Abstract
Background:
Evidence from large randomized clinical trials supports the benefit of sodium-glucose cotransporter-2 inhibitors (SGLT2i) to improve cardiovascular and kidney outcomes in patients with type 2 diabetes mellitus (T2DM) with or at high risk for atherosclerotic cardiovascular disease or chronic kidney disease. Considering this evidence, which has been expanding since the product label indication for empagliflozin to reduce risk of cardiovascular death in 2016, clinician-level variation in the prescription of SGLT2i among US Medicare beneficiaries was evaluated.
Methods:
Antihyperglycemic medication prescribers were identified as those physicians and advanced practice providers prescribing metformin in Medicare part D prescriber data. In this cross-sectional study, the proportion prescribing SGLT2i was assessed overall and across specialties in 2018, with changes assessed from 2014 to 2018. SGLT2i use was compared with other second-line antihyperglycemic medication classes, sulfonylureas and dipeptidyl peptidase-4 inhibitors (DPP4is).
Results:
Among 232,523 unique clinicians who prescribed metformin for Medicare beneficiaries in 2018 (diabetes-treating clinicians), 45,255 (19.5%) prescribed SGLT2i. There was substantial variation across specialties – from 72% of endocrinologists to 14% of cardiologists who prescribed metformin also prescribed SGLT2i. Between 2014 and 2018, the number prescribing SGLT2i increased 5 fold from 9,048 in 2014 to 45,255 in 2018. Among clinicians who prescribed both sulfonylureas and SGLT2i in 2018, SGLT2i was prescribed to a median 33 beneficiaries for every 100 prescribed sulfonylureas (IQR 18, 67). SGLT2i use relative to sulfonylureas increased from 19 (IQR 11, 34) per 100 in 2014 to 33 (IQR 18, 67) per 100 in 2018 (P-trend <0.001).
Conclusions:
Eighty percent of clinicians prescribing metformin to Medicare beneficiaries did not prescribe SGLT2i in 2018. Moreover, sulfonylureas prescriptions were 3 times more frequent than those of SGLT2is, although a pattern of increasing uptake may portend future trends. These findings highlight a baseline opportunity to improve care and outcomes for patients with T2DM.
Keywords: Diabetes, novel agents, cardioprotective medications, SGLT-2 inhibitors
BACKGROUND
Consistent randomized trial evidence supports the use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with type 2 diabetes mellitus (T2DM) with or at high risk for atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease to improve cardiovascular and kidney outcomes, effects that appear to be independent of glucose control.1–5 Their beneficial effects on clinical outcomes represent a paradigm shift in management of patients with T2DM that has traditionally focused on glycemic control. This is of particular relevance to Medicare beneficiaries, most of whom are older, and at an elevated risk of adverse cardiovascular and kidney outcomes.
The first SGLT2i approved for clinical use in the US was canagliflozin, approved in 2013 for the treatment of hyperglycemia in patients with T2DM, followed by dapagliflozin and empagliflozin for the same indication in 2014.6 Beyond glucose control, empagliflozin received a product labeled indication for reduction of risk for cardiovascular death for patients with T2DM and prevalent ASCVD in 2016, the first clinical outcomes indication for any antihyperglycemic therapy.7 Canagliflozin received product label indication to reduce risk for the composite outcome of cardiovascular death, myocardial infarction, and stroke for patients with T2DM and ASCVD in 2018, and dapagliflozin for hypertensive heart failure and cardiovascular death in patients with T2DM and ASCVD or with multiple ASCVD risk factors in 2019.
Despite these regulatory approvals and parallel society-based clinical practice recommendations, little is known about how frequently clinicians prescribe these medications. Specifically, it is important to evaluate whether clinicians caring for patients with T2DM are using them relative to other antihyperglycemic medications given the robustness of clinical trial evidence, product labeled indications and professional society endorsements. Data from a single US academic center study suggested underuse of these medications,8 and preliminary evidence that underuse extends to the national landscape in the US has been reported.9, 10 Furthermore, the study of specialty-specific prescription patterns is relevant, as the proven cardiovascular and kidney benefits of the SGLT2i have continued to expand to include other patient groups.11–13
To characterize the patterns in prescriber practices and temporal trends in the use of SGLT2i in the context of evolving data on their benefits, national clinician-level prescription data for Medicare beneficiaries were used. We evaluated both the proportion of clinicians prescribing other anti-hyperglycemic agents who also prescribed SGLT2i, and assessed the number of beneficiaries for each clinician who received SGLT2i prescriptions relative to the number of beneficiaries receiving other common second-line antihyperglycemic classes.
METHODS
Study Outline
We defined a subset of all national Medicare prescribers as diabetes-treating clinicians based on their prescription of metformin, the most widely used antihyperglycemic drug. We defined the subset of these clinicians, overall and across specialties, who used SGLT2i among their patients and evaluated the proportion of such clinicians prescribing these agents (Figure 1). Moreover, for each prescriber we evaluated the relative number of Medicare beneficiaries on their panels that received SGLT2i as compared with other second line antihyperglycemic agents, such as sulfonylurea and dipeptidyl peptidate-4 inhibitors (DPP4i) (Figure 1).
Figure 1. Study Outline.

