Abstract
We used a mouse peritonitis model to evaluate the in vivo efficacy of telithromycin (HMR 3647) (TEL) and erythromycin (ERY) against four strains of Enterococcus faecalis and three strains of Enterococcus faecium with differing susceptibilities to TEL. TEL was highly active in vivo against Ery-susceptible (Erys) and -intermediate (Eryi) strains (MIC of TEL = 0.015 to 0.062 μg/ml) and showed less efficacy against Ery-resistant (Eryr) isolates (MIC of TEL = 4 to 16 μg/ml), although this was overcome in part by a second subcutaneous dose. Quinupristin-dalfopristin was also noted to have less efficacy against Eryr versus Erys or Eryi E. faecium strains, but this difference was reduced by intravenous administration. In conclusion, TEL was more potent in vivo against enterococci than was ERY; its activity was lowered by the presence of erm(B)-mediated Eryr.
Infections caused by gram-positive organisms are a therapeutic concern because of their increased occurrence and the high rates of resistance among some isolates that cause serious infections (4, 12, 13, 19, 22, 25). Ketolides, including telithromycin (TEL; formerly HMR 3647), have been tested both in vitro and in vivo against gram-negative and gram-positive organisms (1, 3, 5, 8, 18, 23; P. Rajagopalan-Levasseur, E. Vallee, C. Agouridas, J. F. Chantot, and J. J. Pocidalo, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F173, 1995), but multiresistant enterococci have not been well represented in in vivo studies. In a published study, one Enterococcus faecalis and two Enterococcus faecium strains were tested in a mouse septicemia model which demonstrated the ability of a ketolide (HMR 3004) to prolong survival or protect after inoculation of organisms (1). In the present study, we describe the activity of TEL and the determination of the 50% protective doses (PD50s) of TEL, erythromycin (ERY), and quinupristin-dalfopristin (Q-D) against enterococci in a mouse peritonitis model.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [K. V. Singh, K. K. Zscheck, and B. E. Murray, Abst. 38th Intersci. Conf. Antimicrob. Agents Chemother., abst. B-13, 1998].)
Bacterial strains used in the study included the following four E. faecalis strains: TX0921 (HH22) (14), a β-lactamase producer (Bla+) with high-level resistance to gentamicin (Genr); TX0052, an endocarditis isolate; OG1RF (ATCC 47077) (15), a well-known plasmid-free isolate used as a recipient in many laboratories; and V583 (9, 21), a vanB-containing isolate. The three E. faecium strains used in the study were TX16 (2), an endocarditis isolate; TX16.01-Eryc (TX16 cured of ERY resistance [Eryr] by novobiocin), provided by Robert M. Rakita, Houston, Tex.; and a vanA-containing clinical isolate, TX2465 (11; K. V. Singh et al., 38th ICAAC, abstr. B-13). TEL was obtained from Hoechst Marion Roussel, Romainville, France; ERY A was obtained from Abbott Laboratories, North Chicago, Ill.; and Q-D was obtained from Rhône-Poulenc Rorer, Vitry sur Seine, France. MICs were determined by following the National Committee for Clinical Laboratory Standards (NCCLS) guidelines (16, 17). The susceptibility breakpoints of ERY, according to the NCCLS guidelines (16, 17) for enterococci, were ≤0.5 μg/ml for Erys, 1 to 4 μg/ml for Eryi, and ≥8 μg/ml for Eryr. For in vivo testing, enterococci grown on brain heart infusion agar (Difco Laboratories, Detroit, Mich.) plates with or without ERY were used to inoculate brain heart infusion broth, and preparation of inocula and CFU determination were done by following a previously published method (24). Female, 4- to 6-week-old outbred ICR mice (Harlan Sprague Dawley, Houston, Tex.) with a mean weight of 25 g were used. Each dosing group was composed of six animals. For the treatment groups, mice were injected intraperitoneally with 1 ml of premixed bacteria (cell density corresponding to 10 times the minimal lethal dose) in 50% sterile rat fecal extract (10, 24). TEL and other antibiotics were administered orally (p.o.) by gavage, subcutaneously (s.c.) or by the intravenous (i.v.) route immediately following the inoculation of mice. Animals were observed for up to 96 h for E. faecalis and 120 h for E. faecium. The PD50s of TEL and other antibiotics were determined by the method of Reed and Muench (20); Kaplan-Meier survival curves were generated for some. Bacteria were recovered from the spleens of dead mice, and the identity of the organisms was confirmed by phenotypic characteristics or by using pulsed-field gel electrophoresis. Preapproved guidelines of the Animal Welfare Committee of the University of Texas Health Science Center at Houston were followed throughout the course of the animal experiments.
