholar]
- 5. Brehaut JC, Venegas LC, Hudek N, et al. : Using behavioral theory and shared decision-making to understand clinical trial recruitment: interviews with trial recruiters. Trials. 2021;22(1):298. 10.1186/s13063-021-05257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillies K, Campbell MK: Development and evaluation of decision aids for people considering taking part in a clinical trial: a conceptual framework. Trials. 2019;20(1):401. 10.1186/s13063-019-3489-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwartz-Barcott D, Kim HS: Chapter 9: An expansion and elaboration of the hybrid model of concept development. In: Rodgers BL, Knafl KA. (eds.) Concept Development in Nursing. Foundations, Techniques and Applications. Second Edition, Philadelphia: WB. Saunders;2000;129–159. [Google Scholar]
- 8. Knafl K, Deatrick J: Chapter 2: Knowledge Synthesis and Concept Development in Nursing. In: Rodgers BL, Knafl KA. (eds.) Concept Development in Nursing. Foundations, Techniques and Applications. Second Edition, Philadelphia: WB. Saunders;2000;39–54. [Google Scholar]
- 9. Cronin P, Ryan F, Coughlan M: Concept analysis in healthcare research. Int J Ther Rehabil. 2010;17(2):62–68. 10.12968/ijtr.2010.17.2.46331 [DOI] [Google Scholar]
- 10. Beecher C, Devane D, White M, et al. : Concept development in Nursing and Midwifery: An overview of methodological approaches. Int J Nurs Pract. 2019;25(1):e12702. 10.1111/ijn.12702 [DOI] [PubMed] [Google Scholar]
- 11. Jasemi M, Valizadeh L, Zamanzadeh V, et al. : A Concept Analysis of Holistic Care by Hybrid Model. Indian J Palliat Care. 2017;23(1):71–80. 10.4103/0973-1075.197960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Impact Factors: Medical Journal Impact Factors 2019. [Accessed 26 thJune 2019]. Reference Source [Google Scholar]
- 13. Delaney H: A Concept Analysis of trial recruitment. Extended Data. figshare. Dataset.2020. 10.6084/m9.figshare.13109870.v1 [DOI] [Google Scholar]
- 14. Lefebvre C, Manheimer E, Glanville J: Chapter 6: Searching for studies. In: Higgins J, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. updated March 2011. The Cochrane Collaboration,2011. Reference Source [Google Scholar]
- 15. Schulz KF, Altman DG, Moher D, et al. : CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tinnitus Retraining Therapy Trial Research Group, . Scherer RW, Formby C: Effect of Tinnitus Retraining Therapy vs Standard of Care on Tinnitus-Related Quality of Life: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2019;145(7):597–608. 10.1001/jamaoto.2019.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fowler D, Hodgekins J, French P, et al. : Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. Lancet Psychiatry. 2018;5(1):41–50. 10.1016/S2215-0366(17)30476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raghu G, van den Blink B, Hamblin MJ, et al. : Effect of Recombinant Human Pentraxin 2 vs Placebo on Change in Forced Vital Capacity in Patients With Idiopathic Pulmonary Fibrosis: A Randomized Clinical Trial. JAMA. 2018;319(22):2299–2307. 10.1001/jama.2018.6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wildiers H, Tryfonidis K, Dal Lago L, et al. : Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 2018;19(3):323–336. 10.1016/S1470-2045(18)30083-4 [DOI] [PubMed] [Google Scholar]
- 21. Bassler D, Shinwell ES, Hallman M, et al. : Long-Term Effects of Inhaled Budesonide for Bronchopulmonary Dysplasia. N Engl J Med. 2018;378(2):148–157. 10.1056/NEJMoa1708831 [DOI] [PubMed] [Google Scholar]
- 22. Kendler D, Marin F, Zerbini CAF, et al. : Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–240. 10.1016/S0140-6736(17)32137-2 [DOI] [PubMed] [Google Scholar]
- 23. Horwitz S, O’Connor OA, Pro B, et al. : Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–240. 10.1016/S0140-6736(18)32984-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birenbaum A, Hajage D, Roche S, et al. : Effect of Cricoid Pressure Compared With a Sham Procedure in the Rapid Sequence Induction of Anesthesia: The IRIS Randomized Clinical Trial. JAMA Surg. 2019;154(1):9–17. 10.1001/jamasurg.2018.3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shinde S, Weiss HA, Varghese B, et al. : Promoting school climate and health outcomes with the SEHER multi-component secondary school intervention in Bihar, India: a cluster-randomised controlled trial. Lancet. 2018;392(10163):2465–2477. 10.1016/S0140-6736(18)31615-5 [DOI] [PubMed] [Google Scholar]
- 26. Stahl A, Krohne TU, Eter N, et al. : Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2018;172(3):278–286. 10.1001/jamapediatrics.2017.4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. : Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–1339. 10.1016/S0140-6736(18)32167-6 [DOI] [PubMed] [Google Scholar]
- 28. Bridey C, Thilly N, Lefevre T, et al. : Ultrasound-guided versus landmark approach for peripheral intravenous access by critical care nurses: a randomised controlled study. BMJ Open. 2018;8(6):e020220. 10.1136/bmjopen-2017-020220 [DOI] [PMC free article] [PubMed] [Google Scholar]