Abbreviation: SGLT2i, sodium-glucose cotransporter-2 inhibitor.
Data Sources
To study the use of SGLT2i by clinicians providing care to patients with T2DM, data from the annual Medicare Part D Prescriber Public Use Files for the years 2014 through 2018, the most recent years available, were used. The dataset includes information from all prescription medication events for beneficiaries enrolled in the Part D program for each year, representing prescription data for approximately 70 percent of all Medicare beneficiaries.14 All prescribers, including physicians and advanced practice providers, with a valid National Provider Index (NPI) and at least 10 claims for one or more medications, the reporting threshold for data privacy under the Centers for Medicare and Medicaid Services, are included. The data are aggregated and available at a prescriber-medication-level, containing the total number of beneficiaries and the total number of prescriptions dispensed. Information on geographic location as well as specialty descriptions for each provider were also identified. In addition, the Medicare Physician Compare Database was used to identify other prescriber characteristics, including medical school attended and graduation year. These data were used to characterize prescription patterns across clinician groups. The data use in the study are publicly available and the code required for the replication of the analyses are available from the authors upon request.
Covariates
Medications were identified based on their generic names and their combinations. Specifically, prescriptions were identified for all SGLT2i available in the dataset, including empagliflozin, canagliflozin, dapagliflozin, and their combinations with other medications. Prescription for three other antihyperglycemic therapies was also identified – metformin, sulfonylureas, and DPP4i across all their formulations and combinations (Supplemental Table I). We focused our analyses on oral antihyperglycemic agents, thereby not including glucagon-like peptide receptor agonists despite their cardioprotective effects.
In addition, characteristics of clinicians were identified, including their primary specialty (internal medicine, family medicine, endocrinology, cardiology, others), medical school attended, years since graduation from medical school, and practice region. Provider specialty was available as standard Medicare specialty code, which was mapped to the National Plan and Provider Enumeration System and National Uniform Claim Committee taxonomies. Practice region was categorized into the four census regions – Northeast, Midwest, South and West.
Study Population
Each unique prescriber located in the 50 states and District of Columbia in a given year between 2014 and 2018 in the Part D Prescriber dataset was identified. Among these, a population of providers was defined who were likely managing hyperglycemia for patients with T2DM. This was defined as the group prescribing metformin, the first line therapy for glucose control. Additionally, we confirmed that metformin prescribers consistently prescribed sulfonylurea and SGLT2i across specialties (Figure 2). To identify clinicians who routinely managed patients with diabetes in their practice, as opposed to specialists and hospital medicine physicians not providing longitudinal diabetes care, sensitivity analyses identified high-volume metformin prescribers. This group was defined based on a volume threshold based on the number of unique Medicare beneficiaries who received metformin per prescriber in the data. We set the threshold at 25 patients, the median number of unique beneficiaries receiving metformin across all metformin prescribers.
Figure 2. Percentage of prescribers using the anti-hyperglycemic agents metformin, sulfonylureas, sodium glucose cotransporter-2 inhibitors (SGLT2i) across of all prescribers using any of these three medications in selected specialties in 2018.
