Abstract
Adenomyomas are benign lesions that are most frequently found in the gallbladder but can also be rarely found in the biliary tract. Although benign, they present close similarity to malignant lesions and thus deserve important clinical consideration. We present a case of a 74‐year‐old Chinese man who presented acutely with fever and painless obstructive jaundice. CT imaging showed a large calculus within a dilated common bile duct (CBD) and, despite undergoing an endoscopic retrograde cholangiopancreatography (ERCP) with stone clearance, there was a persistent filling defect that was adherent to the wall of the proximal common bile duct. His CA 19–9 was also significantly raised. Intraductal ultrasonography (IDUS) showed a polypoid mass with papillary‐like projections, and ERCP forcep biopsies were unable to exclude a lesion with neoplastic potential. The patient subsequently underwent cholecystectomy with open CBD excision and Roux‐en‐Y hepaticojejunostomy, and histology showed features consistent with a biliary adenomyoma.
Keywords: biliary adenomyoma, biliary polyp, common bile duct
Biliary adenomyomas often present very similarly to malignant lesions and surgical intervention is often performed either because of diagnostic conundrum or to resolve the obstructive biliopathy. This paper illustrates such a case and a brief review of literature was also conducted to help readers better appreciate this rare but benign lesions.
Introduction
Adenomyomas can be found most commonly in the gallbladder but also in other parts of the GI tract such as the stomach, duodenum, and jejunum. Biliary adenomyoma are extremely rare and appear as duct‐like structures with smooth muscle hyperplasia, 1 possibly arising from heterotopic pancreatic tissue and in the presence of reactive inflammatory changes related to gallstones. Though commonly seen in the gallbladder as adenomyomatosis, it is also seen affecting the ampulla of Vater and, rarely, in the extrahepatic bile duct.
Case Report
A 74‐year‐old Chinese man presented to our hospital acutely with fever and painless obstructive jaundice. Although appearing deeply icteric, his abdomen was soft and non‐tender. Laboratory investigations showed serum bilirubin 212 μmol/L, alkaline phosphatase 462 U/L, and alanine transaminase 180 U/L. CT imaging showed a large calculus within the distal common bile duct (CBD). It also revealed a smaller intraluminal focus located more proximally within the CBD, likely representing a second calculus or possibly a polyp given its antidependent position (Fig. 1a). He was started on ceftriaxone and metronidazole, and the pre‐procedure serum carcino embryonic antigen was 2.13 μg/L and serum CA 19–9 was 1392 U/mL.
Figure 1.
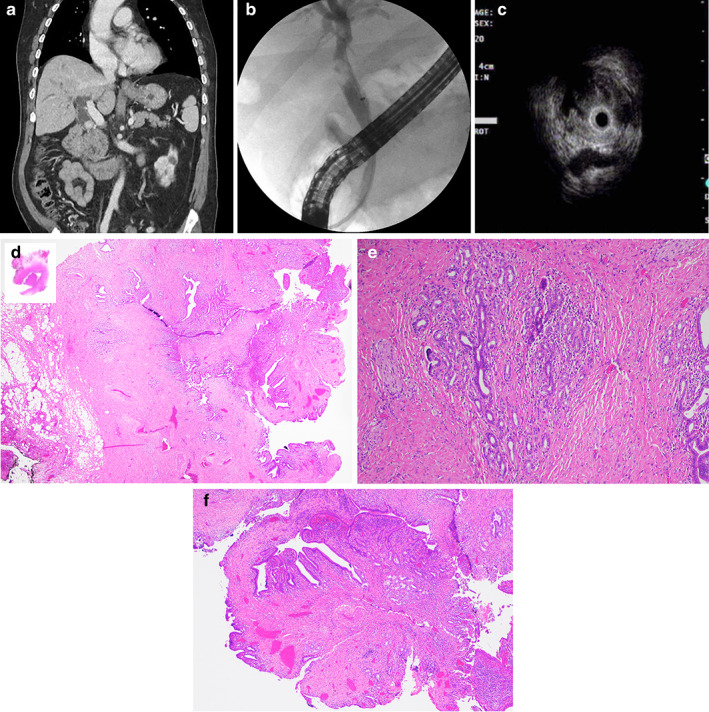
(a) CT image showing a large calculus within the distal common bile duct (CBD) and a smaller intraluminal focus located more proximally within the CBD, likely representing a second calculus or possibly a polyp given its antidependent position. (b) Cholangiogram showing a persistent filling defect that was adherent to the wall of the proximal common bile duct. (c) Intraductal ultrasonography (IDUS) showing a polypoid mass with papillary‐like projections. (d) Cross‐sectional macroscopic examination showing a 1.4‐cm polypoid lesion within the CBD. Microscopic examination of the H&E‐stained slide at low power (20×), showing fibromuscular wall thickening with intramural diverticula or duct‐like spaces. (e) Higher magnification (100×) image showing benign lobular arrangement of biliary glands in association with adjacent smooth‐muscle hyperplasia. Overall, the features are consistent with biliary adenomyoma. (f) A medium (40×) magnification image of the intraluminal polypoid prolapsed portion of the adenomyoma mimicking a neoplastic lesion of the biliary tract.
