Abstract
Objective. To evaluate the utility of intraoperative OCT and its influence on the surgeon’s decision during vitreoretinal surgery.
Methods. This was a pilot, prospective case series conducted at a tertiary care ophthalmology department in Lahore, Pakistan. Sixteen patients undergoing vitreoretinal surgeries were included using the Leica Enfocus microscope integrated intraoperative optical coherence tomography (IOCT). We also investigated the changes in surgical decision making based on the findings revealed by IOCT using a questionnaire.
Results. 16 patients with a mean age of 40.6 ± 19.0 (range: 11-66) years, were included in the study; one case of acute postoperative endophthalmitis could not be imaged. The surgeon had to modify surgical decisions in four (26.7%) cases. IOCT clearly delineated various tissue planes for efficient and safe surgical dissection in pathologies such as posterior vitreous detachment, vitreomacular traction and epiretinal membranes. Furthermore, it also helped identifying perfluorocarbon-retina interface.
Conclusions. The intraoperative OCT modality is a feasible and useful intraoperative imaging technique for various kinds of vitreoretinal disorders. The decision making of the surgeon was modified in a quarter of the cases after the use of this newer modality.
Keywords: intraoperative optical coherence tomography, vitreoretinal surgery, Leica Enfocus, retinal detachment, macular hole
Introduction
Optical coherence tomography (OCT) is an imaging modality that performs cross-sectional imaging of ocular tissues by measuring the magnitude and echo time delay of backscattered light from target ocular structures [1]. This modality has a vital role in the management of retinal diseases and therefore, it is prudent to consider its utilization and integration in the operating room through microscope integrated intra-operative OCT (IOCT) [2].
Previous studies have reported the utility of IOCT in various anterior and posterior segment surgeries. These include the landmark PIONEER and DISCOVER studies that used microscope mounted and microscope integrated OCT modules for IOCT [3,4]. Most of the studies have been reported by resource rich institutions with reasonable patient load and access to newer technologies [5]. Additionally, earlier studies utilized an external OCT system that required the surgeon to pause the surgical procedure to perform imaging and then take subsequent surgical decisions [3].
We report our experience with a microscope integrated IOCT system for real time imaging and tissue-instrument interaction on OCT during various vitreoretinal procedures. We aimed at highlighting the role and feasibility of the IOCT to delineate retinal structures and its ability to influence the surgeon’s decision-making process.
Method
This was a prospective, consecutive case series performed by a single surgeon (KI) in a single center. We evaluated the utility and feasibility of IOCT (Bioptigen/ Leica Microsystems, Wetzlar, Germany) in vitreoretinal surgery. The study was conducted after taking approval from the Institutional Review Board of Layton Rahmatullah Benevolent Trust, Lahore, Pakistan and adhered to the tenets of the Declaration of Helsinki. All patients were recruited after signing a written informed consent. For those who were under 18 years old, an informed consent was taken from the parents.
The inclusion criteria of our study referred to patients with a condition requiring vitreoretinal surgery, willing to undergo IOCT and either themselves or a parent were able to provide a written informed consent. All types of vitreoretinal surgeries were included.
All the surgical cases were operated with the Leica Enfocus operating microscope integrated IOCT system. This is one of the few commercially available systems that employs intraoperative OCT and gives real time per-operative imaging of the retina without having to stop the surgery.
All the diagnosis and advised surgical procedures were recorded in a pre-designed questionnaire. The operating surgeon was asked to record specific findings of the IOCT after each surgery encompassing the following three areas: identifying important vitreoretinal structures during the procedure, the impact in altering the surgeon’s decision and confirmation of the surgeon’s decision preoperatively. Questions were also asked if the surgeon had to modify the surgery considering the findings revealed by the IOCT imaging.
The statistical analysis was performed using Statistical Package for Social Sciences (SPSS version 25.0, IBM Statistics, Chicago, IL, USA). Numerical variables were calculated as mean ± standard deviation (SD), whereas descriptive variables were calculated in frequencies and percentages.
Results
A total of 16 patients, with a mean age of 40.6 ± 19.0 (range: 11-66) years, and a female to male ratio of 1:1.3, were included in the study. The most common vitreoretinal condition treated was retinal detachment (10, 62.5%). The details are provided in Table 1.
Table 1.
