Abstract
(a) Summary for SGLT2i‐induced alterations in 48 chemokines/cytokines in Hep3B and Huh7 cells. (b) SGLT2i‐induced changes in the medium CXCL1 level (each n = 4). (c) SGLT2i‐induced changes in the medium CXCL8 level (each n = 4). (d) SGLT2i‐induced changes in the medium CXCL10 level (each n = 4), (e) SGLT2i‐induced changes in the medium M‐CSF level (each n = 4). The SGLT2i‐induced significant changes in chemokines/cytokines are expressed as the percentage of control (the mean value of the control was set as 100%). CXCL, C‐X‐C motif chemokine ligand; G‐CSF, granulocyte colony‐stimulating factor; IL, interleukin; LIF, leukemia inhibitory factor; M‐CSF, macrophage colony‐stimulating factor; MIP‐1α, macrophage inflammatory protein‐1α; SDF‐1α, stromal‐cell‐derived factor‐1α; TNFα, tumor necrosis factor‐α; VEGF, vascular endothelial growth factor.
Keywords: chemokine, cytokine, hepatoma, sodium‐glucose cotransporter 2, tumor microenvironment
Sodium‐glucose cotransporter 2 inhibitor (SGLT2i) was originally developed as an antidiabetic medication. 1 Recent studies have shown that SGLT2 occurs in tumor cells, 2 and SGLT2i has antitumor effects. 3 SGLT2i‐induced suppression of hepatocellular carcinoma (HCC) was reported in vitro and in vivo studies as well as a case report. 1 , 2 , 3 However, its mechanisms remain unclear. HCC releases various chemokines/cytokines to modulate the tumor microenvironment and regulates the proliferation and invasion of HCC cells. 4 , 5 The aim of this study was to investigate the direct effects of SGLT2i on tumor‐releasing chemokines/cytokines in human HCC cell lines.
Hep3B and Huh7 cells were cultured with Dulbecco's Modified Eagle Medium (DMEM; glucose 4500 mg/L) (FUJIFILM Wako Chemicals, Osaka, Japan) with 10% bovine serum at 37°C for 48 h after passage. Then, the culture medium was replaced with DMEM (glucose 4500 mg/L) with no bovine serum, and cells were treated with SGLT2i (10 μM of canagliflozin dissolved in dimethyl sulfoxide; n = 4 for each cell line) or control vehicle (dimethyl sulfoxide; n = 4 for each cell line) for 24 h. Then, the culture media were collected and subjected to 48 chemokine/cytokine assays using the human cytokine screening 48‐plex panel (Bio‐Plex Pro, Bio‐Rad Laboratories, Inc., Hercules, CA, USA). Each data point was based on the mean value of duplicate assays. All data are expressed as the mean ± SD. The SGLT2i‐induced significant changes in chemokines/cytokines are also expressed as the percentage of control, in which the mean value of the control was set as 100%. Comparisons between two groups were performed using the Wilcoxon rank‐sum test. Statistical analyses were performed using JMP Pro16 (SAS Institute Inc., Cary, NC, USA). A P‐value <0.05 was considered statistically significant.
Of the measured 48 chemokines/cytokines, there was no significant alteration in the medium level of 40 and 39 chemokines/cytokines between the SGLT2i and control groups in Hep3B and Huh7 cells (Tables S1 and S2, Supporting information). However, SGLT2i significantly downregulated six chemokines (C‐X‐C motif chemokine ligand [CXCL] 1, CXCL8, CXCL10, macrophage colony‐stimulating factor [M‐CSF], leukemia inhibitory factor, and stromal cell‐derived factor‐1α) and upregulated two chemokines (interleukin [IL]‐12 and tumor necrosis factor‐α) compared to the control in Hep3B cells (Table SS1). In Huh7 cells, SGLT2i significantly downregulated five chemokines (CXCL1, CXCL8, CXCL10, M‐CSF, and IL‐6) and upregulated four chemokines (granulocyte colony‐stimulating factor, macrophage inflammatory protein‐1α, stromal cell‐derived factor‐1α, and vascular endothelial growth factor) compared to the control (Table S2). Thus, the significant downregulation of CXCL1, CXCL8, CXCL10, and M‐CSF was a common alteration in both SGLT2i‐treated Hep3B and Huh7 cells (Fig. 1a). The medium CXCL1 level decreased by approximately 90% and 55% in SGLT2i‐treated Hep3B and Huh7 cells compared to the control, respectively (Fig. 1b). In addition, approximately 70% and 30% of reduction in the medium level of CXCL8 was observed in SGLT2i‐treated Hep3B and Huh7 cells compared to the control, respectively (Fig. 1c). The medium CXCL10 level decreased by approximately 80% and 45% in SGLT2i‐treated Hep3B and Huh7 cells compared to the control, respectively (Fig. 1d). Moreover, approximately 30% and 15% of reduction in the medium level of M‐CSF was observed in SGLT2i‐treated Hep3B and Huh7 cells compared to the control, respectively (Fig. 1e).