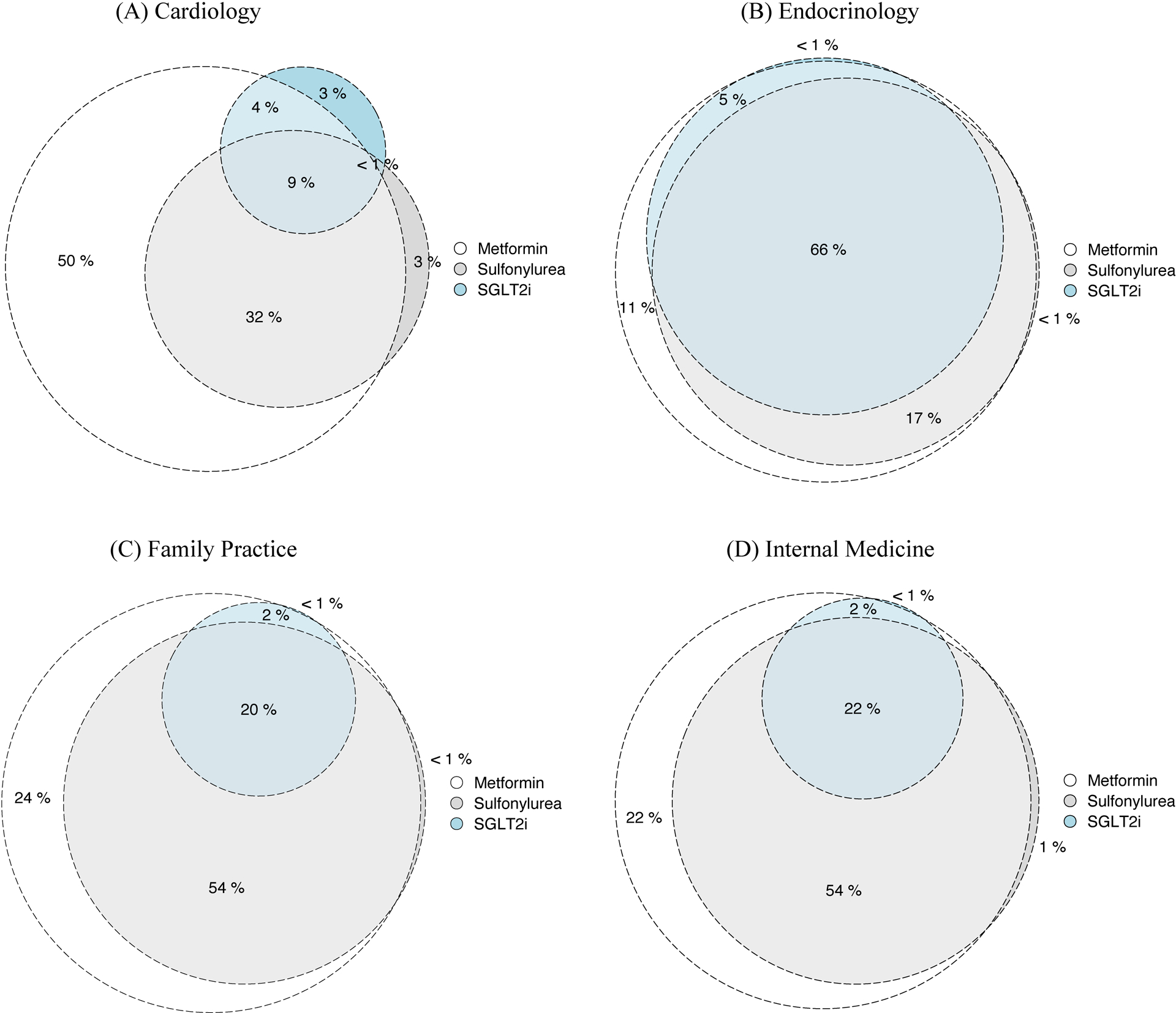
(A) Cardiology, (B) Endocrinology, (C) Family Practice, and (D) Internal Medicine.
In order to place SGLT2i prescribers and their prescriptions in proper context, a subset of the hyperglycemia treating group was identified as clinicians that also prescribed sulfonylureas (Figure 1), the most commonly prescribed second-line antihyperglycemic medication. This group was identified so that the relative prescribing habits for sulfonylureas versus SGLT2i, two second-line classes of glucose-lowering medications could be compared, the former with no intrinsic cardiac or kidney benefits versus the latter which does. Prescription of sulfonylureas allowed for the identification of treatment of patients with a more advanced stage of diabetes than what could be treated with metformin alone. Groups of DPP4i prescribing clinicians were also identified in sensitivity analyses.
Statistical Analyses
The primary analysis was a cross-sectional analysis of patterns of SGLT2i prescription across different clinician subgroups as well as their prescription volumes in 2018. Metrics reported include the number of providers prescribing SGLT2i as well as the number of unique beneficiaries prescribed these medications. For records where a medication was reported but exact counts of beneficiaries receiving medications were marked as <10 in accordance with reporting rules of the Centers for Medicare and Medicaid Services, a count of 5 was assigned for analyses that required beneficiary counts. Using data on characteristics of Medicare prescribers, differences were examined for SGLT2i use across clinician specialty, practice location and duration of practice.
First, the proportion of clinicians prescribing SGLT2i was calculated out of those clinicians prescribing metformin to their patients since metformin is considered the first-line treatment for T2DM and is the most widely prescribe glucose-lowering agent. Individual provider characteristics independently associated with any use of SGLT2i were identified using multivariable logistic regression. In this model, prescribers with any reported SGLT2i prescription were defined as a dependent variable and prescriber characteristics of clinical specialty, nature of medical/advanced practice provider (APP) school (US vs international), and years since medical/APP school graduation, as independent variables. In sensitivity analyses, high-volume metformin prescribers were defined by above-median metformin prescription volume (≥25 beneficiaries a year).
In prescription volume analyses, the number of beneficiaries for a clinician that received SGLT2i prescription was compared to their respective sulfonylurea and DPP4i prescriptions. The Kruskal-Wallis test was used to compare beneficiary counts for SGLT2i prescriptions for individual clinicians across clinician characteristics.
The prescription frequency of SGLT2i was assessed over the 5 years of study 2014–2018 spanning a period that encompassed the cardiovascular clinical outcomes trials results and regulatory approval of multiple cardiovascular indications for the SGLT2i class, and the initial evolution of treatment guidelines emphasizing the early use of SGLT2i in at-risk patients.6 Changes in the proportion of clinicians prescribing SGLT2i were assessed over the 5-year period, overall and by specialty, and for each of the SGLT2i. Trends in number of prescribers and SGLT2i prescription volumes were assessed using the Jonckheere–Terpstra trend test.
Analyses were performed using R 4.0 and Python 3.9, and all statistical tests used a 2-sided level of significance of 0.05. As the data are publicly available and deidentified, they represent non-human subject research under the regulations of the US Department of Health and Human Services (45 CFR 46), precluding informed consent, and were outside the purview of the Yale Institutional Review Board.
