Abstract
The efficacy of antiretroviral drugs against porcine endogenous retroviruses (PERV) that may be harbored in pig organs intended for transplantation was examined in human cells in vitro. The nucleoside analogs zidovudine and dideoxyinosine were found to effectively inhibit PERV replication.
The promise of unlimited supplies of organs for transplantation makes advances in xenotransplantation an exciting area of medicine. However, concerns have been raised about the possible transmission of disease from donor animals to human patients. In particular, pigs which may be suitable for use as donors of hearts and other organs have been found to contain endogenous retroviruses (porcine endogenous retroviruses [PERV]). While the analysis of humans treated with living pig tissues as well as that of monkeys transplanted with pig organs found no evidence of any cross-species transmission of PERV in vivo (10, 13, 15, 16; Z. Long, personal communication), the ability of PERV to infect human cells in vitro has been demonstrated by several groups (13, 15, 16), suggesting that there may be a risk of patient infection during xenotransplantation. To identify treatments that could minimize the risk from such infections, the efficacy of antiretroviral drugs against PERV was determined in human cells in vitro.
To minimize variability between experiments, a single high-titer stock of PERV generated by infection of human 293 cells with culture supernatant from porcine PK-15 cells was used for all drug efficacy studies. For each treatment, 5 × 105 293 cells were plated in six-well plates and infected with 1 ml of viral supernatant in a total volume of 2 ml of medium containing a final concentration of 8 μg of polybrene per ml; the multiplicity of infection under these conditions was approximately 1. Cells were maintained for 3 weeks in the presence or absence of antiretroviral drugs. To determine if the antiretroviral drugs had toxic effects on 293 cells that might interfere with virus infection, uninfected cultures were carried for three passages in the presence of the drugs and counted at each passage. No difference was observed between control and drug-treated cells (data not shown).
The level of PERV virions in supernatant collected from these cultures was quantified using a PCR assay that specifically detects PERV RNA. Real-time PCR was performed and detected using the Taqman chemistry and the 7700 sequence detection system (PE Biosystems) as previously described (11). The primer sequences used for the RNA-specific PCR are as follows: 5′-GAACATCGATGACAAGCTTAGGTATCGATAACAGCCGTTGGTGTGGTCA-3′ (reverse transcription primer) at a final concentration of 100 nM; 5′-AGCTCCGGGAGGCCTACTC-3′ (forward PCR primer) at a final concentration of 300 nM; and 5′-GAACATCGATGACAAGCTTAGGTATCGATA-3′ (reverse PCR primer) at a final concentration of 300 nM. The probe used to detect PERV-specific products was 5′-(5-carboxyfluorescein)-CCACCGTGCAGGAAACCTCGAG ACT-(6-carboxy-tetramethyl rhodamine)-3′ at a final concentration of 100 nM. To quantitate viral load, amounts following a standard curve and consisting of 102, 103, 104, and 105 PERV virus-like particles in 0.25 ml of medium were extracted and tested in the same manner as the test samples. The viral particle numbers for individual samples were obtained by interpolation from the standard curve. Levels of PERV infection achieved in control 293 cells which were not treated with antiretroviral agents ranged from 105 to 107 copies per cell.
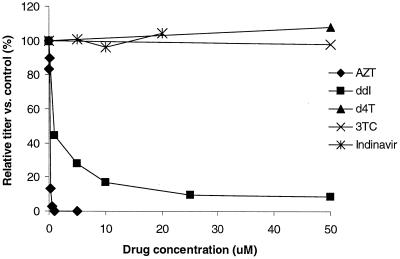
The results of dose-response experiments with the nucleoside analogs zidovudine (AZT), dideoxyinosine (ddI), lamivudine (3TC), and stavudine (d4T) and the protease inhibitor indinavir are shown in Fig. 1. AZT is a potent inhibitor of PERV replication in 293 cells, with a 50% inhibitory concentration (IC50) of approximately 0.25 μM. ddI also inhibited PERV, with an IC50 of approximately 1 μM. These concentrations are similar to IC50s reported in in vitro studies of human immunodeficiency virus type 1 (HIV-1)(1–3). 3TC, d4T, and indinavir were not effective inhibitors of PERV replication.