The utility of Intraoperative OCT in each surgery
| No. | Age (years) | Diagnosis and Relevant Retinal Findings | Vitreoretinal Surgery | Specific Indication of Using Intraoperative OCT | Utility of Intraoperative OCT | ||
|---|---|---|---|---|---|---|---|
| Identification of Important Retinal Structures | Surgeon’s Decision Making | Confirmation of Surgeon’s Surgical Decision | |||||
| 1 | 60 | Retinal Detachment | Pars plana vitrectomy (PPV) with silicon oil tamponade | To rule out any macular pathology such as macular edema or AMD | Yes | No | Yes |
| 2 | 16 | Re-Retinal Detachment | Redo PPV with silicon band | To rule out sub-retinal oil preoperatively and confirmation of retinal attachment at the end of surgery | Yes | No | Yes |
| 3 | 45 | Rhegmatogenous Retinal Detachment | PPV with Silicon Oil | To confirm presence of detachment and to rule out any sub-retinal oil after the procedure | Yes | No | Yes |
| 4 | 40 | Myopic Retinal Detachment with Proliferative vitreoretinopathy (PVR) and multiple retinal breaks. | PPV with Silicon Oil | To confirm the presence of epiretinal membrane (ERM) and completion of posterior vitreous detachment as vitreous was very adherent | Yes | Yes | Yes |
| 5 | 66 | Inferior Bullous Retinal Detachment with extensive macular scarring | Inferior Band + PPV with Silicon Oil | To confirm the cause of macular scarring, which turned out to be active choroidal neovascularization and ensure complete removal of perfluorocarbon | Yes | Yes | Yes |
| 6 | 61 | Diabetic vitreous hemorrhage with Tractional Retinal Detachment (TRD) | PPV with Silicon Oil | To ascertain the location of TRD and residual ERMs | Yes | Yes | Yes |
| 7 | 30 | Emulsified Silicon Oil causing Glaucoma | Removal of Silicon Oil | Removal of silicon oil revealed macular hole, which was confirmed using intraoperative OCT | Yes | No | Yes |
| 8 | 28 | Traumatic Retinal Detachment with ERM | PPV with Silicon oil | To confirm retina attachment and any remnant ERM after ERM Peeling | Yes | No | Yes |
| 9 | 24 | Total Retinal Detachment with PVR | Band + PPV + Silicon Oil | To determine the presence of any ERM and confirmation of complete removal of heavy liquid and retinal attachment at the end | Yes | No | Yes |
| 10 | 57 | Diabetic Vitreous Hemorrhage | PPV | To detect macular edema and other associated pathologies like diabetic TRD and ERM | Yes | Yes | Yes |
| 11 | 60 | Post-operative Endophthalmitis | Core Vitrectomy with Silicon Oil | To determine any retinal traction since the view was hazy | No | No | No |
| 12 | 15 | Retinal Detachment with very adherent PVD | PPV + Silicon Oil | To assess completion of PVD & any residual traction on the macula | Yes | No | Yes |
| 13 | 27 | Diabetic TRD with extensive ERMs | PPV + Silicon Oil | To confirm any remnant retinal tractions and ensure complete removal of ERMs | Yes | No | Yes |
| 14 | 48 | Epiretinal Membrane causing refractory diabetic macular edema | PPV + C3F8 gas | To confirm complete removal of ERM | Yes | No | Yes |
| 15 | 11 | Traumatic Macular Hole | PPV + Internal Limiting Membrane (ILM) Peel with C3F8 gas | To confirm completion of PVD in young highly adherent vitreous and determination of macular hole morphology after ILM Peel | Yes | Yes | Yes |
| 16 | 61 | Idiopathic Macular Hole | PPV + ILM Peel + SF6 gas | To confirm completion of PVD and determination of hole morphology after ILM Peel | Yes | No | Yes |
Successful image acquisition was obtained in 15 cases (93.8%). In these cases, 100% of the images identified important anatomical structures during surgery. A case with acute postoperative endophthalmitis after cataract surgery yielded poor images that could not be analyzed.
IOCT caused a direct change in decision
The IOCT images helped modify the surgeon’s decision making in four cases (26.7%). In two cases of vitrectomy in young patients, the IOCT helped visualize highly adherent vitreous at the posterior pole temporal to the disc, which was not apparent on the surgical microscope. An example is shown in Fig. 1. The vitreous was then detached, thus completing the PVD before proceeding to the next step.
Fig. 1.
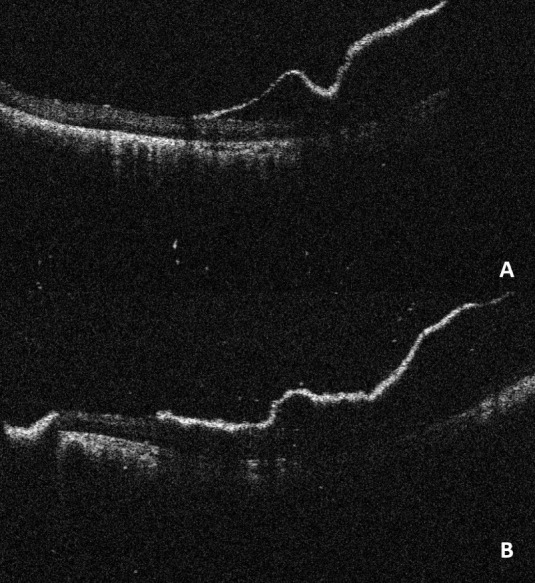
(A) Vitreous is still attached temporal to the disc; (B) Vitreous was then detached before closure
In other two cases, IOCT helped identify mild subretinal fluid in cases of retinal detachment with an apparently reattached retina. In these cases, the subretinal fluid was drained and a reattached retina was confirmed. One of these is shown in Fig. 2.