RESULTS
SGLT2i Prescription Practices of Clinicians Treating Hyperglycemia in Patients with T2DM
In 2018, of the 944,449 unique US clinicians treating Medicare beneficiaries, 232,523 prescribed metformin, defined by prescribing these medications to any Medicare beneficiary. A fifth (19.5%), or 45,255, prescribed any SGLT2i. The proportion of metformin-prescribing clinicians prescribing SGLT2i varied across specialties, including 71.6% endocrinologists, 23.3% internal medicine physicians, 21.4% family medicine physicians, and 13.7% cardiologists (Table 1, Supplemental Table II). Additional characteristics of SGLT2i prescribing clinicians are summarized in Table 1.
Table 1: Prescriber Characteristics among Metformin-Prescribing Clinicians Prescribing sodium-glucose cotransporter 2 inhibitors (SGLT2i) over time.
The table includes metformin-prescribing clinicians that also prescribe SGLT2i for each category, with each cell representing numerator counts and percentages. The denominator of the prescriber counts for metformin-prescribing clinicians are included in Supplemental Table II.
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Number of Prescribers | 9048 (4.3) | 24628 (11.3) | 34106 (15.1) | 40640 (17.7) | 45255 (19.5) |
| Specialty | |||||
| Cardiology | 47 (1.1) | 137 (3.6) | 194 (5.5) | 294 (8.9) | 420 (13.7) |
| Endocrinology | 1452 (32) | 2604 (55.5) | 2955 (60.7) | 3348 (66.7) | 3681 (71.6) |
| Family Practice | 2902 (3.8) | 9042 (11.8) | 12819 (16.6) | 15203 (19.6) | 16544 (21.4) |
| Internal Medicine | 3224 (5.1) | 8398 (13.3) | 11157 (17.7) | 13028 (20.9) | 14230 (23.3) |
| Other | 1423 (2.2) | 4447 (6.3) | 6981 (9.1) | 8767 (10.7) | 10380 (12.1) |
| Region | |||||
| Midwest | 1758 (3.7) | 5136 (10.4) | 7254 (14.4) | 8680 (16.9) | 9857 (19) |
| Northeast | 1972 (4.5) | 4849 (10.9) | 6701 (14.5) | 8191 (17.5) | 9284 (19.7) |
| South | 3771 (5.1) | 10700 (14.1) | 14761 (18.9) | 17279 (21.7) | 18610 (23.1) |
| West | 1547 (3.2) | 3943 (8) | 5390 (10.6) | 6490 (12.5) | 7504 (14.2) |
| Graduation Year | |||||
| < 1980 | 1180 (6.5) | 2882 (16) | 3723 (20.9) | 4335 (24.5) | 4612 (26.6) |
| 1980 – 1989 | 2367 (6.9) | 5980 (17.3) | 7868 (22.9) | 9063 (26.6) | 9784 (29) |
| 1990 – 1999 | 2368 (5.5) | 6321 (14.6) | 8625 (19.9) | 10258 (23.6) | 11044 (25.5) |
| 2000 – 2009 | 1428 (3.7) | 4395 (11.1) | 6366 (15.7) | 7632 (18.6) | 8848 (21.5) |
| 2010 – 2018 | 177 (1.1) | 949 (4.1) | 2164 (7.1) | 3535 (9.4) | 5132 (11.9) |
| School | |||||
| Non-US MD | 2647 (6.2) | 6884 (13.7) | 9405 (18.0) | 11382 (21.2) | 12750 (23.7) |
| US MD/DO | 4252 (5.4) | 11398 (14.2) | 15637 (19.1) | 18584 (22.4) | 20729 (24.8) |
| NP/PA | 1006 (2.4) | 3394 (7.0) | 5538 (10.3) | 7058 (12.0) | 8499 (13.4) |
Abbreviations: DO: Doctor of Osteopathic Medicine, MD: Doctor of Medicine, NP: Nurse Practitioner, PA: Physician Assistant
While a minority of metformin prescribing family medicine and internal medicine physicians prescribed SGLT2i, they represented the largest group of SGLT2i prescribers, owing to their overall larger numbers. Of all SGLT2i prescribers, 36.3% were family practice providers and 31.3% were internists. Endocrinologists and cardiologists represented 8.1% and 1.2% of SGLT2i prescribers, respectively, while APPs represented 18.8% of SGLT2i prescribers. In adjusted analyses, endocrinologists compared with other specialty clinicians treating hyperglycemia of T2DM, and clinicians who were older medical graduates compared with graduates from the most recent decade were most likely to prescribe SGLT2i (Supplemental Figure I).
Of the 45,255 total clinicians who prescribed SGLT2i in 2018, 25,987 (57.4%) prescribed canagliflozin, 24,769 (54.7%) empagliflozin, and 14,476 (32.0%) dapagliflozin. Of these, 29,644 (65.5%) prescribed only one SGLT2i, 11,245 (24.8%) two, and 4,366 (9.6%) prescribed all three. There were differences across specialties, with lower prescription of canagliflozin and higher empagliflozin prescription by cardiologists compared with other specialties (Figure 3B).
Figure 3. Drug-level patterns in sodium-glucose cotransporter 2 inhibitors (SGLT2i) prescribing.