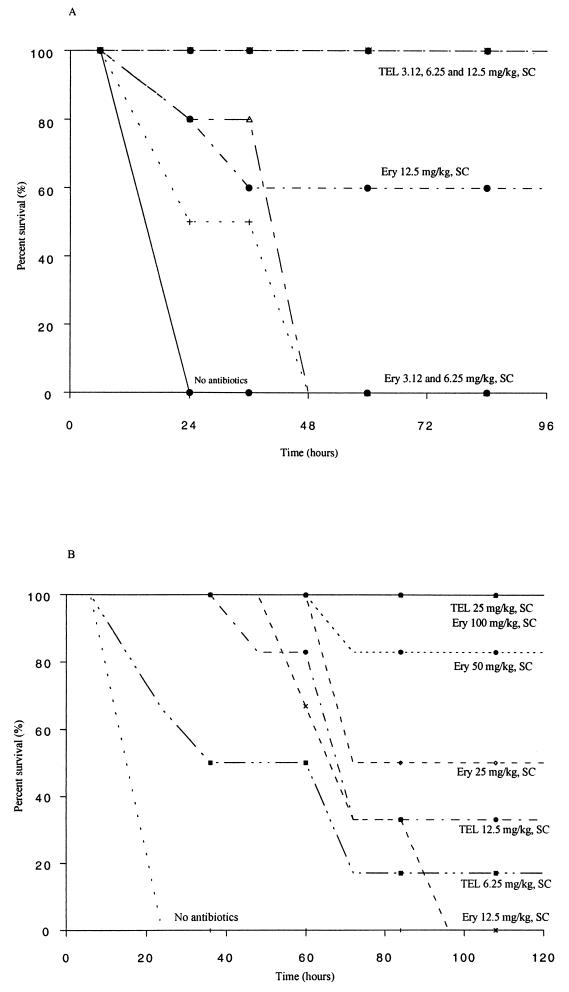
The results of MIC testing for isolates used in the antibiotic protection studies are presented in Table 1 along with PD50s. ERY administered by the oral route showed no protection of OG1RF-inoculated mice even at the highest dose, while TEL demonstrated PD50s of 29.7 mg/kg of body weight when administered by the p.o. route. When administered by the s.c. route (Table 1), TEL displayed a PD50 three times lower (PD50 = 9.4 mg/kg) than s.c. ERY (PD50 = 31.9 mg/kg) in OG1RF-inoculated mice. In TX0921-inoculated mice, the PD50 of TEL administered by the p.o. route was 34.9 mg/kg, while p.o. ERY at 200 mg/kg did not show protection (Table 1). TEL displayed a PD50 ∼21 times lower (PD50 = <0.57 mg/kg) than ERY (PD50 = 12.5 mg/kg) when administered by the s.c route. There was 100% survival of mice at doses of 3.12, 6.25, and 12.5 mg of TEL per kg compared with 60% survival with 12.5 mg of ERY per kg; s.c. ERY did not show any protection in TX0921-inoculated mice at 3.12 and 6.25 mg/kg (Fig. 1A). p.o. TEL and ERY showed no protection (Table 1) at 200 mg/kg against either E. faecalis TX0052 (ERY and TEL MICs of 1,024 μg/ml and 4 to 8 μg/ml, respectively) or V583 (ERY and TEL MICs of 512 to 1,024 μg/ml and 8 μg/ml, respectively) in inoculated mice. Two doses of TEL given by the p.o. route showed some protection (Table 1) against TX0052 (PD50 = 80 mg/kg) but not against V583 (PD50 = >200 mg/kg). One dose of s.c TEL (50 mg/kg) did not show any protection against these strains, but two doses of s.c. TEL showed protection (Table 1) against both (PD50s = 25 to 40 mg/kg). Organisms recovered from the spleens of dead mice were retested for MICs by the agar dilution method, and the MICs of TEL for these isolates were consistent with those observed prior to the inoculation.
