Abstract
Background
Obesity is increasingly prevalent, yet the nutritional management remains contentious. It has been suggested that low glycaemic index or load diets may stimulate greater weight loss than higher glycaemic index or load diets or other weight reduction diets.
Objectives
To assess the effects of low glycaemic index or load diets for weight loss in overweight or obese people.
Search methods
Trials were identified through The Cochrane Library, MEDLINE, EMBASE, CINAHL and manual searches of bibliographies.
Selection criteria
Randomised controlled trials comparing a low glycaemic index or load diet (LGI) with a higher glycaemic index or load diet or other diet (Cdiet) in overweight or obese people.
Data collection and analysis
Two authors independently selected trials, assessed quality and extracted data, including any information provided on adverse effects.
Main results
We identified six eligible randomised controlled trials (total of 202 participants). Interventions ranged from five weeks to six months duration with up to six months follow‐up after the intervention ceased. The decrease in body mass (WMD ‐1.1 kg, 95% confidence interval (CI) ‐2.0 to ‐0.2, P < 0.05) (n = 163), total fat mass (WMD ‐1.1 kg, 95% CI ‐1.9 to ‐0.4, P < 0.05) (n =147) and body mass index (WMD ‐1.3, 95% CI ‐2.0 to ‐0.5, P < 0.05) (n = 48) was significantly greater in participants receiving LGI compared to Cdiets. The decrease in total cholesterol was significantly greater with LGI compared to Cdiets (WMD ‐0.22 mmol/L, 95% CI ‐0.43 to ‐0.02, P < 0.05), as was the change in LDL‐cholesterol (WMD ‐0.24 mmol/L, 95% CI ‐0.44 to ‐0.05, P < 0.05). No study reported adverse effects, mortality or quality of life data.
Authors' conclusions
Overweight or obese people on LGI lost more weight and had more improvement in lipid profiles than those receiving Cdiets. Body mass, total fat mass, body mass index, total cholesterol and LDL‐cholesterol all decreased significantly more in the LGI group. In studies comparing ad libitum LGI diets to conventional restricted energy low‐fat diets, participants fared as well or better on th LGI diet, even though they could eat as much as desired. Lowering the glycaemic load of the diet appears to be an effective method of promoting weight loss and improving lipid profiles and can be simply incorporated into a person's lifestyle. Further research with longer term follow‐up will determine whether improvement continues long‐term and improves quality of life.
Plain language summary
Low glycaemic index or low glycaemic load diets for overweight and obesity
There is a lack of consensus as to the best nutritional management of obesity. We assessed the effects of low glycaemic index or glycaemic load diets in overweight or obese people. Six randomised controlled trials, involving 202 participants, were analysed. Interventions ranged from five weeks to six months duration. Participants receiving the low glycaemic index or load diet lost a mean of one kilogramme more than those on comparison diets. Lipid profile also improved more in participants receiving the low glycaemic index or load diet. No study reported adverse effects, mortality or quality of life data.
Background
Description of the condition
Obesity is defined as an increase in body weight beyond the limitation of skeletal and physical requirement, as the result of an excessive accumulation of fat in the body. On the body mass index scale (Body Mass Index (BMI) = body mass (kg)/height (m2)), obesity can be defined as a BMI of greater than 30. Overweight is the borderline condition between normal weight and obesity and can be classified as a BMI of 25 to 30 (WHO 1997). However, the definition can vary from country to country, or from time to time. The prevalence of overweight and obesity is rapidly increasing worldwide (Strauss 2001; WHO 1997). Obesity is associated with higher rates of abnormal glucose tolerance, hypertension and hyperlipidaemia. Despite its prevalence, the prevention and management of obesity remains contentious. Obesity is the single most frequent risk factor for type 2 diabetes (Mokdad 2001). As a result of the obesity epidemic, the prevalence of type 2 diabetes is increasing and is being diagnosed at increasingly younger ages (Dietz 1998; Silink 2002). The increase in type 2 diabetes in obese people may relate to the relative insulin resistance obesity confers. Poorly controlled diabetes may be complicated by retinopathy, nephropathy, neuropathy and vascular disease. Good glycaemic control is crucial to reduce the complications of disease, to improve the quality and duration of life and to minimise the need for expensive health care.
Description of the intervention
Currently, the nutritional management of overweight and obesity varies greatly due to a lack of consensus among clinicians as to the best approach. This in part reflects the lack of good quality trials. Clinicians have been "hampered by the lack of evidence‐based evaluation and guidance on the range of interventions they might use (for obesity), ranging from diets, drug therapy, surgery, and innovations such as exercise on prescription" (Parliament 2003). Authors of a systematic review of low‐carbohydrate diets found that there was insufficient evidence to make recommendations for or against their use. They found that weight loss was associated with an overall decrease in caloric intake and longer diet duration, rather than reduced carbohydrate content per se (Bravata 2003). In two recent Cochrane systematic reviews in children, a variety of exercise, educational, dietary interventions and lifestyle adjustments were evaluated for preventing and treating obesity in children, indicating the numerous approaches available (Campbell 2002; Summerbell 2003). However, the role of low glycaemic index diets for prevention or management of obesity was not within the scope of any of these reviews.
