Abstract
Objective
To determine treatment persistence and exacerbations in patients initiating inhaler treatment with fixed-dose combinations of inhaled corticosteroids/long-acting beta-2-adrenergic agonists (ICS/LABA) for the treatment of asthma.
Design
Retrospective observational study conducted by review of electronic medical records (database: Fundación RediSS).
Setting
Retrospective cohort study. The follow-up period was 1 year.
Participants
The study included patients aged ≥18 years who started treatment with ICS/LABA and met the inclusion/exclusion criteria.
Main outcomes and measures
The study groups were fluticasone propionate/salmeterol (FP/SAL), beclomethasone/formoterol (BDP/FORM), budesonide/formoterol (BUD/FORM), fluticasone furoate/vilanterol (FF/VI) and fluticasone propionate/formoterol (FP/FORM). The main measurements were persistence, medication possession ratio (MPR) and exacerbations. Statistical significance was established as p<0.05.
Results
In total, 3203 patients were recruited for the study. By groups, 31.1% FP/SAL, 28.6% BDP/FORM, 25.0% BUD/FORM, 8.2% FF/VI and 7.0% FP/FORM. The mean age was 52.2 years, 60.8% were female and 44.9% had persistent-moderate asthma. Treatment persistence was 61.7% (95% CI 60.0% to 63.4%) and by study group it was FP/SAL: 60.7%, BDP/FORM: 61.2%, BUD/FORM: 60.3%, FF/VI: 66.7% and FP/FORM: 67.6% (p=0.046). MPR by study group was FP/SAL: 74.3%, BDP/FORM: 73.8%, BUD/FORM: 74.6%, FF/VI: 79.4% and FP/FORM: 80.6% (p=0.028). The mortality rate was 2.9%. By treatment group, exacerbations were FP/SAL: 21.9% (95% CI 19.3% to 24.5%), BDP/FORM: 22.2% (95% CI 19.5% to 24.9%), BUD/FORM: 22.8% (95% CI 19.9% to 25.7%), FF/VI: 17.9% (95% CI 14.9% to 20.7%) and FP/FORM: 16.0% (95% CI 12.2% to 19.3%), p=0.036.
Conclusions
Patients undergoing treatment with FP/FORM and FF/VI versus FP/SAL, BDP/FORM and BUD/FORM were associated with greater treatment adherence (persistence, MPR) and lower rates of exacerbations. However, further studies will be needed to strengthen the consistency of the results.
Keywords: asthma, respiratory medicine (see thoracic medicine), therapeutics
Strengths and limitations of this study.
Reducing the risk of exacerbations is an important goal in the treatment of asthma. However, in our country there are few studies that evaluate this by analysing the effect of double inhalation therapy at the level of active ingredient (molecule).
The results of the study were obtained in a situation of routine clinical practice, far from the idyllic conditions of randomised clinical trials. This circumstance can be interpreted as a strength of the study, since the data are potentially more generalisable, showing greater external validity of the observed results.
This study has the limitations of retrospective observational studies; for example, the possible under-reporting of information, the difficulty in measuring the confounding variables and the impossibility of establishing a causal relationship between the variables.
Introduction
Asthma is a chronic inflammatory airway disease that courses with bronchial hyper-response and variable airflow blockage.1 In Spain, the prevalence is around 5%, although there are variations between geographical areas.2 Most patients achieve adequate control with inhaled corticosteroids (ICS) and long-acting beta-adrenergic agonists (LABA), although some patients require additional therapy with other medications, including oral corticosteroids (OC).1 2
Reducing the risk of exacerbations is an important goal of asthma treatment.3–5 It is estimated that 30%–52% of patients with asthma have exacerbations of varying intensity.2 6 Factors influencing an increased risk of exacerbations include previous exacerbations, poor asthma control, limitations on activity, lower forced expiratory volume in 1 s (FEV1), exposure to allergens, difficulty handling inhalation devices and treatment adherence.7 Studies show antiasthmatic adherence rates of <65%.1 2 7 The most common causes of treatment discontinuation are side effects, improvements in symptoms and problems in reducing the effect of the drug over time, which lead to discontinuation being advised or spontaneous abandonment.7–10
In Spain, there are few studies evaluating the relationship between the adherence rate and the risk of exacerbations.11 Persistence (or discontinuation) of treatment is a key factor in disease progression and the risk of complications. In addition, there is a growing need to conduct studies representative of the real-life clinical conditions in which medicines are used, so the study may be of interest. The objective of the study was to evaluate treatment persistence and exacerbations in patients initiating inhaler treatment with combinations of ICS/LABA for the treatment of asthma in routine clinical practice.
