Abstract
Due to a lack of published pharmacokinetic (PK) and/or pharmacodynamic (PD) data, decision-making surrounding appropriate dosing of cannabis used for medical purposes is limited. This multiple-dose study evaluated the safety, tolerability, PK and PD of Spectrum Yellow oil [20 mg/mL cannabidiol (CBD)/<1 mg/mL ∆9-tetrahydrocannabinol (THC)]. Participants (n = 43) were randomized to one of five groups: 120 mg CBD and 5.4 mg THC daily, 240 mg CBD and 10.8 mg THC daily, 360 mg CBD and 16.2 mg THC daily, 480 mg CBD and 21.6 mg THC daily or placebo. Study medication was administered every 12 h for 7 consecutive days. Treatment-emergent adverse events (TEAEs); plasma and urine concentrations of THC, CBD and metabolites; and self-reported subjective effects were collected. Nearly all TEAEs (44/45) were of mild or moderate severity; none was serious. The highest incidence of TEAEs (67%) was in the two higher-dose treatment groups. The highest number of TEAEs (17/45) occurred on the first treatment day. Steady-state plasma CBD concentrations were reached by Day 7. On Day 7, CBD exposure showed dose proportionality (AUC0–t slope = 1.03 [0.70, 1.36], Cmax slope = 0.92 [0.53, 1.31]). Most plasma THC concentrations were below the limit of quantification. Across Days 1 and 7, there were no consistent differences in subjective effects between placebo and active study medication. A prudent approach to improve tolerability with Spectrum Yellow oil might involve initial doses no higher than 240 mg total CBD and 10.8 mg total THC daily in divided doses, with titration upward over time as needed based on tolerability.
Introduction
∆9-tetrahydrocannabinol (THC) and THC-like compounds have regulatory approval in a number of regions to treat anorexia associated with weight loss in patients with acquired immunodeficiency syndrome, nausea and vomiting associated with cancer chemotherapy, and neuropathic pain (1–3). Cannabidiol (CBD) is a non-intoxicating cannabinoid that has regulatory approval in the USA, the EU and Australia to treat rare seizure disorders including Dravet and Lennox–Gastaut syndromes (4) and has been investigated as a treatment for anxiety and mood disorders, psychosis, inflammatory disorders and chronic pain (5). Adverse events (AEs) associated with approved cannabinoid pharmaceutical medications are somnolence, decreased appetite and diarrhea for CBD (4) and abdominal pain, dizziness and euphoria for THC (6). Bioavailability of oral THC and CBD is generally low, highly variable and estimated to be ∼6% of dose delivered as a result of significant first-pass metabolism by the liver (7, 8) via cytochrome P450 isozymes CYP2C9, CYP2C19 and CYP3A4. THC is hydroxylated to pharmacologically active 11-hydroxy-THC (11-OH-THC) by CYP2C9, and its direct oxidation produces a pharmacologically inactive acid, 11-carboxy-THC (11-COOH-THC) (9). CBD is hydroxylated by CYP3A4 and CYP2C19 to pharmacologically active 7-hydroxy-CBD (7-OH-CBD), which is subsequently oxidized to pharmacologically inactive 7-carboxy-CBD (7-COOH-CBD) (10).
Despite a growing body of literature surrounding the therapeutic use of cannabinoids, there is a paucity of data regarding the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of non-pharmaceutical cannabis used for medical purposes. Two single-dose studies have compared the PK profiles of THC between oromucosal formulations with an approximate 1:1 ratio of THC to CBD and THC alone. One study found that there was a longer time to peak plasma concentration (tmax) for 11-OH-THC in the 1:1 THC to CBD group (10 mg THC; 10 mg CBD) compared to 10 mg THC (11). In the other study, there were no differences in PK profiles between low-dose 1:1 THC to CBD (5.4 mg THC; 5 mg CBD) and 5 mg THC or high-dose 1:1 THC to CBD (16.2 mg THC; 15 mg CBD) and 15 mg THC, although there was a trend toward decreases in mean 11-OH-THC time to maximum plasma concentration (Cmax) and area under the curve (AUC) after high-dose 1:1 THC to CBD vs. THC alone (12). In a single-dose study that compared the PK profiles of an oral formulation with a 2:1 ratio of THC to CBD (10 mg THC; 5.4 mg CBD) with 10 mg THC, there was a relative decrease in 11-OH-THC AUC in the 2:1 THC to CBD group (13).
Although concurrently measured PD and PK data have not been collected for non-pharmaceutical cannabis used for medical purposes, the broader literature on the PD of THC and CBD can help show their expected acute effects. Subjective effects of oral THC include increased ratings of subjective “high,” increased hunger and alterations in mood, and at doses of 10 mg THC or higher, cognition and psychomotor functions are temporarily impaired; in contrast, oral administration of CBD typically does not produce interoceptive effects, impairment of cognitive or psychomotor function or significant abuse-related subjective response (14–16). In one study that examined the PD of combined THC and CBD, nabiximols (an oromucosal spray that contains THC and CBD in a 1:1 ratio) had a significant abuse potential at higher doses of 21.6 mg THC + 21 mg CBD and 43.2 mg THC + 40 mg CBD compared with placebo (17). A major limitation of this prior work is that the PD effects of both THC and CBD when orally administered have not been characterized in proportions other than an approximate 1:1 ratio.
The lack of repeated- or multiple-dose PK data for non-pharmaceutical cannabis for medical purposes, coupled with a lack of PD data, leaves a gap in the literature related to cannabis used for medical purposes. Multiple-dose studies with a large dose range are needed to closely approximate real-world conditions in individuals who consume cannabis for medical purposes and to best inform physician and patient decision-making regarding safety and dosing. The aim of this study was to provide a thorough evaluation of the safety, tolerability, PK and PD of a cannabis product (Spectrum Yellow oil [20 mg/mL CBD and <1 mg/mL THC]) that is used for medical purposes in multiple countries.
Methods
Compliance with ethical standards
This trial was conducted in accordance with consensus ethics principles, International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki and local Australian laws and regulations. The protocol was approved by the Alfred Hospital Ethics Committee (Project No. 591/19; approved 25 November 2019). Written informed consent was obtained from each participant before any trial-related procedures were performed.
Participants
Adults aged 18–55 years were eligible for the study if they were in good health as assessed by medical history, physical examination, 12-lead electrocardiogram (ECG) and clinical laboratory investigations; had ≥2 lifetime exposures to THC-containing cannabis products and had a body mass index (BMI) 18–30 kg/m2. Women of childbearing potential were required to have a negative pregnancy test at screening and at intake to the research facility.
Exclusion criteria included women who were pregnant, lactating, breastfeeding or planning a pregnancy; women of childbearing potential or men who were sexually active with women of childbearing potential, who were unwilling or unable to use an acceptable method of contraception; use of tobacco/nicotine-containing products >5 occasions within 1 month of screening or during the study; use of prescription drugs or herbal supplements (except hormonal contraception) within 4 weeks of screening; use of any over-the-counter drugs, vitamins or supplements within 72 h prior to study treatment; a positive breath test for ethanol or positive urine drug screen at screening or prior to study treatment; a history of psychosis or schizophrenia, including first-degree relatives; use of any CBD- or THC-containing product within 8 weeks of screening or during the study; and a history of suicidal behavior or current suicidal ideation.
Study design and treatment
This Phase 1, randomized, double-blind, placebo-controlled, multiple-dose trial was conducted between November 2019 and December 2019 at one site in Australia. Spectrum Yellow is a commercially available cannabis-based product that was made with supercritical carbon dioxide extracted cannabis resin in medium-chain triglyceride (MCT) oil (Tweed Inc., Canopy Growth Corporation, Smiths Falls, ON, Canada). Analytical testing of the clinical batch detected a total terpene concentration <0.05%; the measured concentration of 0.9 mg/mL THC was used to estimate dosages of THC.
Participants were randomly assigned to one of five groups in a 1:1:1:1:1 ratio: 120 mg CBD and 5.4 mg THC daily (3 mL, twice daily [Treatment A]); 240 mg CBD and 10.8 mg THC daily (6 mL, twice daily [Treatment B]); 360 mg CBD and 16.2 mg THC daily (9 mL, twice daily [Treatment C]); 480 mg CBD and 21.6 mg THC daily (12 mL, twice daily [Treatment D]); or placebo. Participants in the four active treatment groups received water in addition to study medication, coadministered with a dual syringe, to create equivalence across groups with respect to volume of liquid administered (18 mL, twice daily). Participants in the placebo group received a mixture of water and placebo (Tweed Inc., Canopy Growth Corporation, Smiths Falls, ON, Canada) coadministered with a dual syringe, in order to closely mimic the ratio of oil and water that participants in the active treatment groups received and thus preserve the blind. The placebo was made with MCT oil, coloring agents alfalfa extract and beta carotene extract, and natural cannabis terpenes for flavoring. Analytical testing of placebo confirmed the absence of cannabinoids and a total terpene content of 0.05%.
Participants were confined to a residential research facility and received study medication twice daily after a standardized meal (e.g., for breakfast, 2 cups of cereal; 2 slices of toast; 2 servings of butter or margarine; 2 condiments; 250 mL of milk; 1 sugar sachet) for 6 days, plus a single dose in the morning of Day 7. Participants were discharged after a 32-h blood draw on Day 8 and returned to the facility on Days 9, 10, 11 and 13 for blood draws and study assessments.
Safety assessments
Safety measures included laboratory assessments (hematology, biochemistry and urinalysis), monitoring of vital signs and ECGs, assessment of suicidality (Columbia-Suicide Severity Rating Scale) and monitoring of treatment-emergent adverse events (TEAEs)/serious adverse events (SAEs).
