Abstract
Aims
Periprosthetic joint infection (PJI) is a debilitating condition with a substantial socioeconomic burden. A novel autologous blood glue (ABG) has been developed, which can be prepared during surgery and sprayed onto prostheses at the time of implantation. The ABG can potentially provide an antimicrobial coating which will be effective in preventing PJI, not only by providing a physical barrier but also by eluting a well-known antibiotic. Hence, this study aimed to assess the antimicrobial effectiveness of ABG when impregnated with gentamicin and stem cells.
Methods
Gentamicin elution from the ABG matrix was analyzed and quantified in a time-dependent manner. The combined efficiency of gentamicin and ABG as an anti-biofilm coating was investigated on titanium disks.
Results
ABG-gentamicin was bactericidal from 10 μg/ml and could release bactericidal concentrations over seven days, preventing biofilm formation. A concentration of 75 μg/ml of gentamicin in ABG showed the highest bactericidal effect up to day 7. On titanium disks, a significant bacterial reduction on ABG-gentamicin coated disks was observed when compared to both uncoated (mean 2-log reduction) and ABG-coated (mean 3-log reduction) disks, at days 3 and 7. ABG alone exhibited no antimicrobial or anti-biofilm properties. However, a concentration of 75 μg/ml gentamicin in ABG sustains release over seven days and significantly reduced biofilm formation. Its use as an implant coating in patients with a high risk of infection may prevent bacterial adhesion perioperatively and in the early postoperative period.
Conclusion
ABG’s use as a carrier for stem cells was effective, as it supported cell growth. It has the potential to co-deliver compatible cells, drugs, and growth factors. However, ABG-gentamicin’s potential needs to be further justified using in vivo studies.
Cite this article: Bone Joint Res 2020;9(12):848–856.
Keywords: Periprosthetic joint infection, Stem cell, Implant
Article focus
This study aims to assess the antimicrobial effectiveness of gels made from the patient’s own blood, and to investigate the effects of incorporating gentamicin and stem cells, to prevent infection.
Key message
This novel autologous blood glue (ABG) can be prepared cost-effectively and it has the potential to be incorporated with antibiotics and stem cells from the bone marrow.
Its use as an implant coating with gentamicin in patients with a high risk of infection may prevent bacterial adhesion perioperatively and in the early postoperative period.
Strengths and limitations
This novel autologous gel with gentamicin can be easily prepared in surgery and sprayed onto implants, potentially preventing infection.
This study could benefit from investigating the effect of gentamicin on the differentiation potential of stem cells in the ABG.
This study could also benefit from investigating whether ABG combined with stem cells can improve osseointegration, aided by the effects of gentamicin.
Introduction
Periprosthetic joint infection (PJI) is a serious complication that leads to reduced quality of life, high treatment costs, and in some cases death. It is defined by the presence of microorganisms either attached to the implant, forming a biofilm on the implant surface, or in adjacent tissue. 1,2 As of 2019, it accounts for 0.97% of total hip arthroplasties (THAs) and 1.03% of total knee arthroplasties (TKAs). Although it has a low incidence (up to 2% in primary procedures), its management is incredibly challenging and currently involves either retention of the implant with debridement of infective tissues and antibiotic administration, or removal and arthroplasty of the infected implant. Removal or arthroplasty of the implant may include a prolonged course of systemic antibiotics, with the option to use a spacer made from antibiotic-containing bone cement. 3 Furthermore, arthroplasties are predicted to increase 400% by 2030, anticipating an even higher prevalence for PJI. 4
The majority of PJIs are caused by Gram-positive species: Staphylococcus aureus and coagulase-negative staphylococci. However, Gram-negative bacilli such as Pseudomonas aeruginosa account for up to 23% of PJIs, especially in early-onset infections. 5 These species can form biofilms that are extremely resilient communities of microorganisms embedded in an extracellular polymeric substance. 6 Their intricate structure prevents attack by the host immune defence and reduces the diffusion of antimicrobials into the biofilm, leading to reduced antibiotic efficacy. 7
To prevent biofilm formation, antibiotic bone cements are used for cemented joint arthroplasties but for cementless implants this is not an option. 8 Hence, an ideal anti-infective coating should promote osseointegration while inhibiting bacterial attachment and biofilm formation. Osseointegration could be further improved by using bioactive substances, such as bone marrow stem cells (BMSCs), which are able to differentiate to bone and could encourage bone ingrowth. We have developed a cost-effective autologous gel that is prepared during surgery from the patient’s own blood and can be sprayed onto the implant surface. This gel can be used to spray a layer of stem cells onto the implant surface that potentially enhances osseointegration. 9 These cells may be obtained at surgery from the patient’s bone marrow (BM) and used to encourage osseointegration. The addition of an antimicrobial agent would also be beneficial as it would prevent bacterial colonization at the crucial time of insertion, and instead promote cell attachment to the implant surface.