He underwent endoscopic retrograde cholangiopancreatography (ERCP) and stone fragments were removed with balloon and Dormia basket trawls, but larger stone fragments were not removed because of distal narrowing. Decision was made to stage the procedure because of prolonged procedure time, and a 10‐Fr 8‐cm plastic biliary stent was placed. He underwent a repeat ERCP 2 weeks later with balloon dilation of the papilla and distal CBD, and remnant stones were extracted with the basket and balloon. A final cholangiogram showed no CBD stones but there was a persistent filling defect that was adherent to the wall of the proximal CBD (Fig. 1b). Intraductal ultrasonography (IDUS) was performed, which showed a polypoid mass with papillary‐like projections (Fig. 1c). ERCP forcep biopsy specimens taken from the mass revealed the presence of columnar‐lined epithelium with no features of high‐grade dysplasia or malignancy. However, a biliary mass lesion with neoplastic potential could not be excluded.
He subsequently underwent a cholecystectomy with open CBD excision and Roux‐en‐Y hepaticojejunostomy. On cross‐sectional macroscopic examination of the resected specimen, a 1.4‐cm polypoid lesion was identified within the CBD. Microscopic examination of the H&E‐stained slide at low (20×) magnification revealed fibromuscular wall thickening with intramural diverticula or duct‐like spaces (Fig. 1d), and higher magnification (100×) images showed benign lobular arrangement of biliary glands in association with adjacent smooth muscle hyperplasia (Fig. 1e). Overall, the features were consistent with biliary adenomyoma. At medium (40×) magnification, image of the intraluminal polypoid prolapsed portion of the adenomyoma mimicked a neoplastic lesion of the biliary tract (Fig. 1f).
Discussion
The incidence of biliary adenomyoma is hard to estimate because of the various terminologies used to refer to the same type of lesion. Terms such as adenomyomatosis, adenomyomatous hyperplasia, adenomyosis, or adenomyoma have been used interchangeably. Suffice to say, it is exceedingly rare with an estimated 4 out of 22 000 surgical cases reported at Mayo clinic in a paper published in 1932. 2 A more recent literature review on this topic by Gouveia et al. in 2021 described only 61 cases of biliary adenomyoma in the medical literature, with most cases published as single case reports. A further breakdown of these cases showed that CBD adenomyoma, such as in this case, is much rarer with only 12 such cases (20% of all biliary adenomyoma) reported in the literature. 3
The pathophysiology of biliary adenomyoma is still unclear. Four possible hypotheses have been suggested. The most commonly accepted one, which is recognized by the WHO, is that these lesions may represent a form of incomplete heterotopic pancreas tissue. This “misplaced” pancreatic tissue could have stimulated secondary muscular proliferation, thus distorting the normal architecture of the biliary tree. 1 Another hypothesis is that these lesions develop in diverticula from reactive muscle hyperplasia and secondary gland formation. 4 Yet another hypothesis suggests that adenomyoma may have developed as a result of fibroadenomatous changes due to aging. 5 Finally, chronic inflammation such as CBD stones and cholangitis may have also resulted in the reactive muscle hyperplasia that forms such adenomyomas. Based on current evidence, biliary adenomyomas are not considered to be malignant or have malignant potential.