How the intervention might work
The glycaemic index factor is a ranking of foods based on their overall effect on blood sugar levels (Jenkins 1981). Low glycaemic index foods, such as lentils, provide a slower more consistent source of glucose to the bloodstream, thereby stimulating less insulin release than high glycaemic index foods, such as white bread (Jenkins 1981). Hence, low glycaemic index foods may increase insulin sensitivity by minimising fluctuations in blood glucose levels and reducing the secretion of insulin over the day (Kiens 1996). There is some evidence that even when the kilo joule intake is the same, low glycaemic index food diets may stimulate more weight loss in obese people than high glycaemic index diets (Brand‐Miller 2002). One review highlighted the possible usefulness of low glycaemic index foods in the management of obesity (Pawlak 2002). However, there is controversy about their role. In another review looking at the outcomes of appetite, food intake, energy expenditure and body weight, the authors concluded that there is currently no evidence that low glycaemic index foods are superior to high glycaemic index foods in regard to long‐term body weight control (Raben 2002). However, this review included some studies that may have been underpowered, contained confounding factors or in which follow‐up was too short to observe an effect.
The glycaemic load (GL) of a food is calculated as the carbohydrate content (g) multiplied by the glycaemic index value of the food and divided by 100: GL = CHO (g) x GI /100. The total glycaemic load of a menu is the sum of all the individual glycaemic load values for the foods in the menu (Ebbeling 2003).
Why it is important to do this review
Our systematic review may clarify issues surrounding the role of low glycaemic index or load diets in the management of obesity and overweight. If alterations in the glycaemic index or load of the diet alone can increase insulin sensitivity, decrease weight, or decrease poor health outcomes in obesity and overweight (including type 2 diabetes and its associated complications), then the use of low glycaemic index diets would have significant health and cost benefits for the community.
Objectives
To assess the effects of low glycaemic index or low glycaemic load diets on weight loss in people who are overweight or obese.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Trial design
We considered all randomised controlled trials that compared a low glycaemic index or load diet with a higher glycaemic index or load diet for weight loss in overweight and obesity.
Trial duration
We included trials with dietary interventions lasting two weeks or longer. Efficacy was assessed as short term (if follow‐up was less than six months), intermediate (six months to less than 12 months) and long‐term (12 months and over).
Exclusion criteria
We excluded studies in which the intervention was only a generalised recommendation to increase the proportion of low glycaemic index foods in the diet, or to reduce the glycaemic load, without provision of explicit detail; studies in which the intervention was either not directly supervised or well‐documented (for example by the use of food diaries or the provision of food); studies in which there was a co‐intervention in the experimental group that was not also applied to the control group; studies in which the explicit aim of the study was not weight reduction; and studies in which the final outcome measurements for the intervention and comparator groups were not sampled at the same time point after the intervention. For example, one study defined the endpoint of the trial as the time when participants achieved 10% weight reduction (Pereira 2005) and was excluded from this review because the timing of the assessment ranged from six to ten weeks after the intervention.
Types of participants
Participants were males and females of any age who were classified as overweight or obese using validated and specified criteria. People with diabetes mellitus were excluded.
Types of interventions
We included studies that compared a low glycaemic index, or low glycaemic load, diet with a higher glycaemic index or load diet or any other diet.
Types of outcome measures
Primary outcomes
body mass (kg), body mass index (BMI), BMI adjusted for age;
adiposity (cm2) and fat distribution (total fat mass, fat free mass, truncal to peripheral fat ratio (DXA), visceral fat (MRI), abdominal fat (DXA, MRI), lean body mass, percentage body fat content, skin fold thickness, ponderal index, waist, waist to hip ratio, visceral fat);
adverse effects.
Secondary outcomes
insulin action (fasting plasma insulin, insulin sensitivity, insulin area under the curve, total insulin released per day, insulin:glucose ratio, homeostasis model assessment (of insulin sensitivity) (HOMA), quantitative insulin‐sensitivity check index (QUICKI));
glycaemic control (glycosylated haemoglobin, glucose area under the curve, fasting plasma glucose, glucose tolerance test, post prandial plasma glucose levels, fructosamine);
cardiovascular risk factors ‐ lipid metabolism (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, fat oxidation, plasma levels of enzymes or hormones involved in lipid metabolism), blood pressure, oxidative stress, inflammation of the endothelium, C‐reactive protein;
satiety (questionnaires using validated scales, amount of food eaten ad libitum post ‐intervention phase, post prandial plasma glucose levels);
other metabolic indices (resting metabolic rate, leptin, C‐peptide excretion);
quality of life (using validated instruments such as SF‐36, Euroquol);
mortality.
Timing of outcome assessment (length of intervention)
Studies were classified as short term (less than six months), medium term (six to less than twelve months), or long‐term (12 months and over), according to the timing of the final outcome assessments after the intervention.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library ((Issue 3, 2006);
MEDLINE (until July 2006);
EMBASE (until July 2006);
CINAHL (until July 2006).
The search strategy described (see for a detailed search strategy under Appendix 1) was used for MEDLINE. This strategy was slightly adapted for use with EMBASE, The Cochrane Library and CINAHL. We placed no language restrictions on either the search or the included trials.
Searching other resources
We hand searched the reference lists of review articles and included studies for other potentially eligible studies.