FIG. 1.
Efficacy of antiretroviral drugs against PERV in human cells. 293 cells were infected with PERV in the presence or absence of antiretroviral drugs and passaged for 3 weeks. Supernatants were assayed for the presence of PERV by RT-PCR. The doses tested were as follows: AZT, 0.01 to 10 μM; ddI, 1 to 50 μM; d4T, 50 μM; 3TC, 50 μM; indinavir, 5 to 20 μM. Each dose-response experiment was repeated at least twice; the results shown are from one representative experiment.
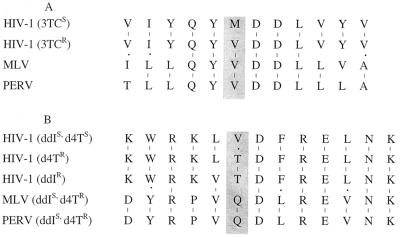
In a previous study, murine leukemia virus (MLV) was found to be resistant to 3TC and d4T; sequence comparisons revealed that the mutations associated with specific drug resistance patterns in HIV-1 occur naturally in the reverse transcriptase (RT) of MLV (14). Comparisons of PERV, MLV, and HIV RT using published sequences show that the RT of PERV is highly related to that of MLV, and sequence comparison in the region associated with resistance to 3TC reveals that like MLV, PERV also contains the single base pair change associated with resistance to this nucleoside analog (Fig. 2A). Resistance of HIV-1 isolates to ddI and d4T has been mapped to single base pair changes flanking amino acids 74 and 75 (4, 5, 7), and some degree of cross-resistance between these two drugs has been noted in clinical settings. PERV and MLV are identical in this region but have several residues that differ from HIV-1, most notably at position 75, where the amino acid change from valine to threonine has been associated with resistance. In PERV and MLV, the amino acid at this position is glutamic acid, and the resistance of PERV and MLV to d4T could be explained by structural changes in this region that alter the binding of the nucleotide analog. Both PERV and MLV contain the Leu-to-Val mutation at position 74 associated with ddIr in HIV-1 but remain sensitive to the drug, suggesting that other changes between PERV or MLV and HIV in this region compensate for the presence of the L74V mutation identified in ddIr HIV. The 3TCr mutation M184V is also associated with some increase in resistance to ddI in HIV-1 (5); it should be noted that both the L74V and the M184V mutations are associated with only modest increases in ddI resistance (five- to eightfold). Although PERV and MLV are identical in this region, they appear to differ by severalfold in their sensitivity to ddI, further supporting the idea that other changes outside this region affect sensitivity. The overall homology between PERV and MLV is 60 to 70%. These results show that while sequence comparisons are useful in predicting sensitivity to antiretroviral drugs, in vitro testing provides important information as well, particularly when sequences that are not well conserved are being examined.
FIG. 2.
Sequence alignment of HIV-1, MLV, and PERV. Regions of RT-flanking mutations known to confer resistance to 3TC, d4T, or ddI in HIV-1 were aligned using published sequences. (A) Protein sequence flanking the known 3TCr mutation M184V in HIV-1. While the region is highly conserved overall between the three viruses, the M184V mutation of HIV-1 occurs naturally in both MLV and PERV (shaded box). (B) Protein sequence flanking the known ddIr mutation L74V and the d4Tr mutation V75T. PERV and MLV sequences are identical in this region, and both viruses remain sensitive to ddI despite the presence of the L74V mutation. At position 75, the valine residue present in d4Ts HIV-1 is replaced by Q in PERV and MLV. Both viruses are d4Tr.
In spite of the considerable divergence of protease sequences between MLV and HIV-1, our previous study showed that unlike PERV, MLV was inhibited by the HIV-1 protease inhibitor indinavir (14). These results are consistent with the fact that resistance to protease inhibitors often involves multiple base pair changes, making it difficult to predict the efficacy of HIV-1 protease inhibitors against other retroviruses strictly on the basis of sequence.