TABLE 1.
PD50s of telithromycin and other antibiotics for enterococci in the mouse peritonitis model
| Antibiotic | Route of therapyb | PD50 (mg/kg) fora:
|
||||||
|---|---|---|---|---|---|---|---|---|
|
E. faecalis
|
E. faecium
|
|||||||
| OG1RF (ATCC 47077) | TX0921 Bla+ Chli Genr Tetr | TX0052 Chli Eryr [erm(B)] Genr Strr | V583 Chli Eryr [erm(B)] Tetr Vanr (vanB) | TX16 Chli Eryr [erm(B)] Genr Strr Tetr | TX16.01-Eryc Genr Strr Tetr | TX2465 Ampr Chli Eryi Vanr (vanA) | ||
| TEL | 0.031 | 0.015 | 4–8 | 8 | 16 | 0.062 | 0.015–0.031 | |
| p.o., 1 dose | 29.7 | 34.9 | >200 | >200 | >200 | 12.5 | 38.6 | |
| p.o., 2 doses | — | — | 80 | >200 | 168 | 9.35 | — | |
| s.c., 1 dose | 9.4 | 0.57 | >50 | >50 | >50 | 13.6 | 10.4 | |
| s.c., 2 doses | — | — | 40 | 25 | 12.5 | 3.12 | — | |
| ERY | 0.5 | 0.5 | 1,024 | 512–1,024 | 1,024 | 2 | 2–4 | |
| p.o., 1 dose | >200 | >200 | >200 | — | >200 | >200 | — | |
| s.c., 1 dose | 31.9 | 12.5 | >200 | >200 | >200 | 27.7 | 35.6 | |
| Q-D | 8 | 8 | 16 | 16 | 1 | 1 | 1 | |
| s.c., 1 dose | — | — | — | — | >200 | 27.9 | — | |
| s.c., 2 doses | — | — | — | — | 36.5 | — | — | |
| i.v., 1 dose | — | — | — | — | 32.1 | 15.2 | 10.2 | |
Values in bold are MICs, (in micrograms per milliliter). Ranges represent the results of different determinations. Chl, chloramphenicol; Str, streptomycin; Tet, tetracycline; Van, vancomycin. MICs were 1 to 4 μg/ml for Eryi and 16 μg/ml for Chli. —, not tested.
Single-dose administration of drug was performed at 0 h immediately after intraperitoneal inoculation of bacteria. Two-dose administration was performed at 0 and 4 h after infection.
FIG. 1.
(A) Survival and dose-response curve following therapy with TEL or ERY of peritonitis caused by E. faecalis strain TX0921 (HH22 Bla+ Genr) given by the s.c. route (single dose). (B) Survival and dose-response curve following therapy with TEL or ERY of peritonitis caused by E. faecium strain TX0016.01-Eryc given by the s.c. route (single dose).