Data collection and analysis
Selection of studies
Two reviewers (DT and LB) independently reviewed the titles, abstract sections and keywords of every record retrieved from the literature searches to identify studies for assessment. We retrieved the full articles when the information suggested that the study might fit the review criteria. We eliminated any trial that clearly did not fulfil the inclusion criteria, for example, was not a randomised controlled trial, was not performed on people who were overweight or obese, had no comparator, included a co‐intervention, or where the intervention was less than two weeks duration. If uncertainty existed, we retrieved the full text of the article for further review. The decision to eliminate a trial was based on agreement by all three reviewers. When a trial was excluded after this point, a record of the article, including the reason for exclusion, was retained (for details see 'Characteristics of excluded studies'). We had planned to measure inter‐rater agreement using Cohen's kappa statistic (Cohen 1960; Fleiss 1981) and to discuss any differences in opinion. However, as the authors identified the same abstracts for further investigation and later for inclusion, this was not done. An adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection is attached (Moher 1999).
Data extraction and management
Two authors (DT and LB) independently extracted the data on the study population, intervention and outcomes for each included study, using a standard data extraction form, which included the following:
general information: published or unpublished, title, authors, source, contact address, country, setting, language, year of publication, duplicate publication, source of funding;
trial characteristics: design, randomisation (and method if stated), allocation concealment, blinding of outcome assessors;
participants: if randomised, inclusion criteria, exclusion criteria, total number in intervention and control groups,sex, age, baseline characteristics, diagnostic criteria, similarity of groups at baseline, withdrawals, losses to follow‐up;
intervention and comparator, duration of trial;
outcomes: Outcomes specified in the methods, other outcomes assessed, length of post‐intervention follow‐up if applicable;
results: For continuous variables, we extracted the number of participants, and the baseline and post‐intervention means with standard deviations (SD) or standard error of the mean (SEM) or 95% confidence interval (95% CI) for the intervention and control groups. We transformed SEM or 95% CI into SD, if appropriate. Any dichotomous outcomes were also recorded.
Any variations in data extraction were resolved by consensus, referring back to the original data.
Assessment of risk of bias in included studies
The methodological quality of each included randomised controlled trial was assessed independently by two authors (DT and EE), based on quality criteria specified by Schulz and Jadad (Jadad 1996; Schulz 1995). The following factors were studied:
Minimisation of selection bias ‐ a) was the randomisation procedure adequate? b) was the allocation concealment adequate?
Minimisation of attrition bias ‐ a) were withdrawals and dropouts completely described? b) was the analysis by intention‐to treat?
Minimisation of detection bias ‐ were the outcome assessors blind to the intervention? Blinding of either the participant or the administrator of the intervention is generally not possible in dietary intervention studies, and it is often not feasible to have an assessor who has had no part in the trial, hence blinding was not assessed as a quality criterion. Blinding of outcome assessors was recorded.
We had planned a sensitivity analysis based on classification of the studies into three categories (Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005): A ‐ low risk of bias: all quality criteria met; B ‐ moderate risk of bias: one or more of the quality criteria only partially met; C ‐ high risk of bias: one or more quality criteria not met,; as well as exploring the effect of the individual quality criteria. However, as there were insufficient studies, this was not done.
Calculation of the level of inter‐rater agreement using the kappa statistic (Cohen 1960; Fleiss 1981) was planned for quality assessment, with any variation in assessment by the authors resolved through discussion, however because there was no variation this was not performed.
Assessment of heterogeneity
All data were initially analysed with a fixed effect model. We tested for heterogeneity between trial results using a standard χ2‐test to observe whether any variation in study results was compatible with the variation expected by chance alone. A significance level of α = 0.1 was used for the test of heterogeneity. The I2 parameter was used to quantify any inconsistency (I2 = [(Q‐df)/Q] x 100%, where Q is the χ2‐statistic and df is its degrees of freedom) (Higgins 2002). A value for I2 greater than 50% was considered to indicate substantial heterogeneity (Higgins 2003). Where heterogeneity was found, we attempted to determine potential sources of heterogeneity with subgroup and sensitivity analyses.
Assessment of reporting biases
The number of studies was too small for us to be able to explore publication bias through assessment of funnel plot asymmetry (Cooper 1994; Tang 2000).
Data synthesis
We summarized the data statistically when they were sufficiently uniform and of sufficient quality. For dichotomous outcomes, we had planned to express the effect size in terms of relative risk with 95% confidence interval (CI), but there were no dichotomous outcomes relevant to this review.
For continuous outcomes, we calculated weighted mean differences. We extracted the baseline and post‐intervention means with standard deviations (SD) (or standard error of the mean (SEM) or 95% CI) for the intervention and control groups and transformed any SEM or 95% CI into SD where appropriate. For trials where the results were given as mean changes from baseline, we recorded the absolute changes in outcome between baseline and post‐intervention for both the control and intervention groups. If required, the mean difference could have been calculated by subtracting the control absolute change from the intervention absolute change. However, it would have only been approximate to calculate the estimate of variance for each of these changes [ = Vpre + Vpost ‐ 2r(SEpre x SEpost)], where Vpre and SEpre are the variance and standard error of the mean baseline value; Vpost and SEpost are the variance and standard error of the mean post‐intervention value; and r is the correlation between baseline and post‐intervention values. The variance of the total change could then have been calculated as the sum of the variance of the change in the intervention group and the variance of the change in the control group. As this involved approximations and owing to the small number of studies, this was not done (Higgins 2005). We used mean results and absolute change results for the outcomes of interest. When post‐intervention measures of dispersion were not given (for example if the results were presented as percentage change from baseline), the baseline measures of dispersion were also used as the post‐intervention values. This is a conservative approach, since variation at baseline should be larger than that at post‐intervention, but this approach was only taken when the pre‐ and post‐ measures of dispersion for the same outcome were similar to each other in other trials. If the results were given on different scales, we used standardised mean differences. When data were only presented graphically, we obtained an estimate of the mean and SD from the graph.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed after excluding the largest trial (McMillan‐Price 2006) to determine its effect on the results. Subgroup analyses were planned according to age (18 years and less, over 18 years); sex (male, female); duration of the trial intervention (less than six months, six to twelve months, more than twelve months), difference between the glycaemic index or glycaemic load of the dietary intervention and that of the comparator, body mass (body mass index 25‐29, greater than 30) and whether the comparator diet was a high glycaemic index or load diet, or an energy restricted reduced fat diet. However, the number of included trials was too small for reliable analysis by subgroups.