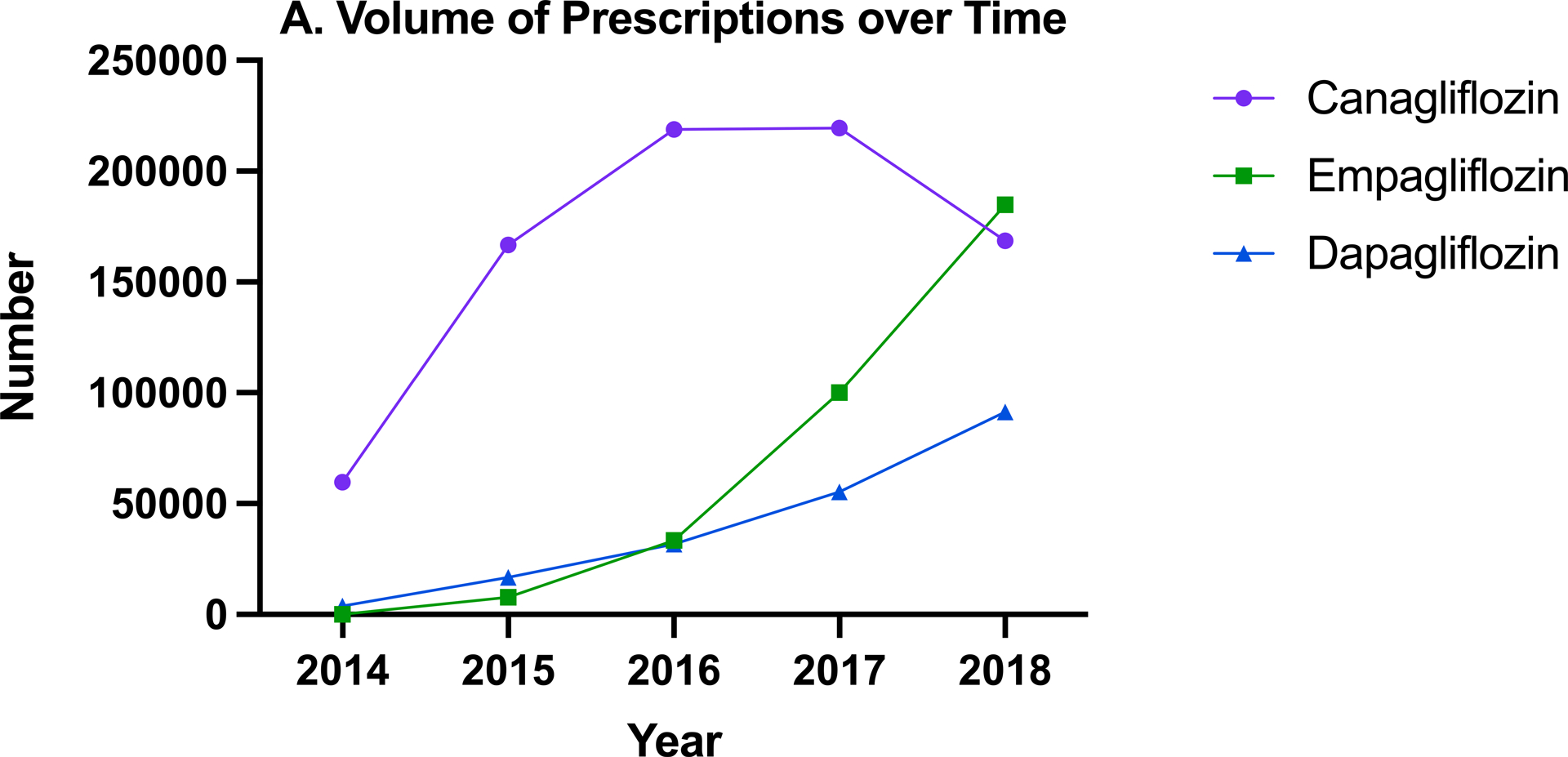

(A) Total number of beneficiaries receiving canagliflozin, empagliflozin, and dapagliflozin prescriptions over time and (B) Percentage of SGLT2i prescribing clinicians who prescribed each generic medication by specialty in 2018
In sensitivity analyses, focusing on 113,981 above-median metformin prescription (≥25 beneficiaries a year), 39,343 (34.5%) also used SGLT2i, with 83.8% and 35.8% prescribers in endocrinology and cardiology, respectively, representing groups with the highest percentage of SGLT2i use. (Supplemental Figure II).
Proportionate use of SGLT2i relative to other second line medications among their Prescribers
In 2018, of the 154,163 metformin-prescribing clinicians who also prescribed sulfonylurea, 40,884 (26.5%) used SGLT2i. Median relative SGLT2i use was defined as the median of the ratio of the number of beneficiaries each clinician prescribed SGLT2i to the number of beneficiaries each clinician prescribed sulfonylureas. Among these 40,884 clinicians 33 patients were prescribed SGLT2i for every 100 prescribed sulfonylureas (IQR 18, 67) in 2018. Cardiologists had the highest use of SGLT2i relative to their sulfonylurea use (59 per 100), followed by endocrinologists (44 per 100 sulfonylureas). In contrast, the family medicine and internal medicine physicians used SGLT2i in 32 and 28 patients respectively per 100 prescribed sulfonylureas (Supplemental Table III).