Commercial blood products from either pooled human or bovine sources that can be used as haemostats are generally referred to as fibrin glues. 10 Their efficacy as a sealant has led to their popular and widespread clinical use. 11,12 However, they have several limitations such as risk of transmissible diseases, 13,14 and several fatal anaphylactic reactions have been reported with the use of bovine-sourced solutions. 15,16 Their further use is also limited by high cost and short shelf life. 17 Glues formed from blood directly obtained from the patient, which we refer to as autologous blood glue (ABG), eliminate risk of disease transmission and the chance of autoimmune reactions or allergic mishaps. 10,17 In addition, there may be some benefit in releasing a number of growth factors from activated platelets, which may promote a cell response including proliferation. 18 We therefore investigated the potential of ABG to perform these functions. If ABG shows positive results, it could potentially provide a cost-effective, autologous anti-infective coating for implants. Specifically, ABG could theoretically prevent infection in several ways: 1) as an innate antimicrobial, due to the rich concentration of leucocytes isolated from the blood contained within the platelet-rich plasma (PRP) layer which may remain active; 2) as an anti-adhesive coating through its sealant properties and also due to the fact that it may initially protect the implant surface from bacterial colonization and resorbing slowly over time; 3) as a drug-eluting agent, by steadily releasing antibiotic agents; and 4) as a mesenchymal stem cell (MSC)-carrier to promote osseointegration, promoting the autologous cells to win the 'race for the surface'.
This study aimed to investigate the potential of an antibiotic-impregnated ABG as an antimicrobial implant coating by: 1) determining the elution of gentamicin from the ABG matrix; 2) quantifying the release kinetics of gentamicin in a time-dependent manner; 3) analyzing gentamicin-impregnated ABG efficiency in preventing biofilm formation on a titanium alloy surface; and 4) investigating stem cell viability in ABG-gentamicin. We hypothesized the in vitro potential of ABG embedded with gentamycin in preventing biofilm formation on a titanium alloy surface, and the potential of this gel to maintain stem cell viability.
Methods
This study was carried out with the approval of the Royal National Orthopaedic Hospital (RNOH) Research Ethics Committee (07/Q0506/10), with written informed consent from all subjects.
Preparation of the autologous blood glue
ABG was prepared by spinning the blood using the ABMC Kit (NTL Biologica, Wantage, UK). Blood was spun at 3,300 rpm for three minutes to obtain the plasma, which was then activated to obtain the thrombin component using acetic acid. Blood was also spun at 3,300 rpm for three minutes to obtain PRP and bone marrow (BM) aspirate. PRP and BM aspirate were combined in equal ratios and this final mixture was then combined with blood serum. Mixing was carried out using a double-barrelled syringe, which enabled the components to form a gel almost immediately after mixing. Each experiment was performed using blood and BM from four healthy donors.
Gentamicin-impregnated ABG
A gentamicin sulphate stock solution of 50 mg/ml (Sigma-Aldrich, Irvine, UK) was diluted in order to obtain final concentrations of 10, 25, 50, and 75 μg/ml in ABG and in deionized water (DW). The gentamicin was then added into the thrombin component of the gel before being mixed with the rest of the components to form the gel.
Gentamicin elution from ABG
The amount of gentamicin eluting from the ABG matrix was determined using a modified Disk Susceptibility Test (Clinical and laboratory Standards Institute (CLSI)). A suspension equivalent to a 0.5 McFarland standard of P. aeruginosa NCTC 12903 (Public Health England, Salisbury, UK) in DW was used to create a bacterial lawn onto Mueller-Hinton (MH) agar (Oxoid; Fisher-Scientific, Loughborough, UK). An aliquot of 50 μl of each concentration of ABG-gentamicin was dropped on the agar, and plates were incubated at 37°C for 24 hours. Each concentration of gentamicin in DW was dropped on blank filter paper disks and acted as a control. The elution of ABG-gentamicin was determined by measuring the zones of inhibition produced on the agar surface, and comparing them to the gentamicin in DW released from the filter paper disks.
Minimum bactericidal concentration of ABG-gentamicin
Minimum bactericidal concentration (MBC) was calculated by inoculating equal proportions of a P. aeruginosa suspension with the different concentrations of ABG-gentamicin, for a period of three, five, and seven days at 37°C. At each timepoint, the suspension was removed, serial diluted, and plated in Columbia agar + 5% sheep blood (Oxoid). Plates were incubated at 37°C for 24 hours and growth assessed by counting the colony-forming units per millilitre (CFU/ml). Bacterial reduction was analyzed by comparison with each concentration of gentamicin in DW. The MBC was defined as the lowest concentration of ABG-gentamicin to reduce the initial P. aeruginosa inoculum by ≥ 99.9%.
ABG-gentamicin release kinetics
The rate of gentamicin released from ABG into fetal calf serum (FCS) (Sigma-Aldrich) was indirectly assessed by measuring the bactericidal effect of gentamicin in FCS over three, five, seven, ten, and 14 days. A two-fold analysis was performed, using a previously described modified disk susceptibility test and a reinfection method. ABG-gentamicin and FCS were inoculated in equal proportions in a 12-well plate and incubated at 37°C. At each timepoint, FCS was extracted from the wells and fresh FCS was added to each well.