Sensitivity analysis
We had planned to perform sensitivity analyses to explore the influence of a number of other factors on effect size, by repeating the analysis:
excluding unpublished studies;
taking study quality, as specified above, into account;
excluding any long or large studies to determine their influence on the results;
excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
However, because of the small number of included studies, we did not perform these analyses.
Results
Description of studies
Results of the search
From the initial search, 892 records were identified. From the abstracts of these, we identified 68 papers for examination of the full text. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study. Main reasons for exclusion were: articles were reviews, duplicate papers, the study had no control group or no randomisation, studies did not compare similar groups, the intervention was less than two weeks, weight loss was not the aim of the study, the trial endpoint was different in the two arms of the trial, the diets were both designed to be 'weight maintaining', or the participants were neither overweight nor obese. Six studies met the inclusion criteria (Bouche 2002; Ebbeling 2003; Ebbeling 2005; McMillan‐Price 2006; Slabber 1994; Sloth 2004). Five reported body mass and two reported body mass index (Ebbeling 2003; Slabber 1994). For an adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection see Figure 1 under 'Additional figures'.
1.
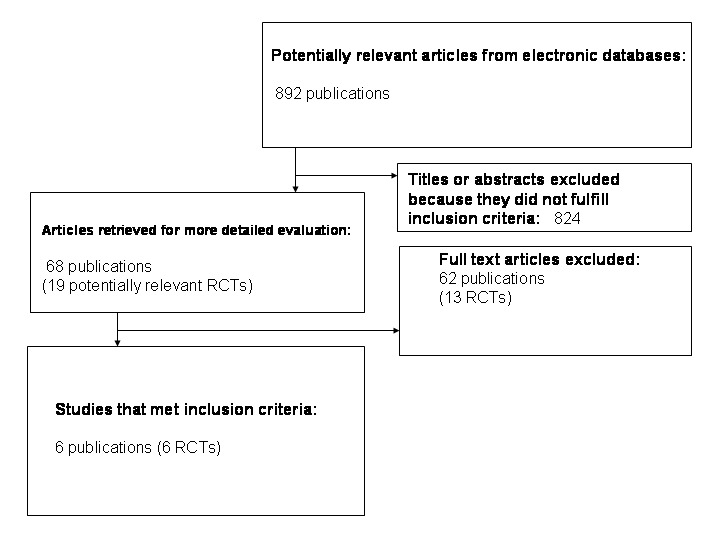
Adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Assessment of inter‐rater agreement
Two authors (DT and LB) reviewed the studies. There was agreement on the studies to be fully assessed. From these, studies eligible for inclusion in the review were selected. All three authors agreed on the final papers chosen for assessment and on the quality assessment of the studies.
Missing data
No authors were contacted for further information or clarification.
Included studies
Details of the characteristics of the included studies are given in the Characteristics of included studies. The following gives a brief overview:
Study types
All six studies selected for the review were randomised controlled trials (Bouche 2002; Ebbeling 2003; Ebbeling 2005; McMillan‐Price 2006; Slabber 1994; Sloth 2004). They were conducted in Australia (McMillan‐Price 2006), France (Bouche 2002), South Africa (Slabber 1994) and the USA (Ebbeling 2003; Ebbeling 2005; Sloth 2004). The duration of the dietary interventions ranged from five‐weeks (Bouche 2002) to 6 months (Ebbeling 2003; Ebbeling 2005) and the maximum length of follow up was 6 months (Ebbeling 2003; Ebbeling 2005).
Participants
The included studies involved a total of 202 participants (the number of participants ranged from 11 participants in a cross‐over trial (Bouche 2002) to 64 participants ( McMillan‐Price 2006). The mean age ranged from 16 years (Ebbeling 2003) to 46 years (Bouche 2002) and more women than men participated. There was a total of 186 participants in the five studies reporting body mass (Bouche 2002; Ebbeling 2005; McMillan‐Price 2006; Slabber 1994; Sloth 2004). Ninety‐three of these participants received the low glycaemic index or load dietary intervention. One study involved children (Ebbeling 2003).