In sensitivity analyses focusing on relative SGLT2i use in high-volume metformin prescribers, among the 37,348 clinicians who prescribed metformin to 25 or more patients and also prescribed sulfonylureas and SGLT2i, 31 patients were prescribed SGLT2i for every 100 prescribed sulfonylureas (IQR 17, 56).
In the evaluation of proportionate usage of SGLT2i relative to a different second-line anti-hyperglycemic agent, DPP4i, of the 118,091 metformin-prescribing clinicians who also prescribed DPP4i, 39,511 (33.5%) used SGLT2i. Among these clinicians, 50 patients were prescribed SGLT2i for every 100 prescribed DPP4i (IQR 33, 100) in 2018. Clinicians who prescribed metformin to 25 or more patients also had a relative SGLT2i prescription of 50 for every 100 prescribed DPP4i (IQR 31, 100).
Trends in Prescription Over Time
The number of unique metformin-prescribing providers prescribing SGLT2i increased from 9,048 in 2014 to 45,255 in 2018. This corresponded to 4.3% of providers treating hyperglycemia in 2014 to 19.5% in 2018.
The percentage of metformin-prescribing providers who prescribed SGLT2i medications increased across all specialties (Figure 4), with the highest percentage use among endocrinologists across the study years, increasing from 32.0% in 2014 to 71.6% in 2018 in this group, followed by internal medicine (5.1% to 23.3%), family practitioners (3.8% to 21.4%), and cardiologists (1.1% in 2014, 13.7% in 2018). In addition to an increase in the number of clinicians prescribing SGLT2i, both the total number of patients prescribed SGLT2i, and the mean number of patients prescribed SGLT2i per provider increased across specialties (Supplemental Figure III). Proportionate utilization of SGLT2i per prescriber also increased from 19 prescribed SGLT2i (IQR 11, 34) per 100 prescribed sulfonylureas in 2014 to 33 (IQR 18, 67) in 2018 (P for trend <0.001). This pattern was also consistent across specialties (Supplemental Table III).
Figure 4.
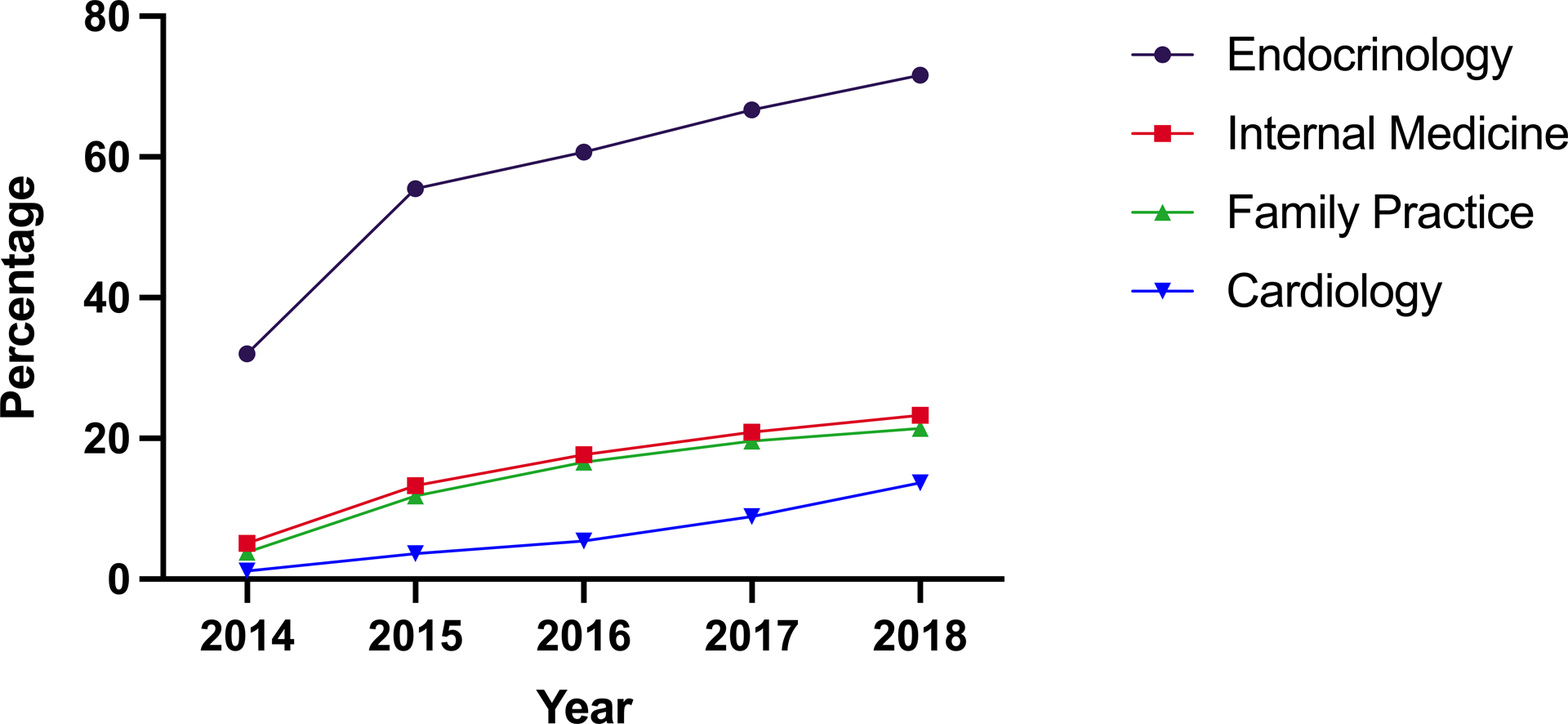
Percentage of metformin-prescribing clinicians who also prescribed sodium-glucose cotransporter-2 inhibitor (SGLT2i), by specialty, over time
There were also notable trends for the individual SGLT2i medications. Between 2014 and 2018, the percentage of SGLT2i prescribing clinicians using canagliflozin decreased from 97.2% to 57.4%, while those prescribing empagliflozin increased from 0.1% to 54.7% and dapagliflozin from 7.0% to 32.0% (Supplemental Figure IV). There was a difference in patterns amongst cardiologists. In 2017, more cardiologists prescribed empagliflozin (60.5%) than canagliflozin (47.6%), and by 2018 the percentage of cardiologists prescribing empagliflozin had increased to 76.0% and there were even more clinicians prescribing dapagliflozin (29.0%) than canagliflozin (28.6%). The total volumes of canagliflozin, empagliflozin, and dapagliflozin prescribed all increased over the five-year period of study (Figure 3A). Although canagliflozin had the highest volume of prescriptions from 2014 to 2017, it was surpassed by empagliflozin in 2018, as the total volume of canagliflozin prescription fell 23.2% from 2017 while the total volume of empagliflozin prescription increased 84.5% during the same period.
DISCUSSION
In a national cohort of clinicians prescribing antihyperglycemic therapy for patients with T2DM, only 1 in 5 prescribed SGLT2i, with only 1 in 7 cardiologists who treated hyperglycemia prescribing these cardioprotective agents. General medicine clinicians, including internists and family practice providers represent the largest category of clinicians prescribing glucose-lowering medications, but over three-fourths of these providers did not prescribe SGLT2i at all. Moreover, clinicians who have incorporated SGLT2i into their practices still prescribe sulfonylureas, agents without any known direct cardiovascular benefits, to about three times as many patients. Even among clinicians who were among the highest volume prescribers of antihyperglycemic agents, over half did not prescribe any SGLT2i. The use of SGLT2i by only a minority of clinicians treating Medicare beneficiaries with common T2DM medications highlights the need to identify challenges to their wider use, be they related to awareness or prescriber/patient reticence due to insurance coverage, costs, and/or potential side effects.