Patients and methods
We performed a retrospective observational analysis of electronic medical records (EMR) obtained from the administrative database of the RediSS Foundation (Health services research network; www.rediss.es), a source of secondary data. The primary data came from various primary care centres in Catalonia (Spain), which are computerised with the OMIAPWIN (EMR). Before export to RediSS Foundation, data are rigorously anonymised and it is not possible to identify the territory, healthcare provider, treating physician or patient, or access any other information that would permit individual identification. This procedure ensures adherence to current law governing the protection of personal data. The population assigned to the centres was mostly urban, of medium-low socioeconomic level.
Patients who sought care and initiated treatment with a fixed-dose ICS/LABA combination between 1 January 2015 and 30 June 2016 (recruitment period, index date) were included in the study. The inclusion criteria were: (a) ≥18 years, (b) patients diagnosed with asthma ≥12 months before the index date, (c) inclusion in the prescription programme (with recorded dose, time interval and duration of each treatment administered; ≥2 prescriptions during the follow-up period), and (d) ensured regular monitoring (≥2 clinical records in the computer system). Exclusion criteria were: (a) patients transferred to other centres, displaced or out of area, (b) permanently institutionalised patients, (c) a history of chronic obstructive pulmonary disease (COPD), pulmonary emphysema, bronchiectasis, cystic fibrosis or bronchial neoplasm, and (d) mixed asthma-COPD phenotype (asthma-COPD overlap).1
Five study groups were differentiated according to the initial fixed-dose combination of ICS/LABA: budesonide/formoterol (BUD/FORM, R03AK07), beclomethasone/formoterol (BDP/FORM, R03AK08), fluticasone furoate/vilanterol (FF/VI, R03AK10), fluticasone propionate/formoterol (FP/FORM, R03AK11) and fluticasone propionate/salmeterol (FP/SAL, R03AK06). The follow-up period from the date of inclusion of the patient was 1 year. Records of patients with asthma were obtained using the International Classification of Primary Care 2 in the European Community (R93)12 and/or the International Classification of Diseases (Ninth Edition) Clinical Modification (493.x for asthma and/or flare-ups). The diagnosis of asthma was always made at the physician’s discretion, according to spirometry values. Exacerbations were defined as an event in the natural course of the disease characterised by acute episodes identified by a progressive increase in breathing difficulties, feeling short of breath, wheezing, chest oppression or a combination of these symptoms, caused by intense airflow obstruction.1 Outpatients or those attending the emergency department (mild-moderate asthma exacerbation) and hospitalised patients (severe asthma exacerbation) were identified. The record of each exacerbation was obtained to assess the rates before and after the index date and the time from diagnosis (in years). In addition, the following variables were collected: body mass index (kg/m2), lung function (FEV1) and asthma severity (intermittent, mild persistent, moderate persistent and severe persistent according to the Spanish Asthma Management Guidelines (GEMA) criteria1) at the start of the study before the index date. All-cause deaths were recorded.
Sociodemographic and comorbidity variables collected were age (continuous and by range), gender and the personal history described in table 1. As a summary variable of general comorbidity: (a) the Charlson13 Comorbidity Index was used as an approximation to severity, (b) the number of chronic comorbidities was obtained, and (c) the individual case-mix index was obtained from the Adjusted Clinical Groups (ACG), which is a patient classification system using isoconsumption of resources.14 The ACG application provides resource utilisation bands (RUB), allowing each patient to be grouped into one of five mutually exclusive categories based on their overall morbidity.
Table 1.