Interventions
Three studies compared a low glycaemic index diet with a higher glycaemic index diet (Bouche 2002; McMillan‐Price 2006; Sloth 2004). One study compared an ad libitum reduced‐glycaemic load diet with a conventional energy‐restricted, reduced‐fat diet (Ebbeling 2003). Another study (Slabber 1994) compared an energy‐restricted low glycaemic index diet with a normal energy‐restricted diet. The remaining study compared an ad libitum low glycaemic index diet with a conventional weight loss restricted‐energy reduced fat diet (Ebbeling 2005). Three studies compared a low glycaemic load or index diet (LGI) with a higher glycaemic diet (Bouche 2002; McMillan‐Price 2006; Sloth 2004). The remaining studies compared the LGI diet with a current best practice weight reducing diet (Ebbeling 2003; Ebbeling 2005; Slabber 1994).
Duration of studies
The LGI dietary interventions ranged from five weeks duration with no follow up (Bouche 2002) to six months duration: an intensive intervention with follow‐up at 12 months after commencement of the intervention. (Ebbeling 2003; Ebbeling 2005). In the Sloth 2004 study the intervention was of 10 weeks duration with no follow up. The interventions in the McMillan‐Price 2006 and Slabber 1994 studies were both 12 weeks long with no follow up.
Outcomes
Original data can be found in Appendix 3.
Primary outcomes
Body mass
Five studies (n = 186) measured body mass (Bouche 2002; Ebbeling 2005; McMillan‐Price 2006; Slabber 1994; Sloth 2004). One study (Ebbeling 2003) included only body mass index. Slabber 1994 included both body mass and body mass index.
Adiposity and fat distribution
Five studies reported total fat mass estimated by dual energy X‐ray absorptiometry (Bouche 2002; Ebbeling 2003; McMillan‐Price 2006; Slabber 1994; Sloth 2004) and two of these also reported fat free mass (a measure of musculo‐skeletal mass) (McMillan‐Price 2006; Sloth 2004).
Adverse effects
No study included adverse effects as an outcome.
Secondary outcomes
Insulin action
One study reported insulin resistance (Ebbeling 2003), and one study reported morning insulin area under the curve, fasting plasma insulin and insulin sensitivity (Bouche 2002). A homeostasis model assessment (of insulin sensitivity) (HOMA) was reported in two studies (McMillan‐Price 2006; Sloth 2004). One study reported the insulin sensitivity index (Ebbeling 2005). McMillan‐Price 2006 and Slabber 1994 reported fasting plasma insulin.
Glycaemic control
Measures relating to plasma glucose concentrations that were reported in the included studies included fructosamine and glucose area under the curve (Bouche 2002) and fasting plasma glucose concentration (Bouche 2002; McMillan‐Price 2006; Slabber 1994; Sloth 2004).
Cardiovascular risk factors
Four studies reported total plasma cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglyceride concentrations (Bouche 2002; Ebbeling 2005; McMillan‐Price 2006; Sloth 2004). Two studies provided data on free fatty acids (Bouche 2002; Sloth 2004). Two studies provided data on blood pressure, recording both systolic and diastolic pressure (Ebbeling 2005; Sloth 2004).
Satiety
In two studies the intervention included ad libitum eating to satiety in the LGI diet group, but not in the comparison diet group (Ebbeling 2003; Ebbeling 2005).
Quality of life
No study included quality of life as an outcome.
Mortality
No study included mortality as an outcome.
Excluded studies
Excluded studies and the reasons for exclusion are given in the Table Characteristics of excluded studies.
Risk of bias in included studies
For details see Appendix 2.
Similarity at baseline
No study included in the review reported any significant differences between treatment groups in the main characteristics of participants at baseline.
Randomisation and allocation concealment
All included trials were described as randomised. However, no additional detail on the method of randomisation was reported by Bouche 2002; Ebbeling 2003, Ebbeling 2005 and Sloth 2004. McMillan‐Price 2006 reported that participants were stratified by weight and sex and then randomly assigned to groups. Slabber 1994 reported that randomisation was by minimisation, a form of block randomisation which aims to provide each group with closely matched participants. Allocation concealment was not reported in any study.
Descriptions of losses to follow‐up
There were no withdrawals or dropouts in the Bouche 2002 study. In the Ebbeling 2005 study, results were reported and analysed only for the 23 participants who completed the study (68% retention rate). In the Slabber 1994 study, 16 of the participants (seven from one treatment group and nine from the other group) also volunteered to receive the alternative treatment after a 12 week washout period. Sloth 2004 provide information on the reasons participants dropped out. The analyses in the Bouche 2002 study were by intention‐to‐treat. In the study by Ebbeling 2003 two participants were lost to follow up (one in the intervention group and one in the control group). Results were analysed both by intention‐to‐treat, and after exclusion of these two participants and were reported as substantially similar. McMillan‐Price 2006 analysed results both with and without intention‐to‐treat. The two remaining studies were not analysed as intention‐to‐treat (Ebbeling 2005; Sloth 2004).
Blinding of outcome assessment
As per the review protocol, blinding was not assessed as a quality criterion. No trial reported blinding of the outcome assessors.
Adequacy of length of follow‐up
Only two studies provided follow‐up beyond cessation of the intervention and these were the longest trials (Ebbeling 2003; Ebbeling 2005). In both trials the duration of the intervention was six months and participants were followed for up at 6 months after completing the intervention (12 months from the commencement of the intervention). The shortest intervention was five weeks (Bouche 2002) with no follow‐up. None of the other three included studies provided follow‐up data (McMillan‐Price 2006; Slabber 1994; Sloth 2004).
We had planned to perform a sensitivity analysis to compare results between studies of high and low quality, however data were insufficient to permit this.