The SGLT2is represent the first class of antihyperglycemic therapies to demonstrate consistent improvement in cardiovascular and kidney outcomes among patients with T2DM, including lower risk of cardiovascular death as labeled for empagliflozin, reduced risk for MACE and for kidney disease progression for canagliflozin and reduced risk for hospitalization for heart failure/cardiovascular death for dapagliflozin.1–5 Given the high prevalence of cardiovascular and chronic kidney disease among patients with T2DM, up to a half of adults with T2DM are estimated to have evidence-based indications for SGLT2i therapy.15 An even larger proportion of Medicare beneficiaries are likely to have a compelling indication for SGLT2i treatment given the high prevalence of both cardiovascular and kidney disease in this older population. Therefore, underuse in the segment of the population characterized in the present dataset represents an opportunity for reduction of possibly preventable cardiovascular events.
One of the challenges contributing to the underuse of SGLT2i may be the cost associated with their use. A typical regimen of metformin and sulfonylureas along with ACE inhibitors and a statin has an average annual cost of $250 under Medicare Part D compared with $6,164 for the same regimen with SGLT2is as the second-line medication in place of sulfonylureas.16 The large out-of-pocket costs with SGLT2i, as well as novelty of these agents may underlie the underutilization of these medications. However, given the cost-effectiveness of these medications for the society and the healthcare system even at current costs through a reduction in healthcare events and risk of death,17 our findings highlight the need to further assess the barriers at a patient-level, particular in primary care where a vast majority of patients receive medical therapy for type 2 diabetes. Moreover, other factors, such as an inertia among clinicians and patients for those stable on other agents to transition to SGLT2i may play a possible role.
The study has limitations that merit consideration. First, the study is based on prescription claims and does not include characteristics of patients, contraindications to therapy, patient preferences, and other reasons for the clinical decision to prescribe one antihyperglycemic therapy over another. Therefore, the study could not estimate the proportion of patients with indications who were prescribed these medications, nor the proportion of patients in whom it was purposefully decided not to prescribe (i.e., prior intolerance, anticipated risk of side effects). However, with nearly half of all patients with T2DM having compelling indications for the therapy,17 likely higher proportion in the Medicare population, the lack of any prescriptions of SGLT2i by four-fifths of all clinicians who treated patients with other oral anti-hyperglycemic agents suggests at least some degree of underuse at 3 years following the first published evidence for major benefits and 2 years following the first labeled indication for cardiovascular protection. While we account for clinician prescribing practices to define their use of SGLT2i, we cannot exclude if some of the clinicians systematically chose to defer the prescription of SGLT2i to other clinicians, but we would presume that this is not a common clinical scenario. Further, while theoretically clinicians could’ve prescribed some of the glucagon-like peptide receptor agonists for cardiovascular risk reduction rather than SGLT2i, a majority of clinicians did not prescribe these drugs either (Supplemental Figure I).