PK assessments
Blood samples were collected in a 4-mL-draw heparin container prior to the morning dose and 1, 2, 4, 6, 8 and 12 h after the morning dose on Day 1; pre-morning dose on Days 2–7; prior to the morning dose and 1, 2, 4, 6, 8, 12 and 16 h after the morning dose on Day 7; and 24, 32, 48, 72, 96 and 144 h after the Day 7 morning dose. Immediately following collection, blood samples were placed on wet ice and centrifuged, and plasma was immediately frozen at −80°C until shipment to the bioanalytical laboratory (iC42 Clinical Research and Development, University of Colorado, Aurora, CO, USA) on dry ice. Samples were stored at the bioanalytical laboratory at −80°C for ∼2 weeks prior to analysis.
Urine samples were collected prior to the morning dose on Days 1–6; and output (all samples provided) was collected between 00:00–12:00 and 12:00–24:00 intervals on Day 7. Urine samples were frozen on dry ice and then stored in a −80°C freezer until shipment to the bioanalytical laboratory (iC42 Clinical Research and Development, University of Colorado, Aurora, CO, USA) on dry ice. Samples were stored at the bioanalytical laboratory at −80°C for ∼2 weeks prior to analysis. All samples (plasma and urine) had undergone one freeze–thaw cycle at the time of analysis.
Analytical methods
Plasma and urine concentrations of CBD, THC and metabolites were quantified using a two-dimensional high-performance liquid chromatography tandem mass spectrometry (LC–LC–MS/MS) assay developed and validated by iC42 Clinical Research and Development (18), and study samples were analyzed in a CLIA (United States Clinical Laboratory Improvement Amendments)-certified laboratory environment accredited by the College of American Pathologists (Northfield, IL, USA). Plasma aliquots of 200 μL of the calibrator, quality control, blank and zero samples were transferred into 1.5-mL low-binding Eppendorf tubes (Eppendorf, Hamburg, Germany). Eight hundred microliters of a protein precipitation solution of 30% water containing 0.2 M ZnSO4/70% methanol (v/v) containing the appropriate isotope-labeled internal standards were added. After vortexing for 10 min and centrifugation (25,000×g, 4°C for 10 min), the supernatants were injected into the LC–LC system (1260 Infinity HPLC components, Agilent, Santa Clara, CA, USA) for online extraction (extraction column C8-material, 3.0·5 mm, 2.7 μm particle size, Advanced Materials Technology, Wilmington, DE, USA). After 1 min, the analytes were backflushed onto the analytical column (Ascentis Express RP-Amide, 3.0 × 150 mm, 2.7 µm particle size, Supelco, Bellefonte, PA, USA) and separated using a gradient of 0.04% formic acid in water (mobile phase A) and acetonitrile: methanol: isopropanol (3:1:1, v/v/v, mobile phase B). Analytes were quantified using an MS-MS detector (series 5500, Sciex, Concord, ON, Canada). MS-MS data were acquired after atmospheric pressure chemical ionization (APCI) in combination with multiple reaction monitoring (MRM) in positive ion mode. Calibration curves were constructed daily from peak area ratios of analytes to the internal standard. Calibrators were fitted using a quadratic equation in combination with 1/x weighting. Calculations were carried out using Sciex MultiQuant (version 3.0.2.). For details, please see the work by Sempio et al. (18).
The assay had been developed and validated following the applicable Clinical Laboratory Standards Institute and United States Food and Drug Administration (FDA) guidelines (19, 20). Plasma and urine concentrations of the following, with lower limit of quantification (LLOQ) in parentheses, were analyzed: THC (0.780 ng/mL), 11-OH-THC (3.125 ng/mL), 11-COOH-THC (0.780 ng/mL), CBD (0.780 ng/mL), 7-hydroxy-CBD (7-OH-CBD; 1.560 ng/mL) and 7-carboxy-CBD (7-COOH-CBD; 1.560 ng/mL). The LLOQs in plasma and urine were both determined following the procedures as set forth in the applicable Clinical Laboratory Standards Institute guidelines (21) and were determined as the lowest concentration consistently achieving accuracy better than ±20% of the nominal concentration, with imprecision ≤20%. All concentrations reported as non-quantifiable were below the LLOQ. Urine concentrations of THC, CBD and metabolites were not normalized to creatinine. Urine samples were not hydrolyzed. The upper limits of quantification were between 100 and 2000 ng/mL. Inter-day analytical accuracy and imprecision ranged from 90.4% to 111% and from 3.1% to 17.4%, respectively. There were no significant matrix interferences and carryover. Sample stability exceeding the maximum storage time (at −80°C) and freeze–thaw cycles the study samples were exposed to were established (18). The calibration and quality control strategy during study sample analysis was in compliance with the applicable United States FDA guidance (20).
PD assessments
Subjective effects were self-reported using the Drug Effects Questionnaire (DEQ), administered prior to the morning dose and 1, 2, 4, 6, 8 and 12 h after the morning dose on Days 1, 3 and 7. Participants were instructed to rate how they were feeling “right now” on six items specifically related to the study product: feeling the effect, liking any of the effects, disliking any of the effects, feeling any good effects, feeling any bad effects and likelihood of taking the study product again. They also rated how much they were experiencing the following 14 adjectives: “sick,” “heart racing,” “anxious,” “relaxed,” “paranoid,” “tired/drowsy,” “alert,” “irritable,” “energetic,” “restless,” “hungry,” “dazed,” “distracted” and “euphoric/happy.” Items were rated on a 100-point visual analog scale, with anchors of “not at all” and “extremely.”
Urine drug screens
Urine drug screens (TOX/See; Bio-rad; Hercules, CA, USA) were collected at inpatient discharge on Day 8 and at outpatient visits on Days 9, 10, 11 and 13. Participants with positive screens for 11-COOH-THC (>50 ng/mL) were instructed to not drive a motor vehicle until a subsequent screen was negative.
Statistical analyses
AEs were tabulated and classified by System Organ Class (SOC) and preferred term using the Medical Dictionary for Regulatory Activities (version 22.1). Safety data were summarized using descriptive statistics. PK parameters for THC, CBD and metabolites were calculated using non-compartmental analysis (Phoenix 64 version 8.1, Pharsight, a Certara Company, USA). Individual PK parameters and plasma concentration over time were summarized using descriptive statistics. Pre-dose Ctrough plasma levels of CBD and THC at each dose were compared across Days 5–7 for assessment of steady state (where the contrast was not statistically significant, P < 0.05) using a mixed effect analysis of variance (ANOVA). On Days 1, 3 and 7, peak post-treatment value (Emax) for each DEQ item was analyzed using ANOVA, with treatment group as fixed effect and participant as random effect. Least square mean (LSmean) estimates and 95% confidence intervals (CIs) reported for each treatment group and for each paired difference between groups were adjusted with Tukey multiple comparison tests.
Results
Participant characteristics
In total, 43 participants were enrolled and randomly assigned to one of five treatment groups (Treatment A, n = 9; Treatment B, n = 8; Treatment C, n = 9; Treatment D, n = 9; placebo, n = 8). Participants were, on average, 27.3 years old (standard deviation (SD) = 6.7) with a BMI of 24.3 (SD = 2.9), and approximately half (51.2%) were female; the majority of participants were White (86.0%) and not Hispanic or Latino (95.3%). All 43 participants were included in the safety and intent-to-treat populations. Three participants who withdrew from the study due to AEs and did not have sufficient PK data, plus the 8 participants in the placebo group, were not included in the PK population (n = 32).
Safety and tolerability
Overall, 49% of participants (21/43) experienced at least one TEAE (Table I). The most common TEAEs included dizziness, presyncope, insomnia, abdominal pain and diarrhea [each reported by three participants (7.0%)]. The number of TEAEs between Treatments A (4) and B (2) and placebo (3) were similar, while there were twice as many TEAEs in Treatments C (6) and D (6) than placebo. TEAEs in the psychiatric disorders SOC only affected participants taking higher doses [Treatments C (2) and D (4)]. The highest number of TEAEs (17/45; 37.8%) occurred on the first day of treatment (Figure 1).
Table I.