For agar diffusion testing, a standard curve of known gentamicin concentrations was initially created and the zones of inhibition measured to create a standard curve. An aliquot of 20 μl of the extracted FCS was dropped on blank antimicrobial disks on P. aeruginosa lawns on MH agar, and zones of inhibition were measured in comparison with the standard curve. Another aliquot of 20 μl of the extracted FCS was inoculated with a 0.5 McFarland P. aeruginosa suspension and incubated at 37°C. After 24 hours, the suspension was serial diluted and enumerated to assess the surviving bacteria. Experiments for the reinfection method were only carried out with 75 μg/ml and 100 μg/ml from days 10 to 14 because these concentrations were deemed to be the most effective.
Inhibitory properties of ABG-gentamicin
A concentration of 75 μg/ml of ABG-gentamicin was used to determine the ability to reduce or eliminate biofilm formation on an implant surface. Titanium alloy disks (Ti6Al4V), measuring 10 mm diameter by 3 mm thickness, were coated with ABG-gentamicin and ABG alone. Disks were then inoculated with P. aeruginosa suspension in a 24-well plate and incubated at 37°C with constant agitation of 80 rpm (SSM1 Orbital shaker; Stuart, Stone, UK). Uncoated disks were used as control. After days 3 and 7, disks were gently washed twice, placed in DW, sonicated for 15 minutes at 50 Hz to 60 Hz (Ultrawave U500H; Ultrawave, Cardiff, UK), and vortexed for 60 seconds. This process detached the adherent bacteria from the disks in order for them to be enumerated. The solution was serially diluted and plated in Columbia agar + 5% sheep blood. Plates were incubated at 37°C and CFU/ml were counted after 24 hours. Clinically significant biofilm-inhibitory properties were determined by a 3-log reduction in CFU/ml when compared to uncoated disks.
Stem cell activity in ABG-gentamicin
Bone marrow aspirate was harvested from iliac crest of healthy adult humans using Jamshidi vacuum aspiration (NTL Biologica). After aspiration, the mononucleated cells were concentrated using a separation tube (ABMC Kit). One 20 ml syringe was filled with 2 ml of citrate anticoagulant (ACD-A, Anticoagulant Citrate Dextrose Solution; Biomet Biologics, Bridgend, UK) and 18 ml of BM. The BM was then spun to separate the cell-free plasma/ACD-A mixture from red blood cells. From a 20 ml BM aspirate that was spun, 1.5 ml of buffy layer would be obtained, which would contain nucleated cells of which a small percentage would be MSCs. To form ABG gel with the BM aspirate, the PRP was mixed with equal volumes of BM aspirate and then combined with the rest of the gel components and 75 μg/ml of gentamicin. The volume of gel formed was 1 ml. The same volume of cells was embedded in a 1 ml fibrin glue gel (Tisseel; Baxter, Newbury, UK) to act as a control. To investigate the effect of gentamicin on BM cells, the following gels were formed: ABG gel with BMSCs; ABG gel with gentamicin; ABG gel with gentamicin and BMSCs; and fibrin glue with BMSCs.
A Presto Blue assay (Bio-Rad, Hemel Hempstead, UK) was used to measure the metabolic activity of the BMSCs trapped in the gel at days 7 and 14. Then 10% Presto Blue solution was added to the culture medium for 30 minutes, and excitation at 560 nm and emission at 590 nm were measured using a Tecan plate reader (Infinite Pro 200 series; Tecan, Mannedorf, Switzerland). The mean absorbance was determined from triplicate samples.
To check the viability of the BMSCs in the gels, a live/dead stain was carried out. The gels with and without cells were washed with phosphate-buffered saline (PBS) and incubated in calcein AM and ethidium homodimer (Thermo Fisher Scientific, Dartford, UK) for 30 minutes at 37°C, 5% CO2, in the dark. After incubation, the gels were washed with PBS and viewed under a fluorescent microscope (Apatome 2; Zeiss, Jena, Germany).
To image the cells in the gels, the gels were fixed in 10% buffered formaldehyde, dehydrated in a series of increasing alcohol concentrations, treated with chloroform for two days to de-fat the tissue, and then embedded in wax. Next, 5 μm-thick sections made using a microtome (Thermo Fisher Scientific) were stained with haematoxylin and eosin (H&E). The percentage area of stem cells in the gel was calculated by counting the number of quadrants with spindle-shaped cells. A grid composed of 1 cm by 1 cm quadrants was superimposed on a 10× magnification image. Quadrants occupied by spindle-shaped cells were counted and divided by the total number of quadrants covering the gel, to calculate percentage area of cells in the gel.
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 25 (IBM, Armonk, New York, USA), expressed as mean ± SD. The data in this study were tested for normality using Shapiro-Wilk test. A Mann-Whitney U test was used for non-parametric data. A p-value < 0.05 was considered significant.