Figure 1.
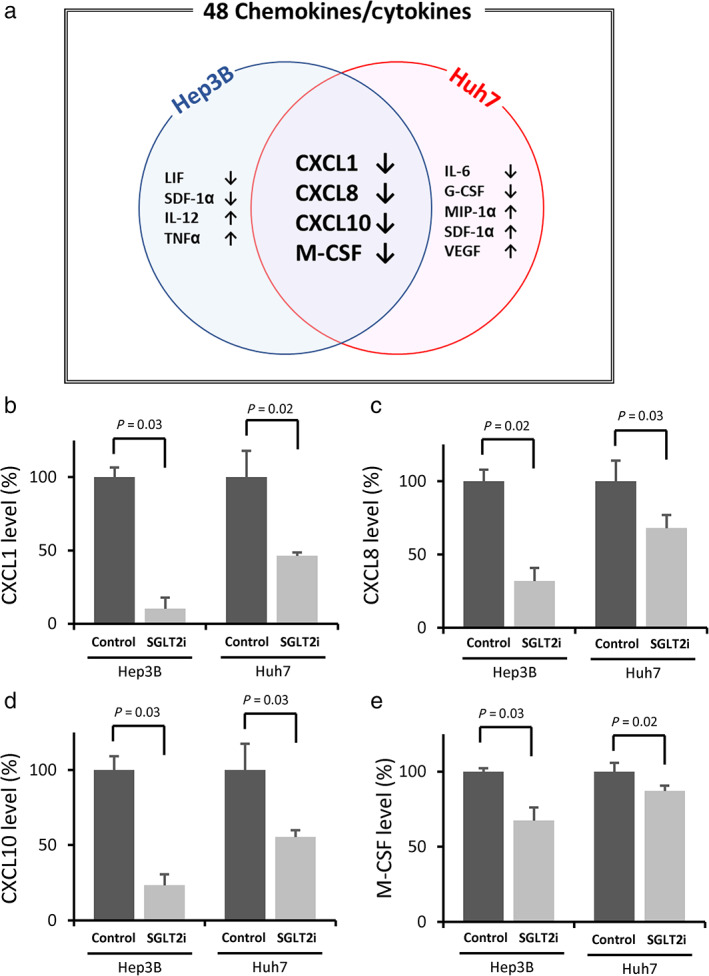
(a) Summary for SGLT2i‐induced alterations in 48 chemokines/cytokines in Hep3B and Huh7 cells. (b) SGLT2i‐induced changes in the medium CXCL1 level (each n = 4). (c) SGLT2i‐induced changes in the medium CXCL8 level (each n = 4). (d) SGLT2i‐induced changes in the medium CXCL10 level (each n = 4), (e) SGLT2i‐induced changes in the medium M‐CSF level (each n = 4). The SGLT2i‐induced significant changes in chemokines/cytokines are expressed as the percentage of control (the mean value of the control was set as 100%). CXCL, C‐X‐C motif chemokine ligand; G‐CSF, granulocyte colony‐stimulating factor; IL, interleukin; LIF, leukemia inhibitory factor; M‐CSF, macrophage colony‐stimulating factor; MIP‐1α, macrophage inflammatory protein‐1α; SDF‐1α, stromal‐cell‐derived factor‐1α; TNFα, tumor necrosis factor‐α; VEGF, vascular endothelial growth factor.