Baseline characteristics of the series studied by study group
| Study groups | FP/SAL | BDP/FORM | BUD/FORM | FF/VI | FP/FORM | P value |
| Patients, n (%) | 996 (31.1) | 917 (28.6) | 802 (25.0) | 263 (8.2) | 225 (7.0) | |
| Sociodemographic features | ||||||
| Mean age, years | 52.3 (19.3) | 53.0 (18.5) | 51.0 (16.8) | 52.5 (17.7) | 52.8 (17.1) | 0.245 |
| Female gender, % | 60.0 | 61.1 | 61.6 | 61.6 | 60.4 | 0.968 |
| General comorbidity | ||||||
| Mean diagnoses | 6.8 (3.9) | 6.4 (3.9) | 6.2 (3.6) | 6.3 (3.6) | 6.8 (3.8) | 0.075 |
| Charlson Comorbidity Index | 0.8 (0.8) | 0.7 (0.8) | 0.7 (0.6) | 0.7 (0.8) | 0.8 (0.9) | 0.097 |
| Mean RUB | 3.0 (0.7) | 2.9 (0.7) | 2.9 (0.8) | 3.0 (0.8) | 2.9 (0.7) | 0.198 |
| 1 (very low comorbidity), % | 4.1 | 3.6 | 8.2 | 6.1 | 4.9 | |
| 2 (low comorbidity), % | 10.3 | 20.4 | 12.5 | 13.3 | 12.0 | |
| 3 (moderate comorbidity), % | 71.1 | 65.5 | 63.1 | 56.7 | 67.6 | |
| 4 (high comorbidity), % | 14.0 | 9.9 | 15.8 | 23.6 | 14.7 | |
| 5 (very high comorbidity), % | 0.5 | 0.5 | 0.4 | 0.4 | 0.9 | 0.111 |
| Associated comorbidity, % | ||||||
| High blood pressure | 29.9 | 28.9 | 29.1 | 29.3 | 29.3 | 0.991 |
| Diabetes mellitus | 13.4 | 12.8 | 13.0 | 13.3 | 12.9 | 0.996 |
| Dyslipidaemia | 41.0 | 41.3 | 41.3 | 40.7 | 39.8 | 0.811 |
| Obesity | 27.9 | 28.6 | 27.7 | 26.2 | 27.6 | 0.963 |
| Ischaemic heart disease | 4.3 | 4.0 | 4.0 | 3.8 | 4.0 | 0.994 |
| Cerebrovascular accident | 7.3 | 7.0 | 7.0 | 6.8 | 6.7 | 0.995 |
| Cardiovascular event | 11.8 | 9.2 | 9.1 | 10.6 | 10.2 | 0.272 |
| Depressive syndrome | 19.5 | 19.6 | 20.2 | 20.9 | 20.4 | 0.982 |
| Malignancies | 10.9 | 10.5 | 10.3 | 11.8 | 10.2 | 0.964 |
| Allergic rhinitis | 61.7 | 63.1 | 61.8 | 63.5 | 62.2 | 0.959 |
| Nasal polyposis | 15.5 | 15.4 | 15.3 | 14.1 | 16.4 | 0.969 |
| Gastro-oesophageal reflux | 39.1 | 40.9 | 38.0 | 41.1 | 40.9 | 0.739 |
| Asthma severity, % | ||||||
| Intermittent | 13.0 | 14.9 | 12.0 | 12.5 | 12.0 | |
| Mild persistent | 25.0 | 21.5 | 24.6 | 25.1 | 25.3 | |
| Moderate persistent | 44.5 | 45.1 | 45.3 | 43.3 | 45.4 | |
| Severe persistent | 17.6 | 18.4 | 18.2 | 19.0 | 18.2 | 0.844 |
| Other variables | ||||||
| BMI, kg/m2 | 28.4 (5) | 28.6 (5.0) | 28.4 (5.0) | 28.6 (5.5) | 27.9 (4.9) | 0.228 |
| FEV1, % predicted | 74.8 | 74.3 | 74.7 | 74.6 | 74.8 | 0.954 |
Values expressed as percentage or mean (SD, standard deviation), p: statistical significance.
BDP/FORM, beclomethasone /formoterol; BMI, body mass index; BUD/FORM, budesonide/formoterol; FEV1, forced expiratory volume in 1 s.; FF/VI, fluticasone furoate/vilanterol; FP/FORM, fluticasone propionate /formoterol; FP/SAL, fluticasone propionate/salmeterol; RUB, resource utilisation bands.
The medicines (active substances) indicated for treatment were obtained according to the Anatomical Therapeutic Chemical Classification System15 classification: oral/systemic corticosteroids (OC, H02AB), short-acting beta-2 agonists (SABA, R03AC), systemic beta-2 agonists (xanthines, R03DA), leukotriene receptor antagonists (R03DC), long-acting muscarinic antagonists (LAMA, R03BB04: tiotropium bromide) and omalizumab (biologicals, R03DX05). In addition, patients receiving chronic doses of oral/systemic corticosteroids were differentiated from those receiving them only for stabilisation of an exacerbation. The choice of drug for a specific patient was at the discretion of the physician, as in routine clinical practice. The information was obtained from drug-dispensing records. The scheduled dose of ICS administered was classified at low, medium or high.1 Treatment persistence was calculated from the index date to the discontinuation date in months. The discontinuation date was the date on which the patient switched to another ICS/LABA or interrupted treatment for ≥60 days without renewing the medication and/or had ≥2 prescriptions dispensed. The rate of treatment persistence was obtained at 6 and 12 months of follow-up. The percentage of therapeutic compliance was calculated based on the medication possession ratio (MPR).16 This was evaluated from the first to the last prescription and represented the number of days of medication dispensed according to the number of days on treatment from the index date.
Records were validated to ensure the quality of the results. A descriptive univariate statistical analysis was carried out. Qualitative data were described using absolute and relative frequencies and quantitative data as means and SD. The 95% CIs used to estimate parameters were based on the total number of subjects with no missing values. The normality of the distribution was assessed using the Kolmogorov-Smirnov test. In the bivariate analysis, analysis of variance, the χ2 test and comparison of means were used for paired data. A multiple linear regression model was used to obtain the variables associated with the number of exacerbations (dependent variables; procedure: consecutive steps). The covariates included in the model were gender, age, general comorbidity (RUB), FEV1, disease duration and asthma severity. Persistence was assessed using Kaplan-Meier curves (log rank procedure; Mantel-Cox). The analysis was made using SPSSWIN V.23. Statistical significance was established as p<0.05.