All-Causality Treatment-Emergent Adverse Events per Treatment Group, by MedDRA System Organ Class and Preferred Term (Safety Population)
| Treatmenta | ||||||
|---|---|---|---|---|---|---|
| SOC PT[n (%) E] |
Overall (n = 43) |
A (n = 9) |
B (n = 8) |
C (n = 9) |
D (n = 9) |
Placebo (n = 8) |
| Participants with at least one TEAE | 21 (48.8) 45 | 4 (44.4) 7 | 2 (25.0) 4 | 6 (66.7) 13 | 6 (66.7) 14 | 3 (37.5) 7 |
| Nervous system disorders | 9 (20.9) 12 | 2 (22.2) 4 | 2 (25.0) 2 | 2 (22.2) 2 | 3 (33.3) 4 | 0 |
| Dizziness | 3 (7.0) 3 | 1 (11.1) 1 | 1 (12.5) 1 | 1 (11.1) 1 | 0 | 0 |
| Presyncope | 3 (7.0) 3 | 0 | 0 | 0 | 3 (33.3) | 0 |
| Headache | 2 (4.7) 2 | 2 (22.2) 2 | 0 | 0 | 0 | 0 |
| Somnolence | 2 (4.7) 2 | 1 (11.1) 1 | 0 | 1 (11.1) 1 | 0 | 0 |
| Amnesia | 1 (2.3) 1 | 0 | 1 (12.5) 1 | 0 | 0 | 0 |
| Lethargy | 1 (2.3) 1 | 0 | 0 | 0 | 1 (11.1) 1 | 0 |
| Psychiatric disorders | 6 (14.0) 9 | 0 | 0 | 2 (22.2) 5 | 4 (44.4) 4 | 0 |
| Insomnia | 3 (7.0) 3 | 0 | 0 | 1 (11.1) 1 | 2 (22.2) 2 | 0 |
| Anxiety | 2 (4.7) 2 | 0 | 0 | 1 (11.1) 1 | 1 (11.1) 1 | 0 |
| Paranoia | 2 (4.7) 2 | 0 | 0 | 1 (11.1) 1 | 1 (11.1) 1 | 0 |
| Hallucination | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Mood altered | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Gastrointestinal disorders | 6 (14.0) 8 | 1 (11.1) 1 | 0 | 0 | 2 (22.2) 3 | 3 (37.5) 4 |
| Abdominal pain | 3 (7.0) 3 | 0 | 0 | 0 | 2 (22.2) 2 | 1 (12.5) 1 |
| Diarrhea | 3 (7.0) 3 | 0 | 0 | 0 | 1 (1.11) 1 | 2 (25.0) 2 |
| Nausea | 2 (4.7) 2 | 1 (11.1) 1 | 0 | 0 | 0 | 1 (12.5) 1 |
| General disorders and administration site conditions | 3 (7.0) 4 | 1 (11.1) 1 | 0 | 1 (11.1) 2 | 0 | 1 (12.5) 1 |
| Discomfort | 1 (2.3) 2 | 0 | 0 | 1 (11.1) 2 | 0 | 0 |
| Eye complication associated with device | 1 (2.3) 1 | 0 | 0 | 0 | 0 | 1 (12.5) 1 |
| Fatigue | 1 (2.3) 1 | 1 (11.1) 1 | 0 | 0 | 0 | 0 |
| Injury, poisoning and procedural complications | 4 (9.3) 4 | 0 | 1 (12.5) 1 | 1 (11.1) 1 | 1 (11.1) 1 | 1 (12.5) 1 |
| Ligament sprain | 1 (2.3) 1 | 0 | 1 (12.5) 1 | 0 | 0 | 0 |
| Vascular access site complication | 1 (2.3) 1 | 0 | 0 | 0 | 0 | 1 (12.5) 1 |
| Vascular access site pain | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Vascular access site swelling | 1 (2.3) 1 | 0 | 0 | 0 | 1 (11.1) 1 | 0 |
| Cardiac disorders | 2 (4.7) 2 | 1 (11.1) 1 | 0 | 1 (1.11) 1 | 0 | 0 |
| Palpitations | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Ventricular extrasystoles | 1 (2.3) 1 | 1 (11.1) 1 | 0 | 0 | 0 | 0 |
| Infections and infestations | 2 (4.7) 2 | 0 | 0 | 0 | 1 (11.1) 1 | 1 (12.5) 1 |
| Upper respiratory tract infection | 1 (2.3) 1 | 0 | 0 | 0 | 1 (11.1) | 0 |
| Viral upper respiratory tract infection | 1 (2.3) 1 | 0 | 0 | 0 | 0 | 1 (12.5) 1 |
| Reproductive system and breast disorders | 2 (4.7) 2 | 0 | 1 (12.5) 1 | 0 | 1 (11.1) 1 | 0 |
| Dysmenorrhea | 2 (4.7) 2 | 0 | 1 (12.5) 1 | 0 | 1 (11.1) 1 | 0 |
| Investigations | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Sensory level abnormal | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Nasal congestion | 1 (2.3) 1 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
E = number of adverse events, MedDRA = Medical Dictionary for Regulatory Activities, n = number of participants with events, PT = preferred term, SOC = system organ class, TEAE = treatment-emergent adverse event.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
Figure 1.
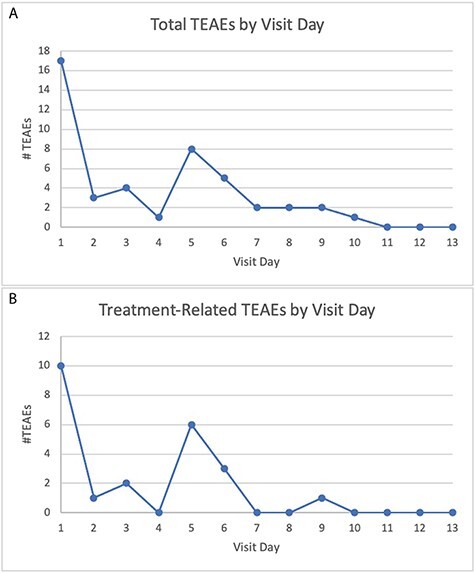
(A) Total and (B) treatment-related treatment-emergent adverse events (TEAEs) by visit day.
Almost all TEAEs were of mild (41/45 TEAEs) or moderate (3/45 TEAEs) severity. There was one severe TEAE: anxiety in Treatment D. All moderate and severe TEAEs were considered related to treatment except for ventricular extrasystoles (Treatment A). There were no SAEs, no life-threatening AEs and no deaths.
Four participants experienced TEAEs that resulted in withdrawal from study treatment: two in Treatment A (in one participant, headache of moderate severity possibly related to study treatment, and fatigue and dizziness of mild severity and not related to study treatment; in another participant, ventricular extrasystoles of moderate severity and unlikely related to study treatment), one in Treatment C (anxiety, hallucination and paranoia, all of mild severity and definitely related to study treatment) and one in Treatment D (anxiety of severe severity and definitely related to study treatment). Three of these participants discontinued due to TEAEs: one each in Treatments A (Day 3), C (Day 4) and D (Day 2); one of these participants (Treatment A) discontinued treatment on Day 5 but did not withdraw from the study and completed outpatient visits.
No clinically significant differences were observed between treatment groups with respect to clinical chemistry laboratory assessments (including no clinically significant changes in transaminases), vital signs, physical examinations, ECGs or suicidality.
Pharmacokinetics
Figure 2 shows the geometric mean plasma concentration–time profiles; Tables II and III summarize the plasma PK parameters, for CBD, THC and metabolites; Table IV summarizes the urinary PK parameters for CBD, THC and metabolites and Table V summarizes plasma THC concentrations.
Figure 2.
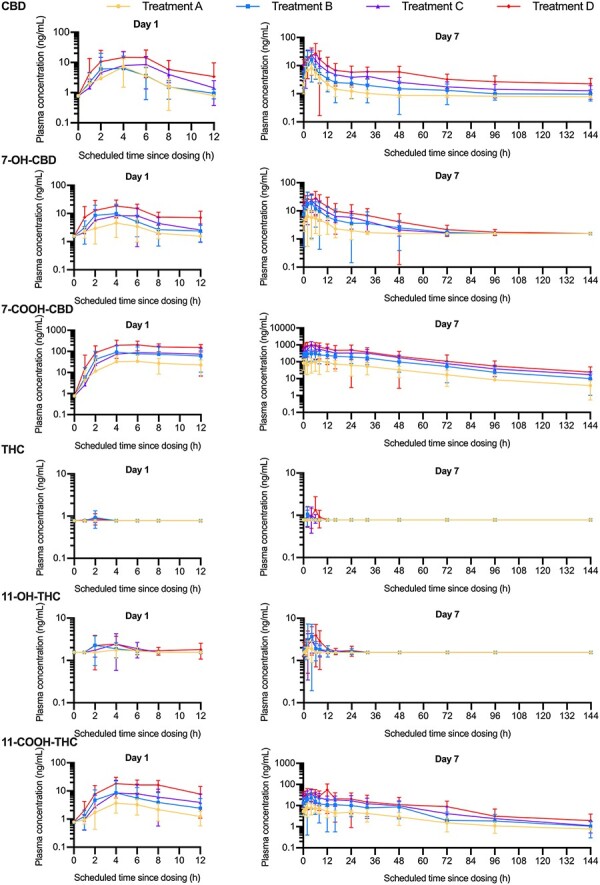
Geometric mean (±standard deviation) plasma concentration–time profiles for cannabidiol (CBD), 7-hydroxy-cannabidiol (7-OH-CBD), 7-carboxy-cannabidiol (7-COOH-CBD), tetrahydrocannabinol (THC), 11-hydroxy-tetrahydrocannabinol (11-OH-THC) and 11-carboxy-tetrahydrocannabinol) (11-COOH-THC) on Day 1 and Day 7 for Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily (semi-logarithmic scale, pharmacokinetic population).
Table II.