Results
Gentamicin elution
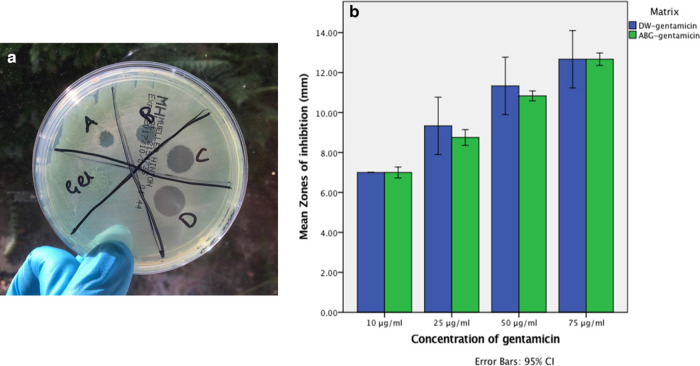
For both ABG-gentamicin and DW-gentamicin, the zones of inhibition increased proportionally with the gentamicin concentration. A standard curve was made for the zones of inhibition of DW-gentamicin concentrations in MH agar (R2 = 0.986). No statistically significant differences were observed in the zones of inhibition between both DW-gentamicin and ABG-gentamicin at 10, 25, 50, or 75 μg/ml. There was, however, a significantly increased release of gentamicin as the concentration increased (p < 0.05, Mann-Whitney U test), except between 50 μg/ml and 75 μg/ml of DW-gentamicin (p = 0.068, Mann-Whitney U test) (Figure 1).
Fig. 1.
Modified disk susceptibility test to determine the gentamicin released from autologous blood glue (ABG). a) Zone of inhibition represented by the different concentrations of antimicrobial activity in the ABG matrix (A: 10 μg/ml, B: 25 μg/ml, C: 50 μg/ml, D: 75 μg/ml). b) Gentamicin release in Mueller-Hinton agar from controls and autologous blood glue (ABG), in concentrations of 10, 25, 50, and 75 μg/ml. CI, confidence interval; DW, deionized water.
Minimum bactericidal concentration of ABG-gentamicin
After three, five, and seven days of incubation, ABG-gentamicin concentrations of 10, 25, 50, and 75 μg/ml were bactericidal to P. aeruginosa when compared to the same gentamicin concentrations in DW. When inoculated with a bacterial suspension, control wells led to a bacterial load of 8.0 × 1010 CFUs/ml at day 3, and too numerous to count (TNTC) on days 5 and 7. In contrast, when wells containing ABG without gentamicin were inoculated, TNTC was achieved at days 3, 5, and 7 when bacteria were enumerated on agar.
Release kinetics
Disk diffusion: using the disk diffusion method, the only concentration that released measurable mean gentamicin into FCS over seven days was 75 μg/ml (50.48 μg/ml (SD 1.65) on days 0 to 3, 28.83 μg/ml (SD 6.46) on days 4 to 5, and 16.00 μg/ml (SD 6.98) on days 6 to 7). FCS extracted from wells containing ABG alone and 10 μg/ml ABG-gentamicin showed no measurable release at any timepoint. Mean release from 25 μg/ml ABG-gentamicin was 13.50 μg/ml (SD 1.38) on days 0 to 3, but showed no measurable release from days 4 to 5 or days 6 to 7. Similarly, 50 μg/ml ABG-gentamicin released significantly lower mean concentrations over five days (25.90 μg/ml (SD 2.09) on days 0 to 3, 11.75 μg/ml (SD 2.25) on days 4 to 5, but no release was detected on days 6 to 7) (Figure 2).
Fig. 2.
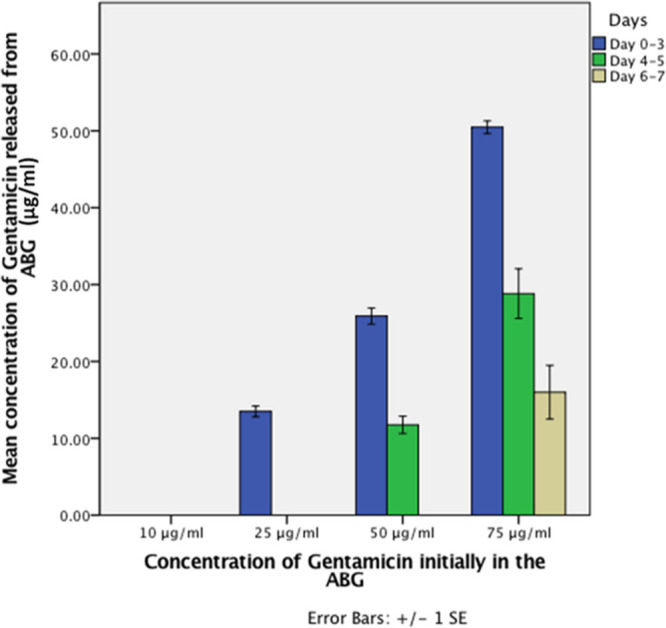
Release kinetics of autologous blood glue (ABG)-gentamicin using a disk diffusion method. The mean concentration of gentamicin within fetal calf serum (FCS), which had been extracted from wells of ABG-gentamicin on days 3, 5, and 7. A concentration of 75 μg/ml of ABG-gentamicin continuously released gentamicin into FCS up to seven days after ABG formation. There was a significant difference between all of the concentrations of gentamicin released from the ABG after three and five days (p < 0.05, Mann-Whitney U test), except between 10 μg/ml and 25 μg/ml at five days. However, after seven days, only the 75 μg/ml concentration showed any effective release of gentamicin. SE, standard error.