Patient and public involvement
Patients and/or the public were not involved in developing this research question, designing the study or providing input to study conduct.
Results
Of an initial population of 8725 subjects diagnosed with asthma (prevalence: 5.4%; 95% CI 5.2% to 5.7%), 3203 patients who met the inclusion/exclusion criteria and could be followed during the study period were analysed. Table 1 shows the baseline characteristics of participants according to the five ICS/LABA study groups. The mean age was 52.2 years, 60.8% were women, the mean RUB was 2.9 points and the mean Charlson Comorbidity Index was 0.7 points. Allergic rhinitis (62.3%), dyslipidaemia (41%), gastro-oesophageal reflux (39.6%) and high blood pressure (28.4%) were the most frequent comorbidities: 44.9% of patients had persistent-moderate asthma, with a mean FEV1 of 74.6%. According to the initial ICS/LABA prescribed, the study groups were as follows: 31.1% (n=996) FP/SAL, 28.6% (n=917) BDP/FORM, 25.0% (n=802) BUD/FORM, 8.2% (n=263) FF/VI and 7.0% (n=225) FP/FORM. There was acceptable comparability in the baseline characteristics of the study groups.
Medication administered and treatment adherence (persistence and MPR) during the follow-up period according to the study groups are detailed in table 2: 95.7% of patients were receiving short-acting beta-2 agonists (SABA) as rescue treatment, 27.7% were receiving OC (20% for regular/chronic use) and 18.2% leukotriene antagonists. There was acceptable homogeneity between the groups. Treatment persistence at 12 months was 61.7% (95% CI 60% to 63.4%) and, by study group, was as follows: FP/SAL: 60.7%, BDP/FORM: 61.2%, BUD/FORM: 60.3%, FF/VI: 66.7% and FP/FORM: 67.6% (p=0.046). The MPR was FP/SAL: 74.3%, BDP/FORM: 73.8%, BUD/FORM: 74.6%, FF/VI: 79.4% and FP/FORM: 80.6% (p=0.028). The mortality rate was 2.9%.
Table 2.
Medication administered and treatment persistence during the follow-up period
| Study groups | FP/SAL | BDP/FORM | BUD/FORM | FF/VI | FP/FORM | P value |
| Patients, n (%) | 996 (31.1) | 917 (28.6) | 802 (25.0) | 263 (8.2) | 225 (7.0) | |
| Medication use, % | ||||||
| Oral corticosteroids | 24.6 | 22.8 | 26.7 | 25.5 | 24.9 | 0.465 |
| Oral corticosteroids for chronic use | 22.0 | 18.5 | 20.2 | 18.6 | 18.7 | 0.373 |
| Systemic antibiotics | 10.0 | 9.2 | 10.1 | 10.3 | 10.1 | 0.959 |
| Short-acting beta-2 agonists | 90.0 | 93.0 | 91.2 | 89.5 | 92.2 | 0.221 |
| Long-acting anticholinergics | 17.0 | 15.2 | 13.8 | 16.0 | 13.8 | 0.414 |
| Systemic beta-2 agonists (xanthines) | 3.8 | 3.5 | 3.2 | 5.7 | 3.1 | 0.431 |
| Leukotriene receptor antagonists | 17.7 | 17.4 | 19.5 | 17.5 | 19.6 | 0.782 |
| Biologicals: omalizumab | 1.3 | 1.4 | 1.2 | 1.5 | 1.3 | 0.997 |
| Inhaled corticosteroid doses, % | ||||||
| Low | 10.5 | 9.8 | 10.1 | 11.1 | 10.7 | |
| Medium | 47.1 | 46.5 | 45.0 | 46.2 | 47.1 | |
| High | 42.4 | 43.7 | 44.9 | 42.7 | 42.2 | 0.547 |
| Other variables | ||||||
| Time from diagnosis, years | 12.5 (4.5) | 12.7 (4.4) | 12.8 (4.2) | 12.6 (3.9) | 12.3 (3.9) | 0.373 |
| Treatment possession, months | 8.9 (3.6) | 8.9 (3.4) | 9.0 (3.3) | 9.6 (3.3)* | 9.7 (3.1)* | 0.046 |
| Duration of treatment, months | 9.9 (3.5) | 9.7 (3.6) | 10.0 (3.5) | 10.2 (3.4)* | 10.3 (3.2)* | 0.036 |
| Medication possession rate, % | 74.3 | 73.8 | 74.6 | 79.4* | 80.6* | 0.028 |
| 95% CI | 71.6 to 77.0 | 70.5 to 76.3 | 71.6 to 77.6 | 74.5 to 84.3 | 75.4 to 85.8 | |
| Treatment persistence, months | ||||||
| 6 | 81.9% | 81.2% | 82.4% | 86.0%* | 87.6%* | 0.014 |
| 12 | 60.7% | 61.2% | 60.3% | 66.7%* | 67.6%* | 0.046 |
| Death, % | 3.0 | 2.7 | 3.1 | 2.3 | 2.7 | 0.954 |
Values expressed as percentage or mean (SD, standar deviation), p: statistical significance.