Plasma Pharmacokinetic Parameters for CBD, THC and Metabolites (Pharmacokinetic Population)
| Pharmacokinetic parameter (unit) | Treatment Aa (n = 8) | Treatment Ba (n = 8) | Treatment Ca (n = 8) | Treatment Da (n = 8) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | |
| CBD | ||||||||
| C max (ng/mL)b | 8.9 (69.6)e | 9.6 (47.5)e | 7.2 (142.1)e | 18.3 (83.5) | 10.9 (106.2) | 23.9 (51.9) | 23.3 (53.6) | 36.3 (112.9) |
| t max (h)c | 4.0 (1.0–6.0)e | 4.0 (1.0–6.1)e | 4.0 (4.0–6.0)e | 4.0 (1.0–6.0) | 6.0 (4.0–6.2) | 4.0 (4.0–6.0) | 5.0 (1.0–12.0) | 5.0 (1.0–8.0) |
| AUC0–12 (h × ng/mL)b | 69.1 (-)g | 54.1 (34.3)h | 74.9 (6.7)i | 100.8 (69.3) | NE | 153.2 (55.0) | 125.9 (42.6)j | 229.4 (79.5) |
| AUC0–t (ng × h/mL)b | 34.8 (77.0)e | 62.2 (31.5)e | 29.7 (138.6)e | 110.2 (69.0) | 51.6 (99.5) | 175.8 (53.0) | 117.5 (45.5) | 263.3 (79.4) |
| t 1/2 (h)d | – | 4.7 (38.2) | – | 5.4 (23.3) | – | 5.2 (24.9) | – | 5.8 (28.7) |
| CL/F (L/h)b | – | 2292.3 (18.6) | – | 2756.1 (43.8) | – | 1761.1 (39.6) | – | 1390.8 (52.5) |
| R ac(AUC) b | – | 0.9 (-)g | – | 1.9 (35.0)i | – | NE | – | 2.3 (57.6)j |
| 7-OH-CBD | ||||||||
| C max (ng/mL)b | 6.4 (39.0)j | 8.8 (41.1)h | 16.1 (57.1)k | 19.9 (78.0) | 13.3 (57.5)e | 23.8 (62.3) | 20.7 (45.7) | 32.7 (74.1) |
| t max (h)c | 4.0 (4.0–6.0)j | 5.0 (1.0–6.1)h | 4.0 (4.0–4.0)k | 4.0 (1.0–6.0) | 4.0 (4.0–6.2)e | 4.0 (4.0–6.0) | 4.0 (4.0–12.0) | 5.0 (1.0–6.0) |
| AUC0–12 (h × ng/mL)b | NE | 70.2 (43.0)h | 99.7 (12.2)f | 140.3 (88.1) | 110.4 (14.6)k | 180.9 (57.8) | 162.3 (32.1) | 263.0 (67.7) |
| AUC0–t (ng × h/mL)b | 30.5 (30.4)j | 78.5 (50.3)h | 75.6 (59.9)k | 156.2 (98.1) | 64.0 (79.7)e | 212.0 (55.5) | 118.6 (57.9) | 309.5 (71.0) |
| t 1/2 (h)d | – | 4.4 (-)g | – | 5.4 (0.2)i | – | 5.2 (15.8)f | – | 5.3 (4.1)f |
| CL/F (L/h)b | – | 2022.8 (47.5)f | – | 2713.8 (48.8)k | – | 1263.7 (41.9)f | – | 1972.8 (112.8)f |
| R ac(AUC) b | – | NE | – | 2.9 (62.3)f | – | 2.0 (50.4)k | – | 2.6 (40.8)k |
| (M/P)AUC0–tb | – | 1.2 (24.9)j | – | 1.4 (44.8) | – | 1.2 (24.6) | – | 1.2 (30.5) |
| 7-COOH-CBD | ||||||||
| C max (ng/mL)b | 37.7 (100.7) | 129.9 (69.8)e | 99.8 (114.9) | 343.9 (54.7) | 105.3 (113.2) | 671.2 (66.2) | 233.8 (48.7) | 1027.1 (58.8) |
| t max (h)c | 5.0 (4.0–6.0) | 8.0 (0.0–8.1)e | 4.1 (4.0–12.0) | 4.0 (4.0–12.0) | 7.0 (4.0–12.0) | 4.0 (4.0–8.0) | 6.0 (4.0–12.0) | 4.0 (4.0–8.0) |
| AUC0–12 (h × ng/mL)b | 318.5 (52.2)k | 1160.4 (71.5)e | 621.7 (66.0)j | 3238.5 (57.8) | 1487.8 (61.3)i | 6471.9 (62.2) | 2025.3 (58.4)k | 9540.4 (63.3) |
| AUC0–t (ng × h/mL)b | 288.9 (86.6) | 1486.7 (69.7)e | 732.0 (101.9) | 4059.8 (61.2) | 731.6 (123.1) | 8019.5 (59.8) | 1747.2 (47.7) | 11,766.7 (66.2) |
| t 1/2 (h)d | – | BLoQ | – | BLoQ | – | 6.3 (-)g | – | BLoQ |
| CL/F (L/h)b | – | 99.7 (71.0)k | – | 69.7 (84.8)i | – | 58.8 (70.3)f | – | 32.3 (-)g |
| R ac(AUC) b | – | 3.8 (30.5)f | – | 4.5 (23.5)j | – | 4.1 (20.8)i | – | 5.4 (44.3)k |
| (M/P)AUC0–tb | – | 23.9 (81.1)h | – | 36.8 (83.9) | – | 45.6 (63.5) | – | 44.7 (47.3) |
| THC | ||||||||
| C max (ng/mL)b | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ |
| t max (h)c | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ |
| AUC0–t (ng × h/mL)b | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ |
| AUC0–12 (h × ng/mL)b | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ | BLoQ |
| t 1/2 (h)d | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
| CL/F (L/h)b | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
| R ac(AUC) b | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
| 11-OH-THC | ||||||||
| C max (ng/mL)b | BLoQ | BLoQ | BLoQ | 8.7 (107.3)i | BLoQ | 3.5 (56.3)f | BLoQ | 7.3 (40.3)j |
| t max (h)c | BLoQ | BLoQ | BLoQ | 4.0 (4.0–4.0)i | BLoQ | 4.0 (1.0–4.0)f | BLoQ | 6.0 (1.0–8.0)j |
| AUC0–12 (h × ng/mL)b | BLoQ | BLoQ | BLoQ | 80.1 (-)g | BLoQ | 33.7 (-)g | BLoQ | 61.6 (17.1)f |
| AUC0–t (ng × h/mL)b | BLoQ | BLoQ | BLoQ | 44.6 (99.3)i | BLoQ | 23.0 (70.4)f | BLoQ | 45.2 (57.8)j |
| t 1/2 (h)d | – | – | – | BLoQ | – | BLQ | – | BLoQ |
| CL/F (L/h)b | – | – | – | – | – | – | – | 331.0 (19.7)i |
| R ac(AUC) b | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
| (M/P)AUC0–tb | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
| 11-COOH-THC | ||||||||
| C max (ng/mL)b | 4.5 (48.0)e | 9.6 (38.3)e | 11.4 (64.9e) | 24.5 (62.7) | 10.7 (124.6) | 36.8 (53.7) | 20.9 (53.9) | 43.8 (68.4) |
| t max (h)c | 4.0 (4.0–6.0)e | 6.0 (1.0–8.0)e | 4.0 (4.0–6.0)e | 4.0 (4.0–6.0) | 6.0 (4.0–11.9) | 4.0 (4.0–6.0) | 4.0 (4.0–12.0) | 5.0 (4.0–8.0) |
| AUC0–12 (h × ng/mL)b | 32.5 (63.4)f | 83.3 (35.8)e | 74.3 (84.8)k | 187.3 (71.9) | 126.4 (65.9)f | 307.8 (54.2) | 141.5 (49.2)e | 371.8 (64.5) |
| AUC0–t (ng × h/mL)b | 25.1 (52.3)e | 103.7 (34.4)e | 61.9 (74.9)e | 210.9 (73.0) | 62.0 (111.9) | 362.7 (54.6) | 130.3 (51.6) | 436.8 (60.2) |
| t 1/2 (h)d | – | NE | – | NE | – | 5.6 (-)g | – | NE |
| CL/F (L/h)b | – | 55.1 (22.4)e | – | 33.5 (-)g | – | 34.1(90.1)i | – | 28.7 (14.5)i |
| R ac(AUC) b | – | 2.4 (15.3)f | – | 3.1 (19.1)k | – | 3.1 (14.0)f | – | 2.7 (42.0)e |
| (M/P)AUC0–tb | – | BLoQ | – | BLoQ | – | BLoQ | – | BLoQ |
AUC0–12 = area under the plasma concentration–time curve from 0 to 12 h time point, AUC0–t = area under the plasma concentration–time curve from 0 to the last quantifiable concentration, BLoQ = below limit of quantification, CL/F = oral clearance of drug from plasma, Cmax = maximum observed plasma concentration, (M/P)AUCτ = AUCτ metabolite/AUCτ parent, NE = not estimable, Rac(AUC) = accumulation ratio based on AUC, Rac(Cmax) = accumulation ratio based on Cmax, tmax = time to reach Cmax.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
Geometric mean (geometric CV%).
Median (range).
Arithmetic mean (arithmetic CV%).
n = 7.
n = 3.
n = 1.
n = 6.
n = 2.
n = 5.
n = 4.
Table III.
Pre-dose Plasma CBD Concentrations by Treatment Group, Days 2–6 (Pharmacokinetic Population)
| Treatment Aa | Treatment Ba | Treatment Ca | Treatment Da | |
|---|---|---|---|---|
| Day | (n = 8) | (n = 8) | (n = 8) | (n = 8) |
| Day 2 | 0.63 (1.06)b | 1.79 (1.01)c | 3.70 (2.48) | 6.44 (3.61) |
| Day 3 | 1.69 (0.96)c | 2.96 (1.56) | 5.50 (4.24) | 9.80 (4.49) |
| Day 4 | 1.75 (0.74)d | 3.40 (2.07) | 5.70 (3.38) | 9.30 (4.95) |
| Day 5 | 1.98 (1.15)e | 4.33 (2.53) | 6.75 (3.78) | 9.73 (4.87) |
| Day 6 | 2.25 (1.51)f | 4.13 (2.09) | 7.54 (3.99) | 12.25 (7.13) |
Mean values (standard deviation) in ng/mL are presented.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
Five samples were below the lower limit of quantification (0.78 ng/mL) and were assigned as zero for analysis.
n = 7.
n = 6.
One sample was below the lower limit of quantification (0.78 ng/mL) and was assigned as zero for analysis.
n = 6; one sample was below the lower limit of quantification (0.78 ng/mL) and was assigned as zero for analysis.
Table IV.