We first demonstrated that SGLT2i directly downregulated the three tumor‐releasing chemokines CXCL1, CXCL8, and CXCL10 in Hep3B and Huh7 cells. Dahlquist et al. had shown that CXCL1 promoted the proliferation and invasion of rat HCC cells in an autocrine manner. 4 In addition, Kahraman et al. reported that autocrine CXCL8 signaling is critical to activate the phosphatidyl‐inositol‐3 kinase/Akt/mammalian target of the rapamycin pathway, which is involved in the development and the progression of liver cancer stem cells. 6 An inhibition of autocrine CXCL8 signaling also represses features of cancer stem cells and increases the sensitivity of the cells to sorafenib treatment. 6 Moreover, Wei et al. found that CXCL10 binds CXC chemokine receptor 3 on B cells and causes plasma cell polarization, suppression of the anti‐tumor T‐cell response, and HCC cell growth. 7 Thus, SGLT2i‐induced downregulation of these chemokines may exert antitumor effects through alterations in tumor characters and tumor immunity.
We also found that SGLT2i downregulated tumor‐releasing M‐CSF in Hep3B and Huh7 cells. Zhu et al. investigated the prognostic value of the tumoral expression of M‐CSF in patients with HCC after curative resection. 5 They found that high tumoral expression of M‐CSF correlates with large tumor size, presence of intrahepatic metastasis, and advanced tumor stage. In addition, the tumoral expression of M‐CSF is an independent risk factor for disease‐free survival and overall survival. 5 Moreover, Chai et al. demonstrated that lower tumoral expression of M‐CSF is associated with decreased tumor volume in a xenograft model of human HCC in nude mice. 8 M‐CSF induces polarization of M1 macrophages and subsequent reduction of macrophage recruitment and better overall survival of patients with HCC. 8 Thus, SGLT2i‐induced downregulation of M‐CSF may exert antitumor effects through the polarization of M1 macrophages.
In this study, we did not investigate the mechanisms for SGLT2i‐induced downregulation of these four chemokines/cytokines. However, we had previously reported that SGLT2 was localized in mitochondria in various human HCC cell lines including Hep3B and Huh7 cells. 2 We had also shown that SGLT2i downregulated ATP synthase F1 subunit alpha, a mitochondrial electron transport system protein, leading to mitochondrial dysfunction and activation of adenosine 5‐monophosphate activated protein kinase (AMPK) in Hep3B and Huh7 cells. AMPK is known to inhibit the various chemokine expressions. 9 Furthermore, mitochondrial dysfunction is reported to regulate the M‐CSF level. 10 Accordingly, SGLT2i may affect mitochondria in HCC cells and modulate the expression of several chemokines/cytokines.
There are several limitations to this study. First, we used a single concentration of SGLT2i in this study. Although the concentration was at the physiologic level, the dose‐dependent alterations in chemokines/cytokines remain unclear. Second, we used an SGLT2i, canagliflozin, but not other SGLT2i variants. Previous studies have shown that dapagliflozin, an SGLT2i, also exerts antitumor effects on breast cancer cells, colon cancer cells, and renal cell carcinoma. 11 , 12 , 13 However, no study has investigated the effect of SGLT2i on chemokine expression, and it remains unclear whether similar results are obtained by treatment with other SGLT2i including dapagliflozin. Third, we used well‐differentiated HCC cell lines. It is still unclear whether SGLT2i alters the expression of chemokine/cytokines in poorly differentiated HCC cell lines. Fourth, we did not evaluate time‐course changes in the expression of chemokine/cytokines. Thus, further studies should be conducted using a wide range of SGLT2i concentrations, several types of SGLT2i, various types of HCC cell lines, and time‐course changes.
In conclusion, SGLT2i directly suppresses tumor‐releasing CXCL1, CXCL8, CXCL10, and M‐CSF in Hep3B and Huh7 cells. The suppression of these chemokines/cytokines may be a possible mechanism for the SGLT2i‐induced antitumor effect of HCC.
Supporting information
Table S1. SGLT2i‐induced alterations in chemokines/cytokines in Hep3B cells.
Table S2. SGLT2i‐induced alterations in chemokines/cytokines in Huh7 cells.
Acknowledgment
We thank Hiroko Nagaki and Hitomi Tankawa for technical assistance in chemokine/cytokine assays.