*Statistically significant results (observed > expected).
BDP/FORM, beclomethasone/formoterol; BUD/FORM, budesonide/formoterol; CI, confidence interval; FF/VI, fluticasone furoate/vilanterol; FP/FORM, fluticasone propionate/formoterol; FP/SAL, fluticasone propionate /salmeterol.
Exacerbations by study group are described in table 3. Overall, 21.5% of patients had some form of exacerbation, and the rates were slightly lower in groups treated with FP/FORM and FF/VI. The percentages of patients with exacerbations according to study group were FP/SAL: 21.9% (95% CI 19.3% to 24.5%), BDP/FORM: 22.2% (95% CI 19.5% to 24.9%), BUD/FORM: 22.8% (95% CI 19.9% to 25.7%), FF/VI: 17.9% (95% CI 14.9% to 20.7%) and FP/FORM: 16.0% (95% CI 12.2% to 19.3%), p=0.036. The differences were most evident in patients with severe exacerbations (7.9%, 6.0%, 7.9%, 6.8% and 4.0%, respectively (p<0.001)). The reductions in exacerbations from baseline to 12 months were: FP/SAL: −6.8%, BDP/FORM: −5.9%, BUD/FORM: −6.1%, FF/VI: −8.6% and FP/FORM: −9.3%, respectively (p=0.037). In the multivariate model, the number of exacerbations during the follow-up was associated with previous exacerbations (β=0.798), FEV1 (β=−0.075) and persistence (β=0.011) (p<0.033). The model determination coefficient was 85.1%. Figure 1 details the percentage of exacerbations according to asthma severity and figure 2 shows the median treatment persistence during the follow-up period.
Table 3.
Exacerbations by study group
| Study groups | FP/SAL | BDP/FORM | BUD/FORM | FF/VI | FP/FORM | P value |
| Patients, n (%) | 996 (31.1) | 917 (28.6) | 802 (25.0) | 263 (8.2) | 225 (7.0) | |
| Follow-up period (1 year) | ||||||
| Exacerbations, % | 21.9 | 22.2 | 22.8 | 17.9* | 16.0* | 0.036 |
| Mean exacerbations | 0.4 (0.8) | 0.4 (0.8) | 0.4 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.087 |
| Number of exacerbations/year, % | ||||||
| 0 | 78.1 | 78.3 | 77.2 | 82.1 | 84.0 | |
| 1 | 15.0 | 14.9 | 13.8 | 9.9 | 7.1 | |
| 2 | 2.9 | 2.6 | 5.5 | 1.9 | 5.3 | |
| 3+ | 4.0 | 4.1 | 3.5 | 6.1 | 3.6 | <0.001 |
| Patients with exacerbations, % | ||||||
| Mild-moderate | 20.1 | 21.7 | 22.2 | 17.5* | 16.0* | <0.001 |
| Severe (hospital admission) | 7.9 | 6.0 | 7.9 | 6.8 | 4.0* | <0.001 |
| Previous year (preindex) | ||||||
| Exacerbations, % | 28.7 | 28.1 | 28.9 | 25.5 | 25.3 | 0.698 |
| Mean exacerbations | 0.5 (0.9) | 0.5 (0.9) | 0.5 (0.9) | 0.5 (1.0) | 0.4 (0.9) | 0.973 |
| Number of exacerbations/year (%) | ||||||
| 0 | 71.3 | 71.9 | 71.1 | 74.5 | 74.7 | |
| 1 | 17.1 | 15.3 | 15.1 | 14.1 | 15.6 | |
| 2 | 5.9 | 8.4 | 9.1 | 3.0 | 2.7 | |
| 3+ | 5.7 | 4.5 | 4.7 | 8.4 | 7.1 | <0.001 |
| Patients with exacerbations, % | ||||||
| Mild-moderate | 27.5 | 27.4 | 28.7 | 24.1 | 25.4 | 0.111 |
| Severe (hospital admission) | 11.7 | 10.8 | 12.2 | 10.6 | 10.7 | 0.217 |
| Differences between the two periods, % | ||||||
| Exacerbations | −6.8 | −5.9 | −6.1 | −8.6* | −9.3* | 0.037 |
| Mild-moderate | −7.4 | −5.7 | −6.5 | −7.6 | −8.4 | 0.282 |
| Severe (hospital admission) | −3.8 | −4.8 | −4.4 | −5.8* | −6.7* | 0.044 |
Values expressed as percentage or mean (SD, standard deviation), p: statistical significance.