Urine Pharmacokinetic Parameters of CBD, THC and Metabolites (Pharmacokinetic Population)
| Pharmacokinetic parameter (units)b | Treatment Aa (n = 8) |
Treatment Ba (n = 8) |
Treatment Ca (n = 8) |
Treatment Da (n = 8) |
|---|---|---|---|---|
| CBD | ||||
| Ae0–12 (mg) | BLoQ | 0.001 (-)c | 0.001 (112.3)d | BLoQ |
| Ae12–24 (mg) | BLoQ | 0.000 (-)c | BLoQ | BLoQ |
| Ae0–24 (mg) | BLoQ | 0.002 (-)c | 0.001 (112.3)d | BLoQ |
| Fe0–12 | BLoQ | 6.02 (-)c | 0.003 (NE)d | BLoQ |
| Fe12–24 | BLoQ | 0.000 (-)c | BloQ | BLoQ |
| Fe0–24 | BLoQ | 0.000 (-)c | 0.000 (112.3)d | BLoQ |
| C LR (L/h) | BLoQ | 2.16 (-)c | 5.13 (29.2)d | BLoQ |
| 7-OH-CBD | ||||
| Ae0–12 (mg) | 0.017 (73.3)e | 0.040 (35.6) | 0.056 (55.5) | 0.024 (113.8) |
| Ae12–24 (mg) | 0.004 (93.0)e | 0.007 (54.9)e | 0.012 (39.7) | 0.005 (141.3) |
| Ae0–24 (mg) | 0.021 (72.6)e | 0.047 (36.4) | 0.069 (51.2) | 0.029 (116.6) |
| Fe0–12 | 0.001 (NE)e | 0.000 (35.6) | 0.000 (55.5) | 0.004 (NE) |
| Fe12–24 | 0.004 (NE)e | 0.004 (NE)e | 0.193 (NE) | 0.014 (NE) |
| Fe0–24 | 0.000 (72.6)e | 0.000 (36.4) | 0.000 (51.2) | 0.000 (116.6) |
| C LR (L/h) | 0.002 (71.3)f | 0.001 (109.9) | 0.001 (65.1) | 0.003 (NE) |
| THC | ||||
| Ae0–12 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| Ae12–24 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| Ae0–24 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| Fe0–12 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| Fe12–24 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| Fe0–24 (mg) | BLoQ | BLoQ | BLoQ | BLoQ |
| C LR | BLoQ | BLoQ | BLoQ | BLoQ |
| 11-COOH-THC | ||||
| Ae0–12 (mg) | 0.002 (48.3)g | 0.005 (58.3)e | 0.006 (95.4)e | 0.007 (114.6)h |
| Ae12–24 (mg) | 0.005 (218.2)d | 0.004 (81.4)f | 0.006 (110.2)e | 0.001 (101.0)e |
| Ae0–24 (mg) | 0.003 (178.0)d | 0.008 (81.6)e | 0.010 (118.6) | 0.004 (273.7) |
| Fe0–12 | 0.000 (48.3)d | 0.000 (58.3)e | 0.000 (95.4)e | 0.000 (114.6)h |
| Fe12–24 | 0.001 (218.2)g | 0.000 (81.4)f | 0.002 (NE)e | 1.2 (NE)e |
| Fe0–24 | 0.001 (178.0)d | 0.001 (81.6)e | 0.001 (118.6) | 0.000 (273.7) |
| C LR (L/h) | 0.003 (67.4)d | 0.002 (77.0)e | 0.001 (116.1)e | 0.001 (202.3)h |
7-COOH-CBD and 11-OH-THC were not measured in urine. Ae0–12 = amount of drug eliminated between 0 and 12 h, Ae12–24 = amount of drug eliminated between 12 and 24 h, Ae0–24 = amount of drug eliminated between 0 and 24 h, BLoQ = below limit of quantification, CLR = renal clearance, Fe0–12 = fraction of administered dose excreted in urine between 0 and 12 h, Fe12–24 = fraction of administered dose excreted in urine between 12 and 24 h, Fe12–24 = fraction of administered dose excreted in urine between 0 and 24 h, NE = not estimable.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
Geometric mean (geometric mean CV%).
n = 1.
n = 2.
n = 7.
n = 6.
n = 4.
n = 5.
Table V.
Summary of Plasma THC Concentrations (ng/mL) by Treatment Group (Pharmacokinetic Population)
| Timepoint | Treatment Aa (n = 8) | Treatment Ba (n = 8) | Treatment Ca (n = 8) | Treatment Da (n = 8) | ||||
|---|---|---|---|---|---|---|---|---|
| BLoQ (n) | Mean (SD) | BLoQ (n) | Mean (SD) | BLoQ (n) | Mean (SD) | BLoQ (n) | Mean (SD) | |
| Day 1 | ||||||||
| Pre-dose | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) |
| 1 h | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 7 (8) | 0.13 (0.35) |
| 2 h | 8 (8) | 0 (0) | 6 (8) | 0.44 (0.93) | 6 (7) | 0.16 (0.42) | 5 (6) | 0.23 (0.65) |
| 4 h | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 7 (8) | 0.10 (0.28) | 7 (7) | 0 (0) |
| 6 h | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 7 (7) | 0 (0) | 6 (6) | 0 (0) |
| 8 h | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) |
| 12 h | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 7 (7) | 0 (0) |
| Day 7 | ||||||||
| Pre-dose | 7 (7) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) |
| 1 h | 6 (6) | 0 (0) | 7 (8) | 0.13 (0.35) | 8 (8) | 0 (0) | 6 (7) | 0.11 (0.30) |
| 2 h | 7 (7) | 0 (0) | 5 (8) | 0.69 (1.01) | 6 (8) | 0.44 (0.90) | 5 (8) | 0.70 (1.05) |
| 4 h | 7 (7) | 0 (0) | 6 (7) | 0.13 (0.34) | 5 (7) | 0.61 (1.31) | 6 (8) | 0.51 (1.05) |
| 6 h | 7 (7) | 0 (0) | 8 (8) | 0 (0) | 7 (8) | 0.19 (0.53) | 4 (7) | 1.57 (2.76) |
| 8 h | 7 (7) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 7 (8) | 0.33 (0.92) |
| 12 h | 7 (7) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) | 8 (8) | 0 (0) |
Timepoints are in relation to the morning dose. Concentrations that were BLQ were assigned as zero for analysis. n = number, BLoQ = below limit of quantitation; lower limit of quantification of THC is 0.78 ng/mL; SD = standard deviation.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
CBD and metabolites
On Day 1, after a single dose of study medication, CBD exposure showed dose proportionality; however, the wide CIs surrounding slope estimates indicate large variability in estimates (AUC0–t slope = 0.81 [0.32, 1.30]; Cmax slope = 0.62 [0.13, 1.12]). The Cmax for 7-OH-CBD increased by 2.5- and 1.3-fold with a doubling in dose between Treatments A and B and Treatments B and D, respectively. The AUC0–12 for 7-OH-CBD in Treatment A was not estimable; therefore, dose proportionality was undetermined. Exposure to 7-COOH-CBD approximately doubled to tripled with each 2-fold increase in dose, where Cmax increased by 2.6- and 2.3-fold, and AUC0–12 increased by 1.9- and 3.2-fold between Treatments A and B and Treatments B and D, respectively. The median tmax for CBD, 7-OH-CBD and 7-COOH-CBD ranged from 4 to 7 h across treatments.
C trough data showed that steady-state plasma CBD concentrations were reached by Day 7 for all treatment groups (Treatment A: LSmean Day 5 [2.02 ng/mL] vs. remaining days [2.18 ng/mL] = −0.1, P = 0.51; Treatment B: LSmean Day 5 [4.32 ng/mL] vs. remaining days [4.32 ng/mL] = −0.006, P = 0.99; Treatment C: LSmean Day 6 [7.54 ng/mL] vs. Day 7 [7.65 ng/mL] = −0.11, P = 0.80; Treatment D: LSmean Day 6 [12.25 ng/mL] vs. Day 7 [11.78 ng/mL] = 0.47, P = 0.71) (also see Table III for mean pre-dose plasma CBD concentrations for Days 2–6). On Day 7, CBD exposure showed dose proportionality (AUC0–t slope = 1.03 [0.70, 1.36], Cmax slope = 0.92 [0.53, 1.31]). By Day 7, there was an almost doubling in exposure of 7-OH-CBD for a 2-fold increase in dose; the Cmax increased by 2.3- and 1.6-fold and the AUC0–12 increased by 2.0- and 1.9-fold between Treatments A and B and Treatments B and D, respectively. For 7-COOH-CBD, there was a tripling in exposure for each 2-fold increase in dose, where the Cmax increased by 2.6- and 3.0-fold, and the AUC0–12 increased by 2.8- and 2.9-fold between Treatments A and B and Treatments B and D, respectively. On Day 7, the median tmax of CBD and 7-OH-CBD ranged from 4 to 5 h, independent of dose, although for 7-COOH-CBD, the median tmax was halved from Treatment A to Treatments B, C and D from ∼8 to ∼4 h, respectively. On Day 7, the mean plasma terminal elimination half-life, t1/2, ranged from 4.7–5.8 to 4.4–5.4 h for CBD and 7-OH-CBD, respectively. Apparent clearance (CL/F) slightly decreased with increasing dose of CBD and 7-OH-CBD, while clearance of 7-COOH-CBD decreased more rapidly with increasing doses.
On Day 7, compared with Day 1, the accumulation ratio based on AUC (Rac (AUC)) for CBD was 0.9- to 2.3-fold (except Rac (AUC) for Treatment C, which was below the limit of quantification [BLoQ]); for 7-OH-CBD, it was 2.0- to 2.6-fold (except Rac(AUC) for Treatment A, which was [BLoQ]) and for 7-COOH-CBD, it was 3.8- to 5.4-fold. On Day 7, 7-COOH-CBD was the major circulating product (23.9-, 36.8-, 45.6- and 44.7-fold higher than CBD for Treatments A, B, C, and D, respectively) followed by 7-OH-CBD (1.2-, 1.4-, 1.2- and 1.2-fold higher than CBD for Treatments A, B, C and D, respectively) and then parent CBD. On Day 7, urinary CBD was detected in one participant in Treatment B and two in Treatment C but was BLoQ for Treatments A and D.