Effects of interventions
Glycaemic index of the intervention and control group diets
The glycaemic index (GI) of the intervention (LGI) diet in the Bouche 2002 study was 41.0 ± 1.0 % compared with a GI of 71.3 ± 1.3 for the control diet, a difference of 30 GI units (P < 0.0001). In the Sloth 2004 study, the weighted glycaemic index for the LGI diet intervention was 78.6 GI units compared with 102.8 GI units in the higher glycaemic index comparison diet, a difference of 24.2 GI units. There was a difference of 25 GI units between the low and high GI diets in McMillan‐Price 2006. The actual values for the LGI intervention were GI 45 ± 1 and GL 89 ± 5 g and for the control diet were GI 70 ± 1 and GL 129 ± 8. In the two Ebbeling studies (Ebbeling 2003; Ebbeling 2005) the low glycaemic load diets were compared with conventional weight reducing diets and Slabber 1994 stipulated a normal low energy diet as the comparator.
Primary outcomes
Body mass
Pooled data from the four studies reporting change in body mass (Bouche 2002; McMillan‐Price 2006; Slabber 1994; Sloth 2004) showed that weight loss was significantly greater in participants receiving the low glycaemic diet compared with those receiving the comparison diet (WMD ‐1.1 kg, 95% CI ‐2.0 to ‐0.2, P < 0.05) (n =163). The fifth study (Ebbeling 2005) reported % change in body mass (WMD ‐0.60 kg, 95% CI ‐4.56 to 3.36).
The decrease in body mass index was greater in participants receiving the low glycaemic index diet compared to the comparator diet (WMD ‐1.3 BMI units, 95% CI ‐2.0 to ‐0.5, P < 0.05) (Ebbeling 2003; Slabber 1994).
Adiposity and fat distribution
The decrease in total fat mass was significantly greater with LGI than with comparison diets (WMD ‐1.1 kg, 95% CI ‐1.9 to ‐0.4, P < 0.05) (Bouche 2002; Ebbeling 2003; McMillan‐Price 2006; Slabber 1994; Sloth 2004).
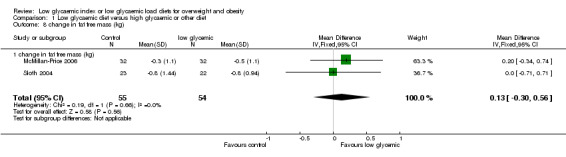
In two studies in which LGI diets and higher GI diets were compared, there was no significant change in fat free mass (muscle mass) after the dietary intervention and no difference between intervention and control groups (WMD 0.1 kg, 95% CI ‐0.3 to +0.6) (McMillan‐Price 2006; Sloth 2004).
Adverse effects
No study reported any adverse effects.
Secondary outcome measures
Insulin action
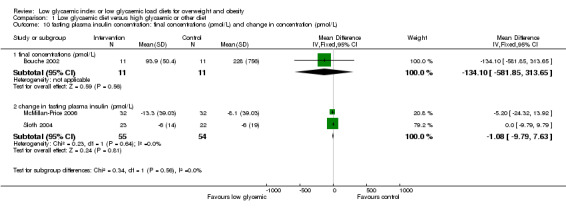
People with diabetes mellitus were excluded from this review. Four studies reported results on outcomes relating to insulin action (Bouche 2002; Ebbeling 2003; McMillan‐Price 2006; Sloth 2004). Insulin resistance decreased in participants receiving an ad libitum reduced glycaemic load diet but not in the group receiving an energy‐restricted, reduced‐fat diet (‐0.4 +0.9 SE versus +2.6 +1.2 SE; P < 0.05) in the one study reporting this outcome (Ebbeling 2003). Bouche 2002 reported a significantly greater decrease in morning insulin area under the curve after the low glycaemic diet compared to the high glycaemic diet. Three studies reported fasting plasma insulin (Bouche 2002; McMillan‐Price 2006; Sloth 2004) with no significant difference between the two diets. Assessment of insulin sensitivity reported by Sloth 2004 (HOMA‐B) and McMillan‐Price 2006 (HOMA) showed no significant differences between treatment groups.
Glycaemic control
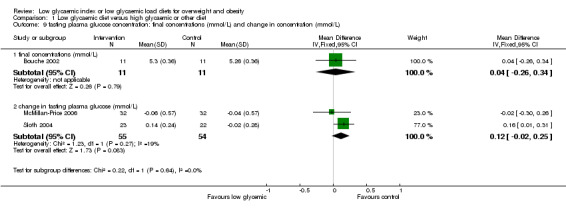
As the protocol for this review excluded people with diabetes mellitus, glycaemic control was reported in only one of the included trials. There was no significant change in fructosamine levels after the low glycaemic index diet (0.04 mmol/L, 95%CI ‐0.23 to +0.31). (Bouche 2002).
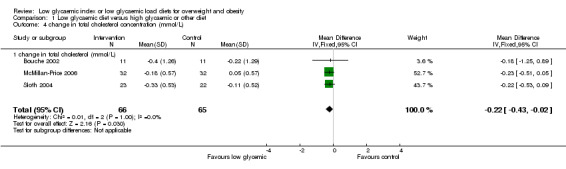
Cardiovascular risk profile
Data from three studies was used to determine the change in total plasma cholesterol concentration after the diet intervention. The fall in total plasma cholesterol was significantly greater in the LGI diet group compared with the comparison group (WMD ‐0.22 mmol/L, 95% CI ‐0.43 to ‐0.02, P < 0.05) (Bouche 2002; McMillan‐Price 2006; Sloth 2004). One study reported percentage change in total plasma cholesterol concentration and there was no difference between groups (‐7.8 %, 95%CI 18.0 to 2.4) (Ebbeling 2005).