Bile duct adenomyoma is rarely diagnosed preoperatively, as cross‐sectional imaging characteristics are not specific enough to differentiate them from other neoplastic lesions. Similarly, endosonographic features may be nonspecific and, when performed, ERCP forcep biopsies may not be adequate to show the prominent smooth muscle bundles. Indeed, the sensitivity of brush cytology and intraductal biopsy in diagnosing malignant biliary strictures is 45 and 48.1%, respectively, and both techniques are almost 100% specific. Combining both modalities increases the sensitivity to 59.4%. 6 Even when localized to the more accessible location of the ampulla of Vater, biopsies were diagnostic only in 62% of patients. 7 One way to overcome the limitation of the diagnostic yield would be to repeat the ERCP forceps biopsy, as it has been shown that a repeated negative biopsy result for malignancy reduces the chance of a malignant lesion to less than 10%. 8 Intraoperative frozen section is another tool that may be used to differentiate such lesions and to decide on the extent of surgery required. Additionally, when suspected, immunohistochemistry evaluation showing cytokeratin 7 expression, no cytokeratin 20 expression, and low proliferative activity (Ki67) would differentiate benign adenomyomas from neoplastic adenomas or carcinomas. 9
As this mass lesion is frequently associated with obstructive jaundice and mimics a neoplastic lesion, invasive surgery often follows. Importantly, it is noted that the recurrence rate of such benign lesions (22%) is 4 times greater after local excision compared to a more radical procedure. 10 In our patient, the raised serum CA 19–9 made the suspicion for a neoplastic lesion even higher, although on hindsight, the raised CA 19–9 was likely related to cholangitis. Following biliary drainage surgery, our patient made a complete recovery with resolution of symptoms and jaundice.
Acknowledgments
We would like to thank our colleagues from Changi General Hospital for their support and help in the care of this patient.
Declaration of conflict of interest: The authors report no conflicts of interest.
Author contribution: Yu Bin Tan contributed to the formal analysis, investigation, methodology, project administration, writing‐original draft, writing ‐ review & editing. Lai Mun Wang contributed to the formal analysis, investigation, writing ‐ original draft. Andrew B E Kwek contributed to the conceptualization, formal analysis, investigation, methodology, project administration, supervision, writing ‐ original draft, writing ‐ review & editing.
Financial support: No funding was obtained for this manuscript.
References
- 1. Albores‐Saavedra J, Henson DE, Sobin LH. Histological Typing of Tumors of the Gallbladder and Extrahepatic Bile Duct. World Health Organization. Berlin: Springer‐Verlag, 1991. [Google Scholar]
- 2. Marshall JM. Tumors of the bile ducts. Surg. Gynecol. Obstet. 1932; 54: 6–12. [Google Scholar]
- 3. Gouveia C, Fidalgo C, Loureiro R, Oliveira H, Maio R, Cravo M. Adenomyomatosis of the common bile duct and ampulla of Vater. GE Port. J. Gastroenterol. 2021; 28: 121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin ED, Bedossa P, Oudinot P. Les lésions de la région oddienne: fréquence et association à des lésions biliares et pancréatiques dans une série de 109 autopsies. Gastroenterol. Clin. Biol. 1987; 11: 574–80, 580. [PubMed] [Google Scholar]
- 5. Fernandez‐Cruz L, Pera C. A histological study of the sphincter of Oddi. Proceedings of the 3rd Gastroenterology Symposium; 8–9 Jun 1976; Nice, France. 10.1159/000400281. [DOI]
- 6. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta‐analysis. Gastrointest. Endosc. 2015; 81: 168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menzel J, Poremba C, Dietl KH, Böcker W, Domschke W. Tumors of the papilla of Vater—inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann. Oncol. 1999; 10(10): 1227–31. [DOI] [PubMed] [Google Scholar]
- 8. Logrono R, Kurtycz DF, Molina CP, Trivedi VA, Wong JY, Block KP. Analysis of false‐negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at 2 university hospitals. Arch. Pathol. Lab. Med. 2000; 124: 387–92. [DOI] [PubMed] [Google Scholar]
- 9. Handra‐Luca A, Terris B, Couvelard A, Bonte H, Flejou JF. Adenomyoma and adenomyomatous hyperplasia of the Vaterian system: clinical, pathological, and new immunohistochemical features of 13 cases. Mod. Pathol. 2003; 16: 530–6. [DOI] [PubMed] [Google Scholar]
- 10. Burhans R, Myers RT. Benign neoplasms of the extrahepatic biliary ducts. Am. Surg. 1971; 37: 161–6. [PubMed] [Google Scholar]