THC and metabolites
All plasma concentrations for THC on Days 1 and 7 in Treatment A were BLoQ, and the majority of plasma concentrations for THC on Days 1 and 7 in Treatments B, C and D were BLoQ (Table V). Because no participant had more than two quantifiable concentrations of THC on either Day 1 or 7, PK parameters for THC were not calculated for any treatment group. No participant in any treatment group had more than two quantifiable concentrations of 11-OH-THC on Day 1, and no participant in Treatment A had more than two quantifiable concentrations of 11-OH-THC on Day 7.
On Day 7, Cmax, tmax and AUC0–12 of 11-OH-THC for Treatments B, C and D appeared to be dose independent. On Day 1, 11-COOH-THC was readily detected in plasma with a median tmax of 4–6 h. On Day 7, 11-COOH-THC Cmax increased by 2.6- and 1.8-fold as the dose doubled between Treatments A and B and Treatments B and D, respectively. A similar apparent increase was observed for 11-COOH-THC AUC0–12 on Day 7, where it increased by 2.3- and 2.0-fold as the dose doubled between Treatments A and B and Treatments B and D, respectively. CL/F slightly decreased with increasing doses. On Day 7, the Rac (AUC) for 11-COOH-THC was 2.4–3.1-fold for all treatment groups. Urinary THC concentrations were BLoQ for all treatments at 0–12 and 12–24 h on Day 7.
Pharmacodynamics
Mean post-treatment peak ratings of most negative effects (dislike, bad effects, heart racing, anxious, paranoid, tired/drowsy, irritable, restless, dazed and distracted) were low on Days 1, 3 and 7 (Table VI). Mean post-treatment peak ratings of “restless” on Day 1 were higher for Treatment D relative to Treatments A, B and C; on Day 3, they were higher for Treatment D relative to Treatment A and on Day 7, they were higher for D relative to placebo and lower for A relative to B and D. Across treatment groups, the median time to post-treatment peak ratings of “restless” occurred at 4.5 h on Days 1 (Q1 = 1.7, Q3 = 8.7), 3 (Q1 = 1.8, Q3 = 8.4) and 7 (Q1 = 2.5, Q3 = 8.6). Mean post-treatment peak ratings of “distracted” on Day 1 were higher for Treatment D relative to placebo and Treatments A and B, and on Days 3 and 7, they were higher for Treatment D relative to B. Across treatment groups, the median time to post-treatment peak ratings of “distracted” occurred at 2.7 h (Q1 = 1.7, Q3 = 8.5) on Day 1, at 4.5 h on Day 3 (Q1 = 1.7, Q3 = 8.3) and at 3.4 h (Q1 = 1.6, Q3 = 6.5) on Day 7. Mean post-treatment peak ratings of other negative effects differed between active study medication and placebo (e.g., “bad effects” on Day 7) and between the three lower-dose treatment groups relative to the highest-dose treatment group (e.g., “dazed” on Day 1; “dislike the effects” on Day 3); however, these other between-group differences were not consistent across Days 1, 3 and 7. Mean post-treatment peak ratings of “take study product again” appeared to differ in Treatments A and B vs. placebo on Days 1 and 7; however, high mean pre-treatment ratings of this item on both days in Treatments A and B (e.g., on Day 7, Treatment A: M = 81.1, Treatment B: M = 70.8, placebo: M = 33.9) suggest that post-treatment between-group differences on this item may simply be due to high pre-treatment ratings in active vs. placebo treatment groups.
Table VI.
Mean Peak Post-treatment Value on Drug Effects Questionnaire (Intent-to-Treat Population)
| Treatmenta | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | |||||||||
| DEQ, LSmean (SE) | Placebo (n = 8) |
A (n = 9) |
B (n = 8) |
C (n = 9) |
D (n = 9) |
Placebo (n = 8) |
A (n = 9) |
B (n = 8) |
C (n = 9) |
D (n = 9) |
| Feelings | 34.5 (9.6) | 24.7 (9.0)b | 32.4 (9.6) | 41.4 (9.0) | 51.2 (9.0)b | 42.1 (9.6) | 35.1 (9.0) | 42.2 (9.6) | 47.1 (9.0) | 44.9 (9.0) |
| Dislike | 18.4 (6.9) | 5.8 (6.5)b | 12.3 (6.9) | 15.4 (6.5) | 30.9 (6.5)b | 19.5 (6.9) | 3.3 (6.5)b | 5.0 (6.9)g | 7.3 (6.5)f | 26.4 (6.5)b,g,f |
| Like | 39.6 (10.2) | 42.0 (9.6) | 50.1 (10.2) | 49.4 (9.6) | 45.3 (9.6) | 44.6 (10.2) | 39.0 (9.9) | 49.6 (10.2) | 43.2 (9.6) | 31.33 (9.6) |
| Good effects | 42.6 (10.9) | 36.9 (10.3) | 48.5 (10.9) | 45.7 (10.3) | 46.3 (10.3) | 40.2 (10.9) | 37.9 (10.2) | 50.1 (10.9) | 37.3 (10.2) | 35.4 (10.2) |
| Bad effects | 19.5 (7.1) | 8.3 (6.7) | 11.5 (7.1) | 14.4 (6.7) | 27.0 (6.7) | 22.4 (7.1) | 8.9 (6.7)b | 5.6 (7.1)g | 9.4 (6.7) | 28.1 (6.7)b,g |
| Take study product again | 43.0 (10.9)c,d | 79.7 (10.3)d | 78.3 (10.9)c | 64.7 (10.3) | 66.9 (10.5) | 52.2 (10.9) | 72.0 (10.3) | 73.6 (10.9) | 55.9 (10.3) | 53.6 (10.3) |
| Sick | 22.0 (6.7)d | 2.9 (6.3)d | 7.4 (6.7) | 9.8 (6.3) | 20.3 (6.3) | 22.0 (6.7)d | 1.7 (6.3)d | 7.6 (6.7) | 7.3 (6.3) | 7.3 (6.3) |
| Heart racing | 4.4 (3.7) | 1.8 (3.5)b | 5.5 (3.7) | 1.4 (3.5)f | 11.7 (3.5)b,f | 5.7 (3.7) | 3.7 (3.5) | 3.2 (3.7) | 1.8 (3.5) | 7.4 (3.5) |
| Anxious | 15.4 (4.4)d | 2.2 (4.2)b,d | 4.5 (4.4)g | 8.8 (4.2) | 18.1 (4.2)b,g | 8.0 (4.4) | 4.01 (4.2) | 3.5 (4.4) | 4.9 (4.2) | 14.0 (4.2) |
| Relaxed | 61.5 (9.1) | 61.4 (8.6) | 71.4 (9.1) | 64.3 (8.6) | 72.1 (8.6) | 56.4 (9.1) | 60.3 (8.6) | 67.4 (9.1) | 65.3 (8.6) | 68.3 (8.6) |
| Paranoid | 9.5 (3.6) | 4.0 (3.4) | 1.4 (3.7)g | 10.4 (3.4) | 12.4 (3.4)g | 9.4 (3.7) | 1.9 (3.4)b | 0.9 (3.7)g | 3.9 (3.4) | 12.4 (3.4)b,g |
| Tired/drowsy | 50.6 (9.0) | 58.9 (8.5) | 54.5 (9.0) | 51.7 (8.5) | 70.9 (8.5) | 57.1 (9.0) | 57.9 (8.7) | 44.0 (9.0)g | 51.8 (8.7) | 72.4 (8.5)g |
| Alert | 39.7 (10.4) | 41.9 (9.8) | 30.9 (10.4) | 44.4 (9.8) | 42.2 (9.8) | 37.7 (10.4) | 33.7 (10.1) | 30.5 (10.4) | 38.4 (10.1) | 21.6 (9.8) |
| Irritable | 3.3 (5.0) | 3.8 (4.7) | 3.6 (5.1) | 11.7 (4.8) | 16.3 (4.8) | 15.4 (5.1) | 9.1 (4.9) | 3.1 (5.1) | 6.4 (4.9) | 12.22 (4.8) |
| Energetic | 40.1 (9.2) | 34.8 (8.6) | 39.1 (9.2) | 32.1 (8.6) | 37.9 (8.6) | 45.1 (9.2) | 30.5 (8.8) | 31.5 (9.2) | 37.6 (8.8) | 33.9 (8.6) |
| Restless | 19.6 (7.2) | 10.7 (6.8)b | 16.6 (7.2)g | 19.3 (6.8)f | 40.2 (6.8)b,g,f | 20.6 (7.2) | 5.01 (7.2)b | 14.7 (7.2) | 21.0 (7.2) | 33.2 (6.8)b |
| Hungry | 48.8 (10.6) | 40.1 (10.0) | 65.9 (10.6) | 45.7 (10.0) | 61.4 (10.0) | 42.1 (10.6) | 45.1 (10.4) | 63.2 (10.6) | 33.9 (10.4) | 47.2 (10.0) |
| Dazed | 22.0 (9.2)i | 20.8 (8.6)b | 14.3 (9.2)g | 25.2 (8.6)f | 57.8 (8.6)b,f,g,i | 29.4 (9.1) | 27.3 (8.8)b | 12.9 (9.1)g | 31.7 (8.8) | 53.9 (8.6)b,g |
| Distracted | 14.0 (8.0)i | 18.6 (7.5)b | 11.0 (8.0)g | 30.0 (7.5) | 45.9 (7.5)b,g,i | 19.4 (8.0) | 24.5 (7.7) | 3.0 (8.0)g | 23.4 (7.7) | 38.9 (7.5)g |
| Euphoric/happy | 39.0 (10.3) | 37.8 (9.7) | 51.6 (10.3) | 50.8 (9.7) | 59.3 (9.7) | 36.7 (10.3) | 34.2 (9.7) | 43.2 (10.3) | 46.3 (9.7) | 51.6 (9.7) |
| Feelings | 30.8 (9.6) | 23.9 (9.4) | 41.4 (9.6) | 48.2 (9.4) | 41.1 (9.4) | |||||
| Dislike | 23.6 (6.9)c | 14.2 (6.9) | 1.8 (6.9)c | 13.8 (6.9) | 19.9 (6.9) | |||||
| Like | 33.4 (10.2) | 42.1 (9.9) | 43.1 (10.2) | 42.8 (9.9) | 30.9 (9.9) | |||||
| Good effects | 31.9 (10.9) | 37.7 (10.5) | 44.4 (10.9) | 41.1 (10.5) | 31.4 (10.5) | |||||
| Bad effects | 23.3 (7.1)c | 11.4 (7.0) | 1.0 (7.1)c | 13.4 (7.0) | 19.5 (7.0) | |||||
| Take study product again | 45.0 (10.9)c,d | 85.0 (10.5)d,e | 76.3 (10.9)c | 49.5 (10.5)e | 63.7 (10.5) | |||||
| Sick | 19.6 (6.7) | 11.5 (6.6) | 0.9 (6.7) | 4.1 (6.6) | 12.1 (6.6) | |||||
| Heart racing | 9.8 (3.7) | 1.6 (3.7) | 0.9 (3.7) | 5.6 (3.7) | 5.6 (3.7) | |||||
| Anxious | 8.1 (4.4) | 2.3 (4.4) | 1.8 (4.4) | 5.8 (4.4) | 5.8 (4.4) | |||||