Fig. 2.
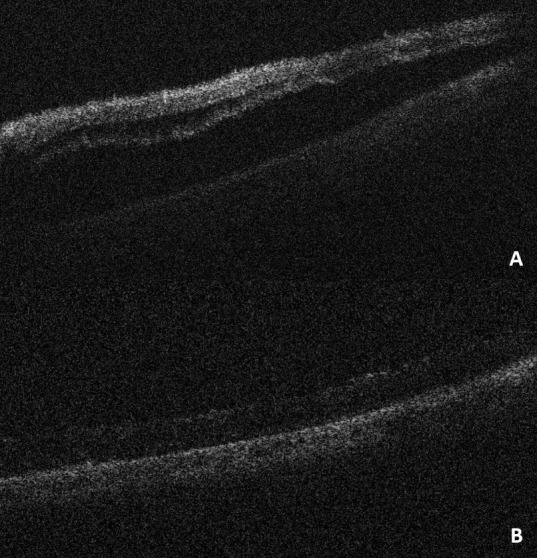
(A) Evidence of subretinal fluid post-surgery without the help of the intraoperative OCT. (B) Re-attached retina post drainage of the subretinal fluid
IOCT aided in the decision-making process
In two cases of proliferative vitreoretinopathy (PVR), the modality helped image the extent and location of epiretinal membranes (ERMs). Additionally, the IOCT helped view subretinal fibrosis and the presence of any subretinal oil intraoperatively.
In cases of retinal detachment, IOCT was helpful in the confirmation of reattachment, and therefore, the injection of oil could be made with confidence. Additionally, it helped confirm the complete removal of perfluorocarbon heavy liquid.
Lastly, we could confirm a complete ILM peel in a case of macular hole. The IOCT showed a decrease in paramacular thickness, as shown in Fig. 3.
Fig. 3.
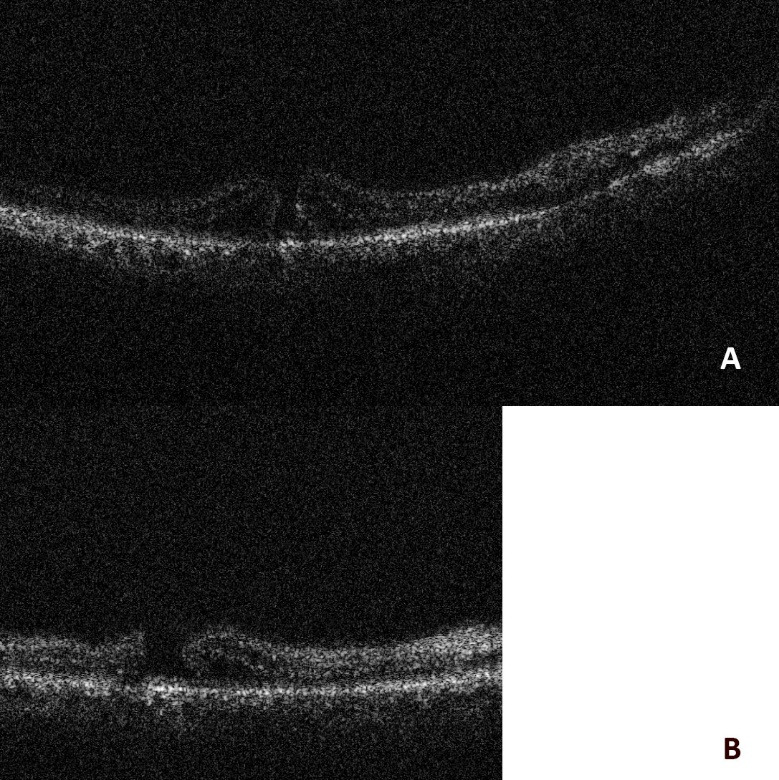
(A) Prior to ILM peeling; (B) Post ILM peeling - the paramacular thickness has decreased
Discussion
The incorporation of OCT in the operating room is expected to yield positive results due to its high resolution and quick scans. Earlier studies have reported the usefulness of IOCT in cases like vitreomacular traction, macular holes and retinal detachment [6-9]. The PIONEER (Prospective Intraoperative and Perioperative Ophthalmic Imaging with Optical Coherence Tomography) study in 2014 was the first large scale study to demonstrate the benefit of IOCT in an ophthalmic surgical setting [4]. Subsequently, the DISCOVER (Determination of Feasibility of Intraoperative Spectral Domain Microscope Combined/ Integrated OCT Visualization during EnFace Retinal and Ophthalmic Surgery) study, in 2018, further demonstrated the use of this technology in specific cases [10].