Reinfection method: when the FCS-containing eluted gentamicin was challenged with a suspension of P. aeruginosa, there was a decrease in bacterial survival with the increase in gentamicin concentration in the ABG. A significant decrease in bacterial survival was observed for 10 μg/ml (p = 0.046, Mann-Whitney U test) and 25 μg/ml (p = 0.008, Mann-Whitney U test) of gentamicin in ABG up to seven days. However, a concentration of 75 μg/ml of gentamicin recorded no significant difference in bacterial load between days 7 and 14 (p = 0.067, Mann-Whitney U test). Therefore, when the concentration of gentamicin was increased from 75 μg/ml to 100 μg/ml a significant difference in bacterial survival was observed at day 14 (p = 0.046, Mann-Whitney U test) (Figure 3).
Fig. 3.
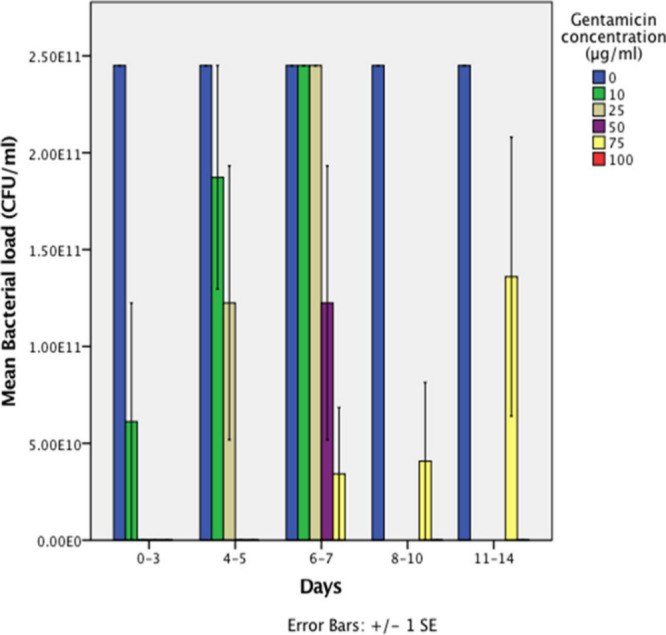
Release kinetics of autologous blood glue (ABG)-gentamicin using a reinfection model. The mean CFUs/ml were calculated after inoculating samples of fetal calf serum (FCS) extracted from wells containing increasing concentrations of ABG-gentamicin, with a 0.5 McFarland suspension of Pseudomonas aeruginosa at different time points. A bar corresponding to ABG-gentamicin concentration of 100 μg/ml for day 14 cannot be observed because the mean value is inferior to 5 x 1010 CFUs/ml and 100 μg/ml was effective at all timepoints while 75 μg/ml was only effective up to five days. Experiments for the reinfection method were only carried out with 75 μg/ml and 100 μg/ml from days 10 to 14 because these concentrations were deemed to be the most effective. SE, standard error.
Biofilm formation
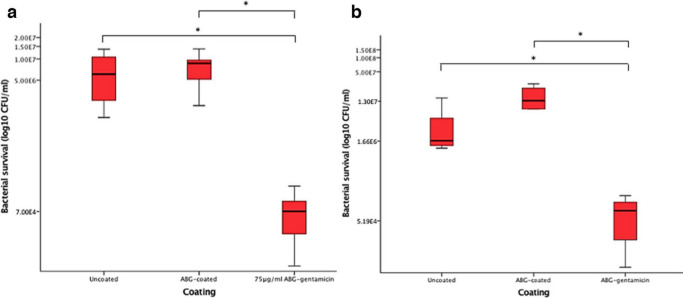
After three days of inoculation with P. aeruginosa, there was no significant increase in the number of bacteria adhering to ABG-coated disks compared to uncoated disks. There was a statistically significant reduction in CFUs/ml in response to the 75 μg/ml ABG-gentamicin coated disks compared to uncoated (p = 0.006, Mann-Whitney U test) and ABG-coated disks (p < 0.001, Mann-Whitney U test). This trend was also observed after seven days. After seven days, there was a statistically significant reduction in CFU/ml on 75 μg/ml ABG-gentamicin coated disks compared to uncoated (p = 0.029, Mann-Whitney U test) and ABG-coated disks (p = 0.016, Mann-Whitney U test) (Figure 4).
Fig. 4.
a) Bacterial recovery after biofilm formation following three days of bacterial inoculation on uncoated, autologous blood glue (ABG)-coated, and 75 μg/ml ABG-gentamicin coated disks. There was statistical significance between ABG-coated and 75 μg/ml (p < 0.001, all Mann-Whitney U test) and uncoated and 75 μg/ml (p = 0.006). b) Bacterial recovery after biofilm formation following seven days of bacterial inoculation on uncoated, ABG-coated, and 75 μg/ml ABG-gentamicin-coated disks. There was statistical significance between uncoated versus 75 μg/ml (p = 0.029) and ABG-coated versus 75 μg/ml (p = 0.016).
Disks coated with ABG-gentamicin had a higher reduction in bacterial numbers after seven days of incubation (3-log reduction in CFU/ml), compared with three days of incubation (2-log reduction in CFU/ml), in ABG-coated disks, but a similar reduction was observed on uncoated disks (2-log reduction in CFU/ml). The most likely explanation for this is the degradation rate of the ABG, which may increase over time in culture (Figure 4).