These results demonstrate that PERV replication can be controlled using standard antiretroviral therapies but that, like MLV, the virus is inherently resistant to some commonly used therapies.
Acknowledgments
We thank M. Stefanski for preparation of PERV supernatant, M. Kaloss and S. Brazinski for analysis of indinavir control samples and for performing sequence comparisons, and I. Mychkovsky, L.-P. Li, and I. Burimski for technical assistance. We are grateful to Merck and Co. for generously supplying indinavir.
REFERENCES
- 1.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deminie C A, Bechtold C M, Stock D, Alam M, Djang F, Balch A H, Chou T-C, Prichard M, Colonno R J, Lin P-F. Evaluation of reverse transcriptase and protease inhibitors in two-drug combinations against human immunodeficiency virus replication. Antimicrob Agents Chemother. 1996;40:1346–1351. doi: 10.1128/aac.40.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flexner C, Hendrix C. Pharmacology of anti-retroviral agents. In: DeVito V T Jr, Hellman S, Rosenberg S A, editors. AIDS: biology, diagnosis, and prevention. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 479–493. [Google Scholar]
- 4.Gao Q, Gu Z, Parniak M A, Li X, Wainberg M A. In vitro selection of variants of human immunodeficiency virus type 1 resistant to 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine. J Virol. 1992;66:12–19. doi: 10.1128/jvi.66.1.12-19.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Z, Gao Q, Li X, Parniak M A, Wainberg M A. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine. J Virol. 1992;66:7128–7135. doi: 10.1128/jvi.66.12.7128-7135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneine W, Tibell A, Switzer W M, Sandstrom P, Rosales G V, Mathews A, Korsgren O, Chapman L E, Folks T M, Groth C G. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998;352:695–699. doi: 10.1016/S0140-6736(98)07145-1. [DOI] [PubMed] [Google Scholar]
- 7.Kozal M J, Kroodsma K, Winters M A, Shafer R W, Efron B, Katzenstein D A, Merigan T C. Didanosine resistance in HIV-infected patients switched from zidovudine to didanosine monotherapy. Ann Intern Med. 1994;121:263–268. doi: 10.7326/0003-4819-121-4-199408150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Lacey S F, Larder B A. Novel mutation (V75T) in human immunodeficiency virus type 1 reverse transcriptase confers resistance to 2′,3′-didehydro-2′,3′-dideoxythymidine in cell culture. Antimicrob Agents Chemother. 1994;38:1428–1432. doi: 10.1128/aac.38.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lea A P, Faulds D. Stavudine: a review of its pharmacodynamic and pharmacokinetic properties and clinical potential in HIV infection. Drugs. 1996;51:846–864. doi: 10.2165/00003495-199651050-00009. [DOI] [PubMed] [Google Scholar]
- 10.Martin U, Steinhoff G, Kiessig V, Chikobava M, Anssar M, Morschheuser T, Lapin B, Haverich A. Porcine endogenous retrovirus (PERV) was not transmitted from transplanted porcine endothelial cells to baboons in vivo. Transpl Int. 1998;11:247–251. doi: 10.1007/s001470050136. [DOI] [PubMed] [Google Scholar]
- 11.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer W M, Chapman L E, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 12.Patience C, Patton G S, Takeuchi Y, Weiss R A, McClure M O, Rydberg L, Breimer M E. No evidence of pig DNA or retroviral infection in patients with short-term extracorporal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 13.Patience C, Takeuchi Y, Weiss R A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 14.Powell S K, Artlip M, Kaloss M, Brazinski S, Lyons R, McGarrity G J, Otto E. Efficacy of antiretroviral agents against murine replication-competent retrovirus infection in human cells. J Virol. 1999;73:8813–8816. doi: 10.1128/jvi.73.10.8813-8816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi Y, Patience C, Magre S, Weiss R A, Banerjee P T, Le Tissier P, Stoye J P. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998;72:9986–9991. doi: 10.1128/jvi.72.12.9986-9991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson C A, Wong S, Muller J, Davidson C E, Rose T M, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]