| Relaxed | 53.1 (9.1) | 59.5 (8.9) | 59.5 (9.1) | 55.9 (8.9) | 67.8 (8.9) | |||||
| Paranoid | 3.5 (3.7) | 3.1 (3.6) | 2.0 (3.7) | 3.1 (3.6) | 3.7 (3.6) | |||||
| Tired/drowsy | 43.8 (9.00) | 62.0 (8.8) | 41.6 (9.0) | 46.9 (8.8) | 60.0 (8.8) | |||||
| Alert | 36.8 (10.42) | 41.2 (10.1) | 26.8 (10.4) | 37.7 (10.1) | 28.6 (10.1) | |||||
| Irritable | 11.0 (5.1) | 7.5 (4.9) | 9.6 (5.1) | 9.3 (4.9) | 20.4 (4.9) | |||||
| Energetic | 39.8 (9.2) | 35.1 (8.8) | 22.1 (9.2) | 29.0 (8.8) | 41.5 (8.8) | |||||
| Restless | 15.6 (7.2)i | 4.2 (7.2)b,h | 28.8 (7.3)h | 21.9 (7.2) | 38.2 (7.2)b,i | |||||
| Hungry | 46.3 (10.6) | 46.4 (10.4) | 49.5 (10.6) | 42.9 (10.4) | 58.3 (10.4) | |||||
| Dazed | 20.4 (9.2) | 22.1 (8.8) | 12.0 (9.2)g,j | 37.5 (8.8)j | 43.7 (8.8)g | |||||
| Distracted | 14.9 (8.0) | 24.5 (7.7) | 10.4 (8.00)g | 30.8 (7.7) | 40.9 (7.7)g | |||||
| Euphoric/happy | 35.0 (10.3) | 36.2 (9.9) | 47.9 (10.3) | 47.6 (9.9) | 56.0 (9.9) | |||||
Items were rated on a 100-point visual analog scale. DEQ = Drug Effects Questionnaire, LSmean = least square mean, SE = standard error.
Treatment A: 120 mg total CBD and 5.4 mg THC daily; B: 240 mg total CBD and 10.8 mg total THC daily; C: 360 mg total CBD and 16.2 mg total THC daily; D: 480 mg total CBD and 21.6 mg total THC daily.
A vs. D (P < 0.05).
B vs. placebo (P < 0.05).
A vs. placebo (P < 0.05).
A vs. C (P < 0.05).
C vs. D (P < 0.05).
B vs. D (P < 0.05).
A vs. B (P < 0.05).
D vs. placebo (P < 0.05).
B vs. C (P < 0.05).
Urine drug screens
At least one participant in each treatment group had a positive urine drug screen for 11-COOH-THC through 72 h after the final dose of study medication (Figure 3). One participant in Treatment B, and two participants in Treatment D had a positive urine drug screen for 11-COOH-THC 144 h after the final dose.
Figure 3.
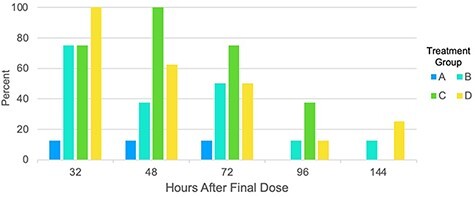
Percentage of urine drug screens positive for 11-carboxy-tetrahydrocannabinol (11-COOH-THC) (>50 ng/mL) in each treatment group. Treatment A: 120 mg total CBD and 5.4 mg THC daily (n = 8); B: 240 mg total CBD and 10.8 mg total THC daily (n = 8); C: 360 mg total CBD and 16.2 mg total THC daily (n = 8); D: 480 mg total CBD and 21.6 mg total THC daily (n = 8).
Discussion
Safety and tolerability
Given that almost all TEAEs were mild or moderate in severity and there were no SAEs, doses of Spectrum Yellow oil ranging from 120 mg CBD and 5.4 mg THC to 480 mg CBD and 21.6 mg THC were well-tolerated. Fewer AEs were reported on Days 2 through 13 relative to Day 1, indicating increased tolerability with repeated dosing. Notably, 3 of the 43 enrolled participants (7%) discontinued study treatment for TEAEs definitely or possibly related to treatment. The finding that CBD and THC were well-tolerated is consistent with conclusions of systematic reviews of medical cannabis and cannabinoids (22) and of CBD (5). The most frequently reported TEAEs of dizziness, presyncope, insomnia, abdominal pain and diarrhea are similar to the TEAEs reported in studies of approved cannabinoid pharmaceutical products (Marinol®, Sativex® and Epidiolex®) (23–25). Participants in Treatments C and D reported the highest incidence of TEAEs, suggesting dose dependence in the incidence of TEAEs. Some TEAEs associated with Epidiolex® appear to similarly be dose-dependent (4), but other approved cannabinoid pharmaceutical products have not reported on the dose dependence of TEAEs.
Pharmacokinetics
After both single and multiple doses of Spectrum Yellow oil, CBD exposure showed dose proportionality; steady-state conditions were achieved within 7 days. After 7 days of dosing, there was no to moderate accumulation of CBD, moderate accumulation for 7-OH-CBD and 11-COOH-THC and higher accumulation for 7-COOH-CBD; a multiple-dose PK study of Epidiolex® also reported higher accumulation of 7-COOH-CBD than accumulation of CBD and 7-OH-CBD (23). 7-COOH-CBD is not pharmacologically active and has not shown an anticonvulsant effect in mice (26). The majority of plasma THC concentrations were below the limit of quantification of 0.78 ng/mL.
Plasma concentrations of CBD, THC and metabolites were lower in this study than has been reported in systematic reviews of the PK profiles of oral CBD (5) and THC (27). Although participants in this study consumed study medication 1 h after a meal, higher-fat content foods in the pre-dose meals could have improved PK profiles, as high-fat food increases CBD and THC exposure by as much as 3–5 times relative to fasted conditions (e.g., 28, 29). Nonetheless, the notable percentage of urine drug screens positive for 11-COOH-THC through 144 h after the final dose of Spectrum Yellow oil suggests accumulation of THC and metabolites in tissues and relatively slow urinary excretion. Overall, our results are consistent with the prevailing notion that oral delivery of cannabinoids is characterized by low and variable absorption. Because previous PK studies have examined cannabinoid formulations with a 1:1 or 2:1 ratio of THC to CBD, additional research is needed to further explore the impact of high levels of CBD concomitantly administered with low levels of THC, such as those studied here, on bioavailability.
Pharmacodynamics
Across Days 1 and 7, there were no consistent differences in subjective effects between placebo and Spectrum Yellow oil. There were no between-group differences on items that typically indicate abuse liability, such as “like the effects” and “good effects,” and the magnitude of post-treatment peak ratings of negative effects was low. Negative subjective effects in the two lower-dose treatment groups did not differ from those in the placebo group, and there were some differences in negative effects between the three lower-dose treatment groups relative to the highest-dose treatment group, such as on items of “restless” and “distracted,” which generally peaked 2.5–4.5 h post-treatment. The lack of between-group differences in subjective effects may be because subjective effects are typically observed at higher doses of THC and CBD (14, 16, 30). However, the small number of participants in each group and the wide variability surrounding estimates of between-group differences suggest that PD results should be interpreted with caution.
Clinical implications
Taken together, these safety, tolerability, PK and PD data support a “start low and go slow” approach, with initial doses of Spectrum Yellow oil similar to those in Treatments A and B and titration upward over time based on tolerability. However, research with individuals with various medical conditions is needed before formal condition-specific dosing guidelines can be promulgated. Individuals who elect to use Spectrum Yellow oil for medical purposes may consider the duration for which urine drug screens showed positive (>50 ng/mL) results for 11-COOH-THC at the studied THC total daily doses ranging from 5.4 to 21.6 mg, as urine drug screens could be used to evaluate driving or workplace impairment.
Trial limitations
This trial is limited by its focus on healthy adults; future studies are needed to characterize Spectrum Yellow oil in patient populations. Sample sizes in each treatment group were small, yielding imprecise estimates of dose proportionality and between-group differences in PD. The measure of PD was designed for substances with abuse potential, not for non-intoxicating substances such as CBD.