When disks were qualitatively analyzed after seven days of incubation, it was possible to observe a glue remaining on the ABG-gentamicin coated disks, whereas the ABG-only coated disks did not appear coated.
BMSC activity in ABG-gentamicin
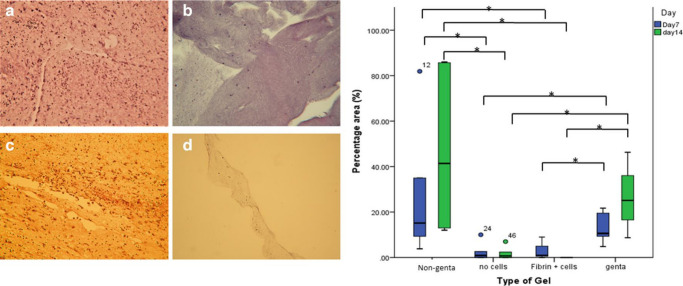
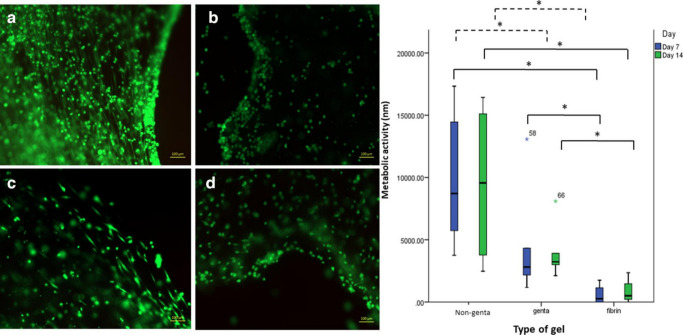
Live/dead staining, Presto Blue analysis, and histology of the gels showed that ABG-gentamicin supported the metabolic activity and growth of BMSCs until day 14. Gentamicin significantly slowed the growth of the cells in the gel, in comparison to the non-gentamicin ABG, but their growth was still significantly higher than cells embedded in fibrin glue, and the cells were alive as demonstrated by H&E and live/dead stain. The cells also maintained their spindle-shaped morphology at days 7 and 14 (Figures 5 and 6).
Fig. 5.
Histology images of the gels after day 14 stained with haematoxylin and eosin (H&E) (×5 magnification). Gels embedded: a) with bone marrow (BM) cells; b) without BM cells; c) with BM cells and gentamicin; and d) with gentamicin but without BM cells. The box plot demonstrates the percentage area occupied by the cells in the gel after seven and 14 days in culture. *p < 0.05, Mann-Whitney U test.
Fig. 6.
Live/dead (calcein Am and ethidium homodimer staining) images of the gels after day 14, at ×5 magnification. Gels embedded: a) with bone marrow (BM) cells; b) without BM cells; c) with BM cells and gentamicin; and d) with gentamicin but without BM cells. The box-plot demonstrates the metabolic activity of the cells in the gentamicin and non-gentamicin autologous blood glue (ABG) gels and fibrin glue after seven and 14 days in culture. There was a significant difference between all ABG gels with fibrin glue. *p < 0.05, Mann-Whitney U test.
Discussion
Due to the ability of bacteria to form a biofilm on implant surfaces, treatment of PJI is extremely challenging, particularly for uncemented implants as antibiotics are routinely used within bone cement. Fibrin-derived glue products from isolated blood have previously demonstrated properties which could make it suitable as a protective seal and carrier of antimicrobials. This project investigated the potential of a novel ABG as an anti-infective implant coating.
In vitro studies have shown that fibrin glue serves as an excellent culture medium and encourages bacterial growth. 19–21 Furthermore, several in vivo studies have also demonstrated equally poor outcomes when comparing uncoated and blood glue-coated infected grafts. 22,23 Although there appears to be no reported increased infection rate with the clinical use of fibrin glue, this may be partly explained by rapid degradation of the currently available fibrin glues by fibrinolysis, thereby eliminating any nidus for infection. 24 The glue impregnated with gentamicin, however, may still form a protective barrier preventing bacteria reaching the implant surface and forming a biofilm. This may be important especially if BMSCs are embedded within the glue, where they would be expected to gain an advantage in attaching to the implant surface and initiating bone ingrowth.
Antibiotic-loaded polymethylmethacrylate (PMMA) has been used prophylactically or in revision surgery to aid in PJI treatment, albeit there are still opposing data stating that they do not confer reduction in these infections, hence its use is still controversial in some practices. PMMA is used as a bone cement to support endoprosthesis fixation, and the antibiotic can only be loaded in very low concentrations, so as not to compromise its biomechanical strength. PMMA is also a biomaterial to which bacteria adhere easily, while ABG has shown to physically prevent attachment and it contains platelet-rich growth factors. Notwithstanding, both approaches have a drawback since the sustained release of antibiotics in subinhibitory concentrations could lead to an increased pathogen resistance. 25
Although a concentration as low as 10 μg/ml ABG-gentamicin was able to reduce bacterial load, a concentration of 75 μg/ml ABG-gentamicin retained bactericidal activity up to seven days after ABG formation. More importantly, when the concentration of gentamicin in ABG increased to 100 μg/ml, a bacterial reduction was achieved up to 14 days. Since the early stages of development of PJI most commonly occur in the first three months after implantation, and the source of bacteria is from the wound site, it is imperative that an anti-infective coating is able to release its antimicrobial agent for periods of time in excess of several days. In addition to preventing infection, it is also very important for gentamicin not to affect the growth of the surrounding cells and cells embedded in the ABG, thereby encouraging the integration of the implant to the surrounding tissue. We have shown in vitro that although gentamicin reduces the growth of BMSCs, it does not kill them and the cells retain their morphology and continue growing up to day 14. The cells are still alive in ABG-gentamicin but have a reduced metabolic activity compared to cells in ABG without gentamicin. Moreover, it would be relevant to show the effect of 100 μg/ml gentamicin in ABG, since it proved to be the most effective concentration in reducing bacterial load up to 14 days.