The IOCT altered the surgeon’s decision in the posterior segment between 29.2% and 43% in the DISOVER and PIONEER studies, respectively [5,10]. Our study agrees with this assessment, having a rate of 26.2%. This modality was especially useful in young adults with adherent vitreous attachments. Induction of posterior vitreous detachment in such cases can be quite difficult and can be missed. If vitreous strands are cut superficially, a fine layer of vitreous remains adherent to the underlying posterior pole near the disc and the macula.
Additionally, IOCT helped us in cases of epiretinal membranes (ERMs) and tractional retinal detachments (TRDs), like in previous studies [5]. The device offered fine details of retinal tissue and overlying membranes helping identify a complete membrane peel. Similarly, during vitrectomy of diabetic TRDs, the system helped clear delineation of tissue planes for efficient and accurate dissection of tissues. Lastly, the IOCT helped confirm a complete reattachment of the retina at the end of surgery.
Other cases of use for this novel system have been demonstrated, including anterior segment surgeries like keratoplasty, Descemet’s membrane stripping endothelial keratoplasty [8], and subretinal injection of stem cells of gene therapy [11,12]. Future studies are still required to understand the complete use of the IOCT system.
This study had various limitations that need discussing. Firstly, this was a pilot study of a prospective long-term study and therefore, the sample size was limited. Secondly, the modality is still relatively novel and understanding the system can take time. Lastly, the surgeon had full knowledge of IOCT systems availability and therefore it may have biased him towards the use of the system.
Conclusion
The IOCT is a feasible imaging technology for various kinds of vitreoretinal disorders that can significantly affect surgical plans.
Conflict of Interest statement
Authors state no conflict of interest.
Informed Consent and Human and Animal Rights statement
Informed consent has been obtained from all individuals included in this study.
Authorization for the use of human subjects
Ethical approval: The research related to human use complies with all the relevant national regulations, institutional policies, is in accordance with the tenets of the Helsinki Declaration, and has been approved by the Institutional Review Board of Layton Rahmatullah Benevolent Trust, Lahore, Pakistan.
Acknowledgements
None.
Sources of Funding
None.
Disclosures
None.
References
- 1.Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT27–OCT36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehlers JP. Intraoperative optical coherence tomography: past, present, and future. Eye. 2016;30(2):193–201. doi: 10.1038/eye.2015.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers JP, Goshe J, Dupps WJ, Kaiser PK, Singh RP, Gans R, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015;133(10):1124–1132. doi: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98(10):1329–1332. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Khan M, Petkovsek D, Stiegel L, Kaiser PK, Singh RP, et al. Outcomes of intraoperative OCT–Assisted epiretinal membrane surgery from the pioneer study. Ophthalmol Retin. 2018;2(4):263–267. doi: 10.1016/j.oret.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan M, Ehlers JP. Clinical utility of intraoperative optical coherence tomography. Curr Opin Ophthalmol. 2016;27(3):201. doi: 10.1097/ICU.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ung C, Miller JB. Intraoperative optical coherence tomography in vitreoretinal surgery. In: Seminars in ophthalmology. Taylor & Francis; 2019. pp. 312–316. [DOI] [PubMed] [Google Scholar]
- 8.Pujari A, Agarwal D, Chawla R, Kumar A, Sharma N. Intraoperative optical coherence tomography guided ocular surgeries: critical analysis of clinical role and future perspectives. Clin Ophthalmol (Auckland, NZ) 2020;14:2427. doi: 10.2147/OPTH.S270708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfau M, Michels S, Binder S, Becker MD. Clinical experience with the first commercially available intraoperative optical coherence tomography system. Ophthalmic Surgery, Lasers Imaging Retin. 2015;46(10):1001–1008. doi: 10.3928/23258160-20151027-03. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers JP, Modi YS, Pecen PE, Goshe J, Dupps WJ, Rachitskaya A, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology. 2018;125(7):1014–1027. doi: 10.1016/j.ophtha.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregori NZ, Lam BL, Davis JL. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina. 2019;39:S9–S12. doi: 10.1097/IAE.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Tang L, Zhou Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58(4):217–226. doi: 10.1159/000479157. [DOI] [PubMed] [Google Scholar]