TEL and ERY administered p.o. showed no protection (Table 1) at 200 mg/kg against the erm(B)-containing E. faecium strain TX16 (ERY MIC = 1,024 μg/ml), but two doses of TEL (TEL MIC = 16 μg/ml) had a PD50 of 168 mg/kg. Similarly, one s.c. dose of TEL (50 mg/kg) did not show any protection against TX16, but two doses of s.c TEL showed a PD50 of 12.5 mg/kg (Table 1). No protection was seen in mice when one dose of Q-D was administered by the s.c. route, but two doses of Q-D showed protection (PD50 = 36.5 mg/kg) in mice (Table 1). One dose of Q-D administered by the i.v. route showed a PD50 of 32.1 mg/kg (Table 1) in TX16-inoculated mice. Against the erm(B)-lacking TX16.01, a single dose of s.c. TEL was highly effective and showed a PD50 twofold lower than ERY and Q-D (Table 1). The time course of survival following therapy of peritonitis with TEL and ERY (one dose given s.c.) is shown in Fig. 1B; TEL showed more protection than ERY at similar or lower doses. As was seen with TX16, i.v. Q-D again showed a lower PD50 than did s.c. Q-D (Table 1). The PD50 of p.o. TEL was 38.6 mg/kg (Table 1) in mice inoculated with TX2465, and s.c. TEL and s.c. ERY showed PD50s of 10.4 and 35.6 mg/kg, respectively (Table 1). Q-D showed a PD50 of 10.2 mg/kg (Table 1) when administered by the i.v. route.
In this study we explored the in vitro and in vivo activities of TEL, ERY, and Q-D against E. faecalis and E. faecium strains. Similar to a previous study (23), we found that TEL inhibited Ery-susceptible (MIC = ≤0.5 μg/ml) and -intermediate (MIC = 1 to 4 μg/ml) enterococci at ≤0.031 μg/ml; for two E. faecalis (TX0052 and V583) and one E. faecium (TX16) strain for which the MICs of ERY were 1,024 μg/ml, the MICs of TEL were 4 to 8 μg/ml and 16 μg/ml, respectively. TEL displayed excellent in vivo activity against two Erys E. faecalis and two E. faecium strains (one Erys and one vanA Eryi ampicillin-resistant [Ampr] strain) when administered by the p.o. and s.c. routes, while ERY showed protection only when administered by the s.c. route, with higher PD50s, these strains. Paralleling the increased MICs of TEL for the Eryr enterococci, single doses of TEL and ERY failed to protect mice when administered by the p.o. or s.c. route, while two doses of s.c. TEL showed protection in mice against these strains. In vivo efficacy of HMR 3004 and HMR 3647 (TEL) was demonstrated earlier against other gram-positive bacteria, including one Erys and two Eryi enterococci (1,6).
While the bactericidal and in vivo activities of Q-D against Eryr E. faecium have been questioned previously (7), we also found that s.c. Q-D was ineffective when given as a single dose for an Eryr E. faecium strain (TX16), while the PD50 was 27.9 mg/kg for a derivative of this strain that was cured of its Ery resistance. Based on the manufacturer's recommendation that the i.v. administration was more appropriate to achieve the desired ratios of the individual components, we also tested i.v. Q-D and found this route to be much more effective, with two doses by the s.c. route and one i.v. dose generating similar PD50s (36.5 and 32.1 mg/kg, respectively) against the Eryr strain TX 16.
In summary, the greater in vitro activity of TEL versus ERY against test bacteria was also reflected in its in vivo activity in a mouse peritonitis model against both E. faecalis and E. faecium strains, suggesting that this ketolide could be a promising drug for use against some multiresistant enterococci.