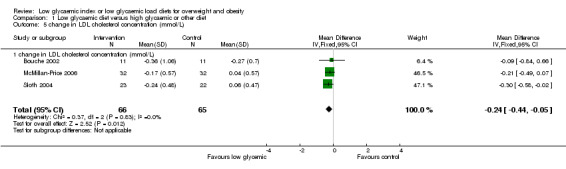
Three studies reported the change in LDL‐cholesterol concentration. The decrease in LDL‐cholesterol was greater after the low glycaemic diet than after the comparison diet (WMD ‐0.24 mmol/L, 95% CI ‐0.44 to ‐0.05, P < 0.05) (Bouche 2002; McMillan‐Price 2006; Sloth 2004).
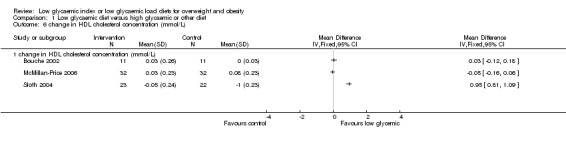
Three studies reported the change in HDL‐cholesterol concentration, but as there was heterogeneity (I2 = 98.5%), the results have been reported separately. In one of these studies, the change in HDL‐cholesterol concentration increased significantly after the low glycaemic diet compared to the comparison diet (+0.95 mmol/L, 95% CI +0.81 to +1.09, P < 0.05) (Sloth 2004). When this study was excluded in the meta‐analysis, there was no heterogeneity (I2 = 0%)(WMD ‐0.02 mmol/L, 95% CI ‐0.11 to +0.07)(Bouche 2002; McMillan‐Price 2006).
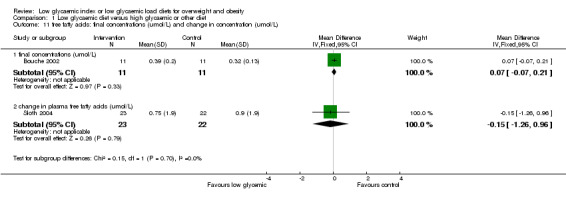
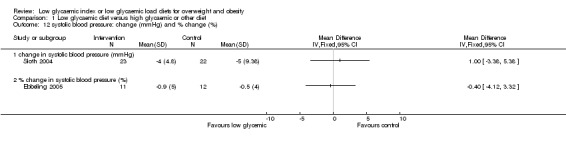
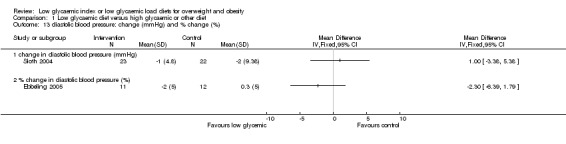
In the two studies reporting plasma free fatty acid concentrations (Bouche 2002; Sloth 2004), there was no significant difference between treatment groups. There was no significant difference in blood pressure between the treatment groups (Ebbeling 2005; Sloth 2004).
Satiety
Satiety was not specified as an outcome measure in any study. Two studies (Ebbeling 2003; Ebbeling 2005) stipulated ad libitum eating (to satiety) for participants receiving the low glycaemic index or load diet, but not for the comparison conventional low fat, reduced energy diet.
Quality of life
No study reported quality of life outcomes.
Mortality
No study assessed mortality as a primary or secondary outcome.
Heterogeneity
There was heterogeneity (I2 = 98.5%) between the three results for the change in plasma HDL‐cholesterol (Bouche 2002; McMillan‐Price 2006; Sloth 2004), due to the favourable significant increase in one of the studies (Sloth 2004) in the low glycaemic diet group compared to the high glycaemic diet. When this study was excluded in the meta‐analysis, there was no heterogeneity (I2 = 0%)(Bouche 2002;McMillan‐Price 2006). Different comparison diets in the studies may have contributed to heterogeneity in the results.
Subgroup analysis
Not performed due to the small number of included studies.
Sensitivity analysis
Not performed due to the small number of included studies.
Assessment of publication bias
There were too few included studies to be able to assess bias from a funnel plot.
Follow‐up
Two studies reported results at 12 month follow‐up from the commencement of the study (Ebbeling 2003; Ebbeling 2005). In the Ebbeling 2005 study there was no significant difference in the percentage change in total cholesterol or LDL‐cholesterol between the two diet groups. However the percentage increase in HDL‐cholesterol was significantly greater in the group originally randomised to the LGI diet compared to the higher glycaemic diet group (11.1%, 95% CI 13.7 to 18.5, P < 0.05).
Discussion
Summary of main results
This review indicates that weight loss was greater in overweight and obese people given low glycaemic index or load diets than in people given comparison diets, including higher glycaemic index or load diets and conventional weight loss diets. Similarly, loss of total fat mass and decrease in body mass index were significantly greater in the group receiving a low glycaemic index or load diet. The loss of 1‐2 BMI units is clinically significant as is the weight loss observed with LGI diets (up to 7 kg during the intervention period). Improvements in the cardiovascular risk profile (indicated by a decrease in total cholesterol and LDL cholesterol) were significantly better with a low glycaemic index or load diet than a comparison diet.