The initial high output of gentamicin over the first three days is in broad agreement with previous release kinetics studies using fibrin glues and various impregnated antibiotics. Although there are two theories about the mechanism behind antibiotic release from blood glues, which involve simple diffusion of the drug through the cross-linked clot interstices or release of the drug as the glue itself dissolves, the rapid initial release seen in both the current study and most others implies that diffusion predominates. However, wide variation exists between total activity duration (ranges between approximately two and seven days) and the degree of release in the initial burst (between 24% and 85% in the first 24 hours). 19,20,26–30 The glue and the gentamicin and cell combination is cost-effective, and can be easily and quickly prepared at the time of surgery.
A sustained release of gentamicin from ABG could not only prevent adhesion of gentamicin-sensitive bacteria during the operative period and postoperative period preventing biofilm formation, but diffusion from the glue may also eliminate bacteria residing in the surrounding tissues after implantation. This certainly justifies in vivo testing of 100 μg/ml ABG-gentamicin as an anti-infective coating on implanted prostheses. It has been previously shown that a cement spacer loaded with a concoction of antibiotics and antifungals is effective in lowering infection recurrence and rate of survival when compared to single antibiotic loading. 31 In addition, since the incidence of polymicrobial PJIs is increasing, it is imperative that further in vitro testing with ABG impregnated with alternative antimicrobial agents is performed. Moreover, as the gel was developed as a carrier for cells to promote osseointegration and bone formation, these gels will be potentially sprayed onto the surface of implants to improve osseointegration of the implant. In future work it will be important to show whether gentamicin would affect the differentiation ability of BMSCs in ABG in vitro as well as in vivo, and to show whether this BMSC combination with ABG improves osseointegration in vivo.
In conclusion, in this study we have developed an autologous glue that can be prepared during surgery. The autologous gel on its own is not antimicrobial, but antibiotic incorporation allows it to be bactericidal for up to 14 days. This may be important for applications to cementless implants, where the glue combined with both antibiotics and stem cells may be effective in preventing infection and promoting osseointegration.
Author contributions
R. Ramalhete: Conducted the experiments, Wrote and edited the paper.
R. Brown: Conducted the experiments, Collected and analyzed the data, Edited the paper.
G. Blunn: Conceptualized the study, Supervised the project, Reviewed and edited the paper.
J. Skinner: Generated ideas for the project, Recruited patients for the study, Reviewed the data and the paper.
M. Coathup: Conceptualized and supervised the project, Analyzed the data, Reviewed the paper.
I. Graney: Conceptualized the project, Reviewed the data and the paper.
A. Sanghani-Kerai: Supervised the project, Carried out the experiments, Collected and analyzed the data, Wrote and edited the paper.
G. Blunn and A. Sanghani-Kerai contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
This study was funded by NTL Biologica, an advance regenerative medicine organisation based in the UK.
Ethical review statement
This study was carried out with the approval of the Royal National Orthopaedic Hospital (RNOH) Research Ethics Committee (07/Q0506/10), with written informed consent from all subjects.