Acknowledgments
This work was supported by a grant from Hoechst Marion Roussel.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arduino R C, Jacques-Palaz K, Murray B E, Rakita R M. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect Immun. 1994;62:5587–5594. doi: 10.1128/iai.62.12.5587-5594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boswell F J, Andrews J M, Ashby J P, Fogarty C, Brenwald N P, Wise R. The in vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 4.Cormican M G, Jones R N. Emerging resistance to antimicrobial agents in gram-positive bacteria. Drugs. 1996;51:6–12. doi: 10.2165/00003495-199600511-00004. [DOI] [PubMed] [Google Scholar]
- 5.Credito K L, Ednie L M, Jacobs M R, Appelbaum P C. Activity of telithromycin (HMR 3647) against anaerobic bacteria compared to those of eight other agents by time-kill methodology. Antimicrob Agents Chemother. 1999;43:2027–2031. doi: 10.1128/aac.43.8.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelstein P H, Edelstein M A C. In vitro activity of the ketolide HMR 3647 (RU 6647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob Agents Chemother. 1999;43:90–95. doi: 10.1128/aac.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantin B, Leclercq R, Garry L, Carbon C. Influence of inducible cross-resistance to macrolides, lincosamides, and streptogramin B-type antibiotics in Enterococcus faecium on activity of quinupristin-dalfopristin in vitro and in rabbits with experimental endocarditis. Antimicrob Agents Chemother. 1997;41:931–935. doi: 10.1128/aac.41.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Roblas R, Calvo R, Esteban J, Bryskier A, Soriano F. The bactericidal activities of HMR 3647 and erythromycin against gram-positive bacilli and development of resistance. J Antimicrob Chemother. 1999;43:285–289. doi: 10.1093/jac/43.2.285. [DOI] [PubMed] [Google Scholar]
- 9.Free L, Sahm D F. Investigation of the reformulated Remel Synergy Quad plate for detection of high-level aminoglycoside and vancomycin resistance among enterococci. J Clin Microbiol. 1995;33:1643–1645. doi: 10.1128/jcm.33.6.1643-1645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollapudi S V S, Gupta A, Thadepalli H, Perez A. Use of lymphokines in treatment of experimental intra-abdominal abscess caused by Bacteroides fragilis. Infect Immun. 1988;56:2369–2372. doi: 10.1128/iai.56.9.2369-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montecalvo M A, Horowitz H, Gedris C, Carbonaro C, Tenover F C, Issah A, Cook P, Wormser G P. Outbreak of vancomycin-, ampicillin-, and aminoglycoside-resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrob Agents Chemother. 1994;38:1363–1367. doi: 10.1128/aac.38.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray B E. Diversity among multidrug-resistant enterococci. Emerg Infect Dis. 1998;4:37–47. doi: 10.3201/eid0401.980106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray B E, Mederski-Samoraj B. Transferable β-lactamase: a new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Investig. 1983;72:1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray B E, Singh K V, Ross R P, Heath J D, Dunny G M, Weinstock G M. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–5223. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—fifth edition; approved standard. NCCLS document M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility testing; tenth informational supplement (aerobic dilution). NCCLS document M100–S10. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 18.Piper K E, Rouse M S, Steckelberg J M, Wilson W R, Patel R. Ketolide treatment of Haemophilus influenzae experimental pneumonia. Antimicrob Agents Chemother. 1999;43:708–710. doi: 10.1128/aac.43.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittet D, Wenzel R P. Nosocomial bloodstream infections: secular trends in rates, mortality, and contribution to hospital deaths. Arch Intern Med. 1995;155:117–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 20.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 21.Sahm D F, Kissinger J, Gilmore M S, Murray P R, Mulder R, Solliday J, Clarke B. In-vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989;33:1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(Suppl. 3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 23.Schulin T, Wennersten C B, Moellering R C, Jr, Eliopoulos G M. In vitro activity of the new ketolide antibiotic HMR 3647 against gram-positive bacteria. J Antimicrob Chemother. 1998;42:297–301. doi: 10.1093/jac/42.3.297. [DOI] [PubMed] [Google Scholar]
- 24.Singh K V, Qin X, Weinstock G M, Murray B E. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J Infect Dis. 1998;178:1416–1420. doi: 10.1086/314453. [DOI] [PubMed] [Google Scholar]
- 25.Witte W, Wirth R, Klare I. Enterococci. Chemotherapy. 1999;45:135–145. doi: 10.1159/000007174. [DOI] [PubMed] [Google Scholar]