Declaration of conflict of interest: Takumi Kawaguchi received lecture fees from Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Taisho Pharmaceutical Co., Ltd., and Otsuka Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.
Author contribution: Dan Nakano and Takumi Kawaguchi participated in study conception and design. Tsubasa Tsutsumi, Masako Hayakawa, and Sachiyo Yoshio participated in acquisition of data. Dan Nakano, Takumi Kawaguchi, Hironori Koga, and Takuji Torimura participated in interpretation of data. Dan Nakano, Takumi Kawaguchi, Tsubasa Tsutsumi, and Sachiyo Yoshio drafted the manuscript. Dan Nakano, Takumi Kawaguchi, and Masako Hayakawa participated in analysis. Hironori Koga and Takuji Torimura participated in the critical revision of the manuscript.
Financial support: This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant‐in‐Aid for Young Scientists (B) JP19K17446 and by the Research Program on Hepatitis from Japan Agency for Medical Research and Development (AMED) under 21fk0210094.
Data availability statement
The data presented in this study are available on request from the corresponding author.
References
- 1. Kawaguchi T, Nakano D, Okamura S et al. Spontaneous regression of hepatocellular carcinoma with reduction in angiogenesis‐related cytokines after treatment with sodium‐glucose cotransporter 2 inhibitor in a cirrhotic patient with diabetes mellitus. Hepatol. Res. 2019; 49: 479–86. [DOI] [PubMed] [Google Scholar]
- 2. Nakano D, Kawaguchi T, Iwamoto H, Hayakawa M, Koga H, Torimura T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: multi‐omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PLoS One. 2020; 15: e0232283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaji K, Nishimura N, Seki K et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int. J. Cancer. 2018; 142: 1712–22. [DOI] [PubMed] [Google Scholar]
- 4. Dahlquist KJV, Voth LC, Fee AJ, Stoeckman AK. An Autocrine Role for CXCL1 in Progression of Hepatocellular Carcinoma. Anticancer Res. 2020; 40: 6075–81. [DOI] [PubMed] [Google Scholar]
- 5. Zhu XD, Zhang JB, Zhuang PY et al. High expression of macrophage colony‐stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J. Clin. Oncol. 2008; 26: 2707–16. [DOI] [PubMed] [Google Scholar]
- 6. Kahraman DC, Kahraman T, Cetin‐Atalay R. Targeting PI3K/Akt/mTOR Pathway Identifies Differential Expression and Functional Role of IL8 in Liver Cancer Stem Cell Enrichment. Mol. Cancer Ther. 2019; 18: 2146–57. [DOI] [PubMed] [Google Scholar]
- 7. Wei Y, Lao XM, Xiao X et al. Plasma cell polarization to the immunoglobulin G phenotype in hepatocellular carcinomas involves epigenetic alterations and promotes hepatoma progression in mice. Gastroenterology. 2019; 156: 1890–1904.e16. [DOI] [PubMed] [Google Scholar]
- 8. Chai ZT, Zhu XD, Ao JY et al. microRNA‐26a suppresses recruitment of macrophages by down‐regulating macrophage colony‐stimulating factor expression through the PI3K/Akt pathway in hepatocellular carcinoma. J. Hematol. Oncol. 2015; 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye J, Zhu N, Sun R et al. Metformin inhibits chemokine expression through the AMPK/NF‐kappaB signaling pathway. J. Interferon Cytokine Res. 2018; 38: 363–9. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Fu Q, Wang Z et al. Sodium hydrosulfide mitigates dexamethasone‐induced osteoblast dysfunction by interfering with mitochondrial function. Biotechnol. Appl. Biochem. 2019; 66: 690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J, Zhu J, Yu SJ et al. Sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharmacother. 2020; 132: 110821. [DOI] [PubMed] [Google Scholar]
- 12. Saito T, Okada S, Yamada E et al. Effect of dapagliflozin on colon cancer cell [Rapid Communication]. Endocr. J. 2015; 62: 1133–7. [DOI] [PubMed] [Google Scholar]
- 13. Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y. Therapeutic effect of sodium glucose co‐transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med. Sci. Monit. 2017; 23: 3737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SGLT2i‐induced alterations in chemokines/cytokines in Hep3B cells.
Table S2. SGLT2i‐induced alterations in chemokines/cytokines in Huh7 cells.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