Second, the time period for the study, ending in 2018, may have been too early for adoption of the evidence into practice. However, our findings provide an early assessment of the prescriber-level uptake of these medications. Moreover, results from more recent studies continue to suggest use of these medications in fewer than 10% of those with compelling indications, highlighting the continued limited use.17 Third, because the study is restricted to Medicare Part D enrollees, prescription patterns for younger patients are not captured. Fourth, beneficiary counts of 10 or lower were reported as such, without reporting of the actual number of beneficiaries. Therefore, the median imputation strategy is expected to have uncertainty as the beneficiary counts could range from 1 to 10, though the effect is expected to be small. Moreover, our central findings focus on any prescriber use of these medications.
CONCLUSIONS
A large majority of US clinicians who prescribed common antihyperglycemic T2DM medications did not use SGLT2i in their practice as recent as 2018, with far fewer prescriptions for SGLT2i relative to sulfonylureas and DPP4is, which are not proven to have any cardiovascular benefits. These findings represent a baseline, highlighting the opportunity to proactively identify challenges clinicians and patients face in the wider use of SGLT2i to realize their potential public health benefits.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
Dr. Khera received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under the award K23HL153775. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures:
Dr. Krumholz works under contract with the Centers for Medicare & Medicaid Services to support quality measurement programs, was a recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Martin Baughman Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a member of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co-founder of HugoHealth, a personal health information platform, and co-founder of Refactor Health, a healthcare AI-augmented data management company. Dr. McGuire has received honoraria or consultancy fees from Afimmune, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eisai, Esperion, GlaxoSmithKline, Janssen Research and Development LLC, Lexicon, Lilly USA, Merck Sharp & Dohme, Metavant, Novo Nordisk, Pfizer and Sanofi US. Dr. Lipska works under contract with Centers for Medicare & Medicaid Services (CMS) to develop and evaluate publicly reported quality measures. Dr. Inzucchi has received honoraria or consultancy fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, vTv Therapeutics, Esperion, and Abbott. The other authors do not report any relevant disclosures. Dr. Khera is the coinventor of U.S. Provisional Patent Application No. 63/177,117, “Methods for neighborhood phenomapping for clinical trials”, and is a founder of Evidence2Health, a precision health and digital health analytics platform.
Non-standard Abbreviations and Acronyms
- SGLT2i
Sodium-glucose cotransporter-2 inhibitor
- T2DM
Type 2 diabetes mellitus (T2DM)
- ASCVD
atherosclerotic cardiovascular disease
- DPP4i
Dipeptidyl peptidate-4 inhibitors
Footnotes
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 2.Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I and Sabatine MS. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. Reply. N Engl J Med. 2019;380:1881–1882. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 6.Nainggolan L FDA Approves Canagliflozin, a First-in-Class Diabetes Drug. 2013. Available at: https://www.medscape.com/viewarticle/781709. Accessed December 30, 2020.
- 7.Wood S FDA Approves CV Death Reduction Claim for Empagliflozin in Type 2 Diabetes. 2016. Available at: https://www.tctmd.com/news/fda-approves-cv-death-reduction-claim-empagliflozin-type-2-diabetes. Accessed December 30, 2020.
- 8.Vaduganathan M, Sathiyakumar V, Singh A, McCarthy CP, Qamar A, Januzzi JL Jr., Scirica BM, Butler J, Cannon CP and Bhatt DL. Prescriber Patterns of SGLT2i After Expansions of U.S. Food and Drug Administration Labeling. J Am Coll Cardiol. 2018;72:3370–3372. [DOI] [PubMed] [Google Scholar]
- 9.Gilstrap LG, Blair RA, Huskamp HA, Zelevinsky K and Normand SL. Assessment of Second-Generation Diabetes Medication Initiation Among Medicare Enrollees From 2007 to 2015. JAMA Netw Open. 2020;3:e205411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, Khatana SAM, Dayoub EJ, Fanaroff AC, Giri J, et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status With Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients With Diabetes in the US. JAMA Netw Open. 2021;4:e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services (CMS). Medicare Fee-For Service Provider Utilization & Payment Data Part D Prescriber Public Use File: A Methodological Overview. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Downloads/Prescriber_Methods.pdf. Accessed December 30, 2020.
- 15.Wong ND, Fan W and Pak J. Estimating the number of preventable cardiovascular disease events in the United States using the EMPA-REG OUTCOME trial results and National Health and Nutrition Examination Survey. Diab Vasc Dis Res. 2020;17:1479164120945674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeJong C, Masuda C, Chen R, Kazi DS, Dudley RA and Tseng CW. Out-of-Pocket Costs for Novel Guideline-Directed Diabetes Therapies Under Medicare Part D. JAMA Intern Med. 2020;180:1696–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong D, Si L, Jiang M, Shao H, Ming WK, Zhao Y, Li Y and Shi L. Cost Effectiveness of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists, and Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: A Systematic Review. Pharmacoeconomics. 2019;37:777–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