*Statistically significant results (effects observed > expected).
BDP/FORM, beclomethasone/formoterol; BUD/FORM, budesonide/formoterol; FF/VI, fluticasone furoate/vilanterol; FP/FORM, fluticasone propionate/formoterol; FP/SAL, fluticasone propionate /salmeterol.
Figure 1.
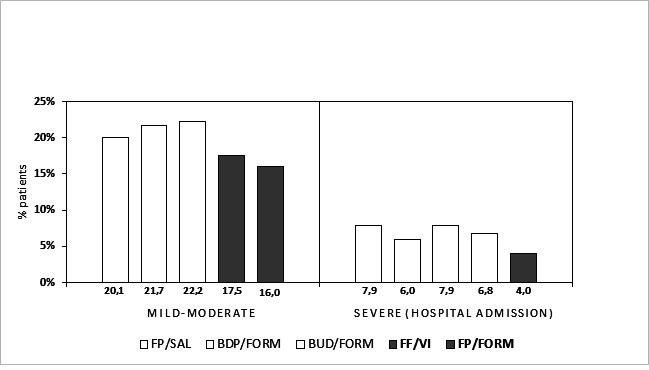
Percentage of patients with exacerbations according to their severity. Values expressed as a percentage of patients with exacerbations during the follow-up year. In grey: statistically significant results (p<0.05). Groups: fluticasone propionate/salmeterol (FP/SAL), beclomethasone/formoterol (BDP/FORM), budesonide/formoterol (BUD/FORM), fluticasone furoate/vilanterol (FF/VI) and fluticasone propionate/formoterol (FP/FORM).
Figure 2.
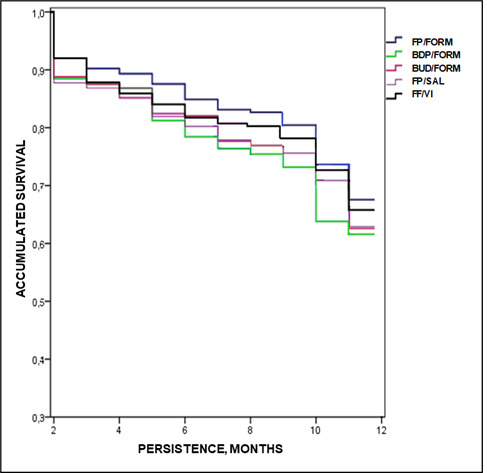
Median treatment persistence during the follow-up period. Kaplan-Meier curve: log rank procedure (Mantel-Cox): χ2=9.643; p=0.039. Groups: fluticasone propionate/salmeterol (FP/SAL), beclomethasone/formoterol (BDP/FORM), budesonide/formoterol (BUD/FORM), fluticasone furoate/vilanterol (FF/VI) and fluticasone propionate/formoterol (FP/FORM).
Discussion
The results of the study show that patients initiating fixed-dose treatment with FP/FORM and FF/VI were associated with increased persistence and MPR, resulting in fewer exacerbations compared with other ICS/LABA. Only patients receiving FP/FORM were associated with a lower rate of severe asthma exacerbations. FP/FORM and FF/VI, which are newer combinations, were less often prescribed (7% and 8.2%, respectively).