ICMJE COI statement
Ian Graney, who is an author on this original research article, is the Chief Executive Officer of NTL Biologica. Apart from University College London (UCL) receiving the grant all other authors or represented academic institutions do not have any other conflict of interest associated with this article.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Cyteval C, Bourdon A. Imaging orthopedic implant infections. Diagn Interv Imaging. 2012;93(6):547–557. [DOI] [PubMed] [Google Scholar]
- 2. Arciola CR, Campoccia D, Ehrlich GD, Montanaro L. Biofilm-Based Implant Infections in Orthopaedics In: Donelli G, ed. Biofilm-based Healthcare-associated Infections. Vol. 1. Cham: Springer International Publishing Switzerland, 2015:29–46. [DOI] [PubMed] [Google Scholar]
- 3. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed SS, Haddad FS. Prosthetic joint infection. Bone Joint Res. 2019;8(11):570–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh P-H, Lee MS, Hsu K-Y, et al. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis. 2009;49(7):1036–1043. [DOI] [PubMed] [Google Scholar]
- 6. St Denis TG, Dai T, Izikson L, et al. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2(6):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miquel S, Lagrafeuille R, Souweine B, Forestier C. Anti-Biofilm activity as a health issue. Front Microbiol. 2016;7:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanghani-Kerai A, Coathup MJ, Brown R, et al. The development of a novel autologous blood glue aiming to improve osseointegration in the bone-implant interface. Bone Joint Res. 2020;9(7):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurian A, Reghunadhan I, Nair KGR. Autologous blood versus fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery: a randomised controlled trial. Br J Ophthalmol. 2015;99(4):464–470. [DOI] [PubMed] [Google Scholar]
- 11. Fujimoto K, Yamamura K, Osada T, et al. Subcutaneous tissue distribution of vancomycin from a fibrin glue/Dacron graft carrier. J Biomed Mater Res. 1997;36(4):564–567. [DOI] [PubMed] [Google Scholar]
- 12. Osada T, Yamamura K, Yano K, et al. Distribution and serum concentration of sisomicin released from fibrin glue-sealed Dacron graft in the rat and human. J Biomed Mater Res. 2000;52(1):53–57. [DOI] [PubMed] [Google Scholar]
- 13. Hino M, Ishiko O, Honda KI, et al. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108(1):194–195. [DOI] [PubMed] [Google Scholar]
- 14. Gröner A. Pathogen safety of plasma-derived products - Haemate P/Humate-P. Haemophilia. 2008;14 Suppl 5(Suppl 5):54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oswald A-M, Joly L-M, Gury C, et al. Fatal intraoperative anaphylaxis related to aprotinin after local application of fibrin glue. Anesthesiology. 2003;99(3):762–763. [DOI] [PubMed] [Google Scholar]
- 16. Beierlein W, Scheule AM, Dietrich W, Ziemer G. Forty years of clinical aprotinin use: a review of 124 hypersensitivity reactions. Ann Thorac Surg. 2005;79(2):741–748. [DOI] [PubMed] [Google Scholar]
- 17. Mittal K, Gupta S, Khokhar S, et al. Evaluation of autograft characteristics after pterygium excision surgery: autologous blood coagulum versus fibrin glue. Eye Contact Lens. 2017;43(1):68–72. [DOI] [PubMed] [Google Scholar]
- 18. Qian Y, Han Q, Chen W, et al. Platelet-Rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem. 2017;5:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kara S, Vural A, Unver A, et al. Fibrin sealant as a carrier for sustained delivery of antibiotics. J Clin Exp Invest. 2014;5(2):194–199. [Google Scholar]
- 20. Thompson DF, Davis TW. The addition of antibiotics to fibrin glue. South Med J. 1997;90(7):681–684. [DOI] [PubMed] [Google Scholar]
- 21. Tanemoto K, Fujinami H. Experimental study on bacterial colonization of fibrin glue and its prevention. Clin Ther. 1994;16(6):1016–1027. [PubMed] [Google Scholar]
- 22. Ney AL, Kelly PH, Tsukayama DT, Bubrick MP. Fibrin glue-antibiotic suspension in the prevention of prosthetic graft infection. J Trauma. 1990;30(8):1000–1006. [DOI] [PubMed] [Google Scholar]
- 23. Itokazu M, Yamamoto K, Yang WY, et al. The sustained release of antibiotic from freeze-dried fibrin-antibiotic compound and efficacies in a rat model of osteomyelitis. Infection. 1997;25(6):359–363. [DOI] [PubMed] [Google Scholar]
- 24. Kram HB, Bansal M, Timberlake O, Shoemaker WC. Antibacterial effects of fibrin glue-antibiotic mixtures. J Surg Res. 1991;50(2):175–178. [DOI] [PubMed] [Google Scholar]
- 25. van Vugt TAG, Arts JJ, Geurts JAP. Antibiotic-Loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of biofilm formation. Front Microbiol. 2019;10:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redl H, Schlag G, Stanek G, Hirschl A, Seelich T. In vitro properties of mixtures of fibrin seal and antibiotics. Biomaterials. 1983;4(1):29–32. [DOI] [PubMed] [Google Scholar]
- 27. Tredwell S, Jackson JK, Hamilton D, Lee V, Burt HM. Use of fibrin sealants for the localized, controlled release of cefazolin. Can J Surg. 2006;49(5):347–352. [PMC free article] [PubMed] [Google Scholar]
- 28. Marone P, Monzillo V, Segù C, Antoniazzi E. Antibiotic-impregnated fibrin glue in ocular surgery: in vitro antibacterial activity. Ophthalmologica. 1999;213(1):12–15. [DOI] [PubMed] [Google Scholar]
- 29. Knafl D, Thalhammer F, Vossen MG. In-vitro release pharmacokinetics of amikacin, teicoplanin and polyhexanide in a platelet rich fibrin—layer (PRF)—a laboratory evaluation of a modern, autologous wound treatment. PLoS One. 2017;12(7):e0181090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greco F, de Palma L, Spagnolo N, et al. Fibrin-antibiotic mixtures: an in vitro study assessing the possibility of using a biologic carrier for local drug delivery. J Biomed Mater Res. 1991;25(1):39–51. [DOI] [PubMed] [Google Scholar]
- 31. Yang C, Wang J, Yin Z, et al. A sophisticated antibiotic-loading protocol in articulating cement spacers for the treatment of prosthetic joint infection: a retrospective cohort study. Bone Joint Res. 2019;8(11):526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]