Conclusion
Over a week of twice-daily dosing, daily doses of CBD up to 480 mg and of THC up to 21.6 mg were generally safe and became better tolerated after the first day of treatment. Steady-state conditions for CBD were achieved within 7 days, but quantifiable plasma THC concentrations were sporadic. A prudent approach to improve tolerability with Spectrum Yellow oil might involve initial doses no higher than 240 mg total CBD and 10.8 mg total THC daily in divided doses, with titration upward over time as needed based on tolerability.
Acknowledgments
We acknowledge Clinical Network Services and Nucleus Network for collecting study data; Roland Jbeily, Justin Verwoert and Ryan Yablonsky for facilitating shipment of study products; staff at iC42 Clinical Research and Development for analyzing blood and urine samples; staff at Nuventra for helping interpret PK results; Aleksandra Trajkovic for assisting with study operations; Scott Young for testing study syringes and Stephanie Louis for reviewing an earlier version of the manuscript.
Contributor Information
Erica N Peters, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Irina Mosesova, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Laura MacNair, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Ryan Vandrey, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21218, USA.
M Hunter Land, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Mark A Ware, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Cynthia Turcotte, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Marcel O Bonn-Miller, Canopy Growth Corporation, One Hershey Drive, Smiths Falls, ON K7A 0A8, Canada.
Funding
This study was funded by Canopy Growth Corporation.
Conflict of Interest
The product investigated in this study is part of the Spectrum Therapeutics line of medical cannabis products, and Spectrum Therapeutics is a wholly owned subsidiary of Canopy Growth Corporation. Canopy Growth Corporation funded the study, provided the study products, designed the study, and interpreted the findings. An external contract research organization, in collaboration with an Australian Phase 1 unit, executed the study; more specifically, the external organization obtained Ethics approval, led participant recruitment, and collected, managed, and analyzed all study data. All biological samples were shipped to the University of Colorado for independent analysis of cannabinoids and associated metabolites. Dr. Peters is an employee of Canopy Growth Corporation, during which time she has received stock options. She also serves as a consultant to Battelle. Ms. Mosesova is an employee of Canopy Growth Corporation, during which time she has received stock options. Dr. MacNair is an employee of Canopy Growth Corporation, during which time she has received stock options. Dr. Vandrey has received consulting fees or honoraria from Canopy Growth Corporation, MyMD Pharmaceuticals Inc., FSD Pharma, and Present Life Corporation. Mr. Land is an employee of Canopy Growth Corporation, during which time he has received stock options, and was a prior employee of GW Pharmaceuticals. Dr. Ware is an employee of Canopy Growth Corporation, during which time he has received stock options. Ms. Turcotte is an employee of Canopy Growth Corporation, during which time she has received stock options. Dr. Bonn-Miller is an employee of Canopy Growth Corporation, during which time he has received stock options. He served on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray Inc.
References
- 1. Lutge, E.E., Gray, A., Siegfried, N. (2013) The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database of Systematic Reviews, 4, CD005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith, L.A., Azariah, F., Lavender, V.T., Stoner, N.S., Bettiol, S. (2015) Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database of Systematic Reviews, 11, CD009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mucke, M., Phillips, T., Radbruch, L., Petzke, F., Hauser, W. (2018) Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews, 3, CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2018) EPIDIOLEX (Cannabidiol) oral Solution-2018 Version Greenwich Biosciences Inc./FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (Accessed Oct 10, 2020).
- 5. Millar, S.A., Stone, N.L., Bellman, Z.D., Yates, A.S., England, T.J., O’Sullivan, S.E. (2019) A systematic review of cannabidiol dosing in clinical populations. British Journal of Clinical Pharmacology, 85, 1888–1900.doi: 10.1111/bcp.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(2017) MARINOL (dronabinol)-2017 Version AbbVie Inc/FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (Accessed Oct 10, 2020).
- 7. Ohlsson, A., Lindgren, J.E., Wahlen, A., Agurell, S., Hollister, L.E., Gillespie, H.K. (1980) Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology Therapeutics, 28, 409–416.doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 8. Agurell, S., Carlsson, S., Lindgren, J.E., Ohlsson, A., Gillespie, H., Hollister, L. (1981) Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia, 37, 1090–1092. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe, K., Yamaori, S., Funahashi, T., Kimura, T., Yamamoto, I. (2007) Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sciences, 80, 1415–1419.doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 10. Hawksworth, G., McArdle, K. Metabolism and pharmacokinetics of cannabinoids. In: Guy, G.W., Whittle, B.A., Robson, P.J. (eds.) The Medicinal Uses of Cannabis and Cannabinoids. Pharmaceutical Press: London, 2004; pp 205–228. [Google Scholar]
- 11. Guy, G.W., Robson, P.J. (2004) A Phase I, double blind, three-way crossover study to assess the pharmacokinetic profile of Cannabis Based Medicine Extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK0215). Journal of Cannabis Therapeutics, 3, 121–152.doi: 10.1300/J175v03n04_02. [DOI] [Google Scholar]
- 12. Karschner, E.L., Darwin, W.D., Goodwin, R.S., Wright, S., Huestis, M.A. (2011) Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clinical Chemistry, 57, 66–75.doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nadulski, T., Pragst, F., Weinberg, G., Roser, P., Schnelle, M., Fronk, E.M., et al. (2005) Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Therapeutic Drug Monitor, 27, 799–810.doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- 14. Vandrey, R., Herrmann, E.S., Mitchell, J.M., Bigelow, G.E., Flegel, R., LoDico, C., et al. (2017) Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. Journal of Analytical Toxicology, 41, 83–99.doi: 10.1093/jat/bkx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoedel, K.A., Szeto, I., Setnik, B., Sellers, E.M., Levy-Cooperman, N., Mills, C., et al. (2018) Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behavior, 88, 162–171.doi: 10.1016/j.yebeh.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 16. Spindle, T.R., Cone, E.J., Goffi, E., Weerts, E.M., Mitchell, J.M., Winecker, R.E., et al. (2020) Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug and Alcohol Dependence, 211, 107937.doi: 10.1016/j.drugalcdep.2020.107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoedel, K.A., Chen, N., Hilliard, A., White, L., Stott, C., Russo, E., et al. (2011) A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Human Psychopharmacology, 26, 224–236.doi: 10.1002/hup.1196. [DOI] [PubMed] [Google Scholar]
- 18. Sempio, C., Almaraz-Quinones, N., Jackson, M., Zhao, W., Wang, G.S., Leehey, M., et al. (2021) Simultaneous quantification of 17 cannabinoids by LC-MS/MS in human plasma. Journal of Analytical Toxicology. (Under Review). [DOI] [PubMed] [Google Scholar]
- 19. CLSI . Liquid-chromatography-mass spectrometry methods. In: C62-A Cd (ed). Approved Guideline. Clinical and Laboratory Standards Institute: Wayne, PA, 2014. [Google Scholar]
- 20. Bioanalytical Method Validation . Guidance for Industry. U.S - 2018 Version. Department of Health and Human Services FDA. https://www.fda.gov/media/70858/download (Accessed Dec 19 2020).
- 21. CLSI . Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline. In: CLSI Document EP17-A2. 2nd edition. Clinical and Laboratory Standards Institute: Wayne, PA, 2012. [Google Scholar]
- 22. Wang, T., Collet, J.P., Shapiro, S., Ware, M.A. (2008) Adverse effects of medical cannabinoids: a systematic review. Canadian Medical Association Journal, 178, 1669–1678.doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor, L., Gidal, B., Blakey, G., Tayo, B., Morrison, G. (2018) A Phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs, 32, 1053–1067.doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stott, C.G., White, L., Wright, S., Wilbraham, D., Guy, G.W. (2013) A Phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. European Journal of Clinical Pharmacology, 69, 1135–1147.doi: 10.1007/s00228-012-1441-0. [DOI] [PubMed] [Google Scholar]
- 25. Parikh, N., Kramer, W.G., Khurana, V., Cognata Smith, C., Vetticaden, S. (2016) Bioavailability study of dronabinol oral solution versus dronabinol capsules in healthy volunteers. Clinical Pharmacology, 8, 155–162.doi: 10.2147/CPAA.S115679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whalley, B.J., Stott, C., Gray, R.A. (2017) The human metabolite of cannabidiol, 7-hydroxy-cannabidiol, but not 7-carboxy-cannabidiol, is anticonvulsant in the maximal electroshock threshold test (MEST) in mouse [abstract no. 1.435]. AES Conference, Washington, D.C. [Google Scholar]
- 27. Poyatos, L., Perez-Acevedo, A.P., Papaseit, E., Perez-Mana, C., Martin, S., Hladun, O., et al. (2020) Oral administration of cannabis and delta-9-tetrahydrocannabinol (THC) preparations: a systematic review. Medicina (Kaunas), 56, 309.doi: 10.3390/medicina56060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lunn, S., Diaz, P., O’Hearn, S., Cahill, S.P., Blake, A., Narine, K., et al. (2019) Human pharmacokinetic parameters of orally administered delta(9)-tetrahydrocannabinol capsules are altered by fed versus fasted conditions and sex differences. Cannabis and Cannabinoid Research, 4, 255–264.doi: 10.1089/can.2019.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crockett, J., Critchley, D., Tayo, B., Berwaerts, J., Morrison, G. (2020) A Phase I, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia, 61, 267–277.doi: 10.1111/epi.16419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spindle, T.R., Cone, E.J., Schlienz, N.J., Mitchell, J.M., Bigelow, G.E., Flegel, R., et al. (2018) Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Network Open, 1, e184841.doi: 10.1001/jamanetworkopen.2018.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]