ICS/LABA are the basis of persistent asthma treatment, although the literature reviewed shows a low rate of adherence to medication (<65% per year).11 16 17 A review of 19 studies shows treatment adherence ranged from 22% to 63% and that 24% of exacerbations and 60% of asthma-related hospitalisations were attributable to poor adherence.18 Zhang et al19 found a persistence of 33.6% in children with persistent asthma on monotherapy. Our results are similar or perhaps slightly higher than those reported but are still low. There may be several possible explanations: (a) the method of measuring persistence/MPR, (b) the dose indicated at the beginning of the study, (c) ours is a more recent study, (d) the patients who sought care assiduously attended check-ups, and/or (e) are subject to specific nursing follow-up. In addition, in the studies reviewed, therapeutic non-compliance was associated with young patients with mild asthma, while fixed combinations improved adherence, as confirmed by our results.18 20
Our results show that 21.5% of patients had some form of exacerbation, and that the rate was slightly lower in patients treated with FP/FORM and FF/VI. The risk of exacerbations was associated with clinical severity (FEV1), previous exacerbations, limitations on activity, allergic rhinitis, insufficient preventive anti-inflammatory treatment and/or poor compliance with the prescribed treatment.20 Schmidt et al,21 in a yearlong prospective observational study, found that treatment with FP/FORM was associated with clinical improvements in asthma (degree of control, severe exacerbation, quality of life and lung function). Usmani et al22 studied the patients with controlled asthma and found that a reduction in the dose of FP/FORM did not affect exacerbations and was well tolerated. A comparative review of the rate of severe asthma exacerbations observed in clinical trials of different fixed-dose combinations of ICS/LABA by Papi et al20 found that the incidence of exacerbations with FP/FORM was lower than that for other combinations of ICS/LABA (especially those that result in hospitalisation) and that the difference cannot be explained solely by the characteristics of the studies (design, population, etc) and could be related to the pharmacological (molecular) characteristics of the combination. A clinical trial of FF/VI also found a lower rate of asthma exacerbations, although it was similar to FP/SAL.23 Another recent trial found that FF/VI showed better asthma control than habitual optimised treatment, but that there were no differences in the rate of asthma exacerbations.24 With design limitations, our data are in line with the literature consulted.20 25 However, the different definitions of asthma exacerbation make comparisons difficult; in our study, the definition of exacerbation was at the clinician’s discretion and was based on the use of health resources.26 27
The study has some limitations, principally those typical of retrospective studies (under-reporting/absence of information). In this sense, the categorisation of the disease (asthma) and the possible classification bias of patients, including the possible inaccuracy of diagnostic coding about the diagnosis of asthma and other comorbidities, are some examples, attributable to the information system. To reduce this bias, a validation of the variables was carried out before analysis. The definition of exacerbation was analysed based on the use of resources (administered drugs, hospital admissions) in the absence of a specific coding system. However, it is a consensus criterion in retrospective studies and should affect all the cohorts analysed in a similar way. In addition, other unmeasured factors could have influenced the results, for example, the socioeconomic level of patients, environmental/work exposure, evolution of the prescribed pharmacological dose, verification of the inhalation technique, including bronchoconstrictor therapy and/or the differentiation of phenotypes. In addition, based on the demand for medical care, non-disease factors may have influenced the results, such as access to health resources, comorbidity or patient specifics, which could cause worsening episodes not to be reported by the patient and therefore remain untreated. It is possible that through a prospective study, some of these factors can be minimised. The capturing patient behaviour through treatment persistence cannot be directly assessed through structured data available in electronic databases. Time to discontinuation is assumed to be a proxy to estimate patient persistence. In addition, the external validity of the results with respect to the representativeness of the population and the small number of patients per study group should also be considered as limitations. When using an efficient inhaler therapy,24 25 the factors that most influence compliance include the type of device, the technique used and the health education instructions received. However, these limitations should affect all the analysed cohorts in a similar way and should not affect the external validity of the results.
The future perspectives offered by this study are those of its replication in other health institutions and interventional strategies aimed at promoting patient self-care (structured and individualised educational programmes). In conclusion, patients receiving FP/FORM and FF/VI were associated with increased treatment adherence (persistence, MPR) and lower rates of exacerbations. However, further studies will be needed to strengthen the consistency of the results.
Supplementary Material
Footnotes
Contributors: AS-M acts as guarantor. AS-M and TF-S participated in the planning, coordination, conception and design of the study. AS-M and TF-S were responsible for data acquisition. AS-M performed the statistical analysis. AS-M, BGR, ST-L, TF-S and JLVG reviewed the results report. AS-M, BGR, ST-L, TF-S and JLVG participated in the critical interpretation of the data obtained, the writing of the manuscript (review) and the final approval of the version to be published. Additionally, AS-M, BGR, ST-L, TF-S and JLVG were responsible for ensuring the adequacy of all aspects of the study.
Funding: The associates of Vectura licensed a formoterol/fluticasone inhaler to Mundipharma Pharmaceuticals. Mundipharma has since developed, registered, marketed and distributed this product and sponsored this study. This study was designed and completed prior to acquisition of Vectura by Philip Morris International.
Competing interests: AS-M is an independent consultant funded by Mundipharma with respect to this manuscript. ST-L and TF-S are employees of Mundipharma.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article. The data sets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This protocol was reviewed and approved at the RediSS Foundation (Ethical Research Committee; International University of Catalonia; code: ANT-PER-2017-01).
References
- 1.Spanish Asthma Management Guidelines (GEMA4.3). Version 2018. Available: https://www.gemasma.com [Accessed Jan 2021].
- 2.Vila-Rigat R, Panadès Valls R, Hernandez Huet E, et al. Prevalence of work-related asthma and its impact in primary health care. Arch Bronconeumol 2015;51:449–55. 10.1016/j.arbres.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract 2017;5:918–27. 10.1016/j.jaip.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenson EK, Tchou MJ, Wheeler DS. Management of acute asthma exacerbations. Curr Opin Pediatr 2017;29:305–10. 10.1097/MOP.0000000000000480 [DOI] [PubMed] [Google Scholar]
- 5.Quirce S, Delgado J, Entrenas LM, et al. Quality indicators of asthma care derived from the Spanish guidelines for asthma management (GEMA 4.0): a multidisciplinary team report. J Investig Allergol Clin Immunol 2017;27:69–73. 10.18176/jiaci.0121 [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald JM, Barnes PJ, Chipps BE, et al. The burden of exacerbations in mild asthma: a systematic review. ERJ Open Res 2020;6:00359-2019. 10.1183/23120541.00359-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbà J, Ramírez G, Sicras A, et al. Identification of factors involved in medication compliance: incorrect inhaler technique of asthma treatment leads to poor compliance. Patient Prefer Adherence 2016;10:135–45. 10.2147/PPA.S95303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicras-Mainar A, Huerta A, Sánchez D, et al. [Use of resources and costs associated with non-adherence to inhaled corticosteroid treatment in asthma]. Semergen 2018;44:13–22. 10.1016/j.semerg.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 9.Vasbinder EC, Belitser SV, Souverein PC, et al. Non-adherence to inhaled corticosteroids and the risk of asthma exacerbations in children. Patient Prefer Adherence 2016;10:531–8. 10.2147/PPA.S92824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dima AL, Hernandez G, Cunillera O, et al. Asthma inhaler adherence determinants in adults: systematic review of observational data. Eur Respir J 2015;45:994–1018. 10.1183/09031936.00172114 [DOI] [PubMed] [Google Scholar]
- 11.Sicras-Mainar A, Traseira-Lugilde S, Fernández-Sánchez T, et al. [Persistence to treatment and resources use with inhaled fixed-dose combinations of corticosteroids and long-acting β-adrenergic agonists for the treatment of asthma: A population-based retrospective study]. Semergen 2018;44:472–84. 10.1016/j.semerg.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 12.Lamberts H, Wood M, Hofmans-Okkes AM, eds. The international classification of primary care in the European community. With a multi-language layer. Oxford: Oxford University Press, 1993. [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 14.Sicras-Mainar A, Navarro-Artieda R. [Adjusted clinicals groups: a patient classification system through risk adjustment]. Rev Peru Med Exp Salud Publica 2013;30:308–14. [PubMed] [Google Scholar]
- 15.World Health Organization . The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). Available: http://www.who.int/classifications/atcddd/en/ [Accessed 10 Apr 2020].
- 16.Mattke S, Martorell F, Hong SY, et al. Anti-inflammatory medication adherence and cost and utilization of asthma care in a commercially insured population. J Asthma 2010;47:323–9. 10.3109/02770900903497196 [DOI] [PubMed] [Google Scholar]
- 17.Voshaar T, Kostev K, Rex J, et al. A retrospective database analysis on persistence with inhaled corticosteroid therapy: comparison of two dry powder inhalers during asthma treatment in Germany. Int J Clin Pharmacol Ther 2012;50:257–64. 10.5414/CP201665 [DOI] [PubMed] [Google Scholar]
- 18.Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care 2015;60:455–68. 10.4187/respcare.03200 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Taylor SD, Sazonov V, et al. Suboptimal persistence with inhaled corticosteroid monotherapy among children with persistent asthma in the UK. Prim Care Respir J 2011;20:97–101. 10.4104/pcrj.2010.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papi A, Mansur AH, Pertseva T, et al. Long-Term fluticasone Propionate/Formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. J Aerosol Med Pulm Drug Deliv 2016;29:346–61. 10.1089/jamp.2015.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt O, Petro W, Hoheisel G, et al. Real-life effectiveness of asthma treatment with a fixed-dose fluticasone/formoterol pressurised metered-dose inhaler - Results from a non-interventional study. Respir Med 2017;131:166–74. 10.1016/j.rmed.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Usmani OS, Kemppinen A, Gardener E, et al. A randomized pragmatic trial of changing to and stepping down Fluticasone/Formoterol in asthma. J Allergy Clin Immunol Pract 2017;5:1378–87. 10.1016/j.jaip.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 23.Woodcock A, Bleecker ER, Lötvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest 2013;144:1222–9. 10.1378/chest.13-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 2017;390:2247–55. 10.1016/S0140-6736(17)32397-8 [DOI] [PubMed] [Google Scholar]
- 25.Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev 2010;4:CD005533. 10.1002/14651858.CD005533.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis A, Torvinen S, Dekhuijzen PNR, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res 2016;16:251. 10.1186/s12913-016-1482-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev 2017;4:CD012226. 10.1002/14651858.CD012226.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article. The data sets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.