Of the six studies included in this review (202 participants), two included obese people (Ebbeling 2003;Slabber 1994) and compared low glycaemic index or load diets with conventional weight reducing low fat diets. Four studies included people with borderline normal weight (BMI=25) or overweight (BMI greater than 25 to 30) and compared a low glycaemic index or load diet with a higher glycaemic index or load diet. Only one study involved children.
In the two studies in which all the participants were obese (Ebbeling 2003; Slabber 1994), the effects of the low glycaemic index or load diets were more apparent. For example the decrease in total fat mass was greater in the group receiving the low GI than the comparator diet (‐4.2 kg, 95% CI ‐7.4 to ‐1.0, P < 0.05) (Ebbeling 2003). Similarly, the decrease in body mass index was greater with the LGI diet than the comparator diet in both studies (Ebbeling 2003) (‐1.8 BMI units, 95% CI ‐3.4 to ‐0.2, P < 0.05) and (Slabber 1994) (‐1.1 BMI units, 95% CI ‐2.0 to ‐0.2, P < 0.05). Ebbeling 2003 also reported a significantly greater decrease in body mass with the LGI diet (‐2.9 kg, 95% CI ‐5.4 to ‐0.5, P < 0.05). Hence low glycaemic diets appear to be effective even in obese people who need to lose considerable amounts of weight. The added advantages of LGI diets are that they are simple to follow and they are more likely to result in satiety than other weight loss diets. Furthermore, provided people consume the right type of low GI foods, there is no need to limit the actual quantity of food to achieve weight loss. This is more conducive to good quality of life than a very restrictive diet.
Overall completeness and applicability of evidence
The degree of overweight and obesity in the populations included in these studies was wide, suggesting that the results would be applicable in other developed communities. Only one study included children (n =16), so care would need to be taken in generalising results in the paediatric population.
Potential biases in the review process
Only six relevant randomised trials were identified and each had methodological limitations, including failure to conceal allocation and lack of blinding, which is difficult in dietary interventions. Furthermore, a range of comparator diets was used and the duration of the intervention was short. The two longest studies had a six month intervention with six month follow‐up. Considering the brevity of the interventions the results observed are notable. One of the major challenges in weight management is sustainability of weight loss or maintenance of weight. Longer trials with increased lengths of follow‐up will determine whether the improvements we report with LGI diets can be maintained and incorporated into lifestyle long‐term.
Authors' conclusions
Implications for practice.
Overweight or obese people on low glycaemic index diets lost more weight than those on high glycaemic index diets or conventional energy restricted weight loss diets, with the change in body mass, total fat mass and body mass index all significantly decreasing after the low glycaemic index diet compared to the comparison diet. It may be easier to adhere to a low glycaemic index diet than a conventional weight loss diet, since there is less need to restrict the intake of food as long as low glycaemic index carbohydrates are predominantly consumed. In studies comparing ad libitum reduced glycaemic index or load diets to conventional restricted low fat diets, even though participants could eat as much as desired on the low glycaemic index or load diets, they fared as well, or better, in the outcomes than those on the comparison diet. Hence, lowering the glycaemic index of foods in the diet appears to be an effective method of losing weight, particularly for the obese.
Implications for research.
Further research with longer duration of follow‐up is required to determine whether the improvements can be maintained long term. Future studies should investigate health‐related quality of life (and adverse effects), since any change in diet is an interference with a person's life style.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2008 | Amended | Converted to new review format. |
Acknowledgements
Sunita Chauhan for assistance in developing the search strategy.
Appendices
Appendix 1. Search strategy
| Search strategy |
| Search for Glycaemic index 1. exp Glycemic Index/ 2. (glyc?emic index or (glyc?emic adj5 load$)).tw. 3. dietary carbohydrate$.tw. 4. (diet adj5 glyc?emic$).tw. 5. (gi adj10 (diet$ or food$ or carbohydrate$)).tw. 6. (food adj5 glyc?emic$).tw. 7. exp Dietary Carbohydrates/ 8. exp Blood Glucose/ 9. blood glucose.tw. 10. blood sugar.tw. 11. 9 or 10 12. 3 and 11 13. 7 and 8 14. 1 or 2 or 4 or 5 or 6 15. 12 or 13 or 14 Search for Obesity 16. Obesity/ 17. exp Weight Gain/ 18. exp Weight Loss/ 19. body mass index/ 20. (overweight or over weight).tw. 21. adipos$.tw. 22. fat overload syndrom$.tw. 23. (overeat or over eat).tw. 24. (overfeed or over feed).tw. 25. weight cycling.tw. 26. weight reduc$.tw. 27. weight losing.tw. 28. weight maint$.tw. 29. weight decreas$.tw. 30. weight watch$.tw. 31. weight control$.tw. 32. obes$.tw. 33. weight gain.tw. 34. weight loss.tw. 35. body mass index.tw. 36. weight chang$.tw. 37. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. 15 and 37 Filter for randomized controlled trials* 39. randomized‐controlled trial.pt. 40. controlled‐clinical trial.pt. 41. randomized‐controlled‐trials.sh. 42. random allocation.sh. 43. double‐blind method.sh. 44. single‐blind method.sh. 45. 39 or 40 or 41 or 42 or 43 or 44 46. animal.sh. 47. human.sh. 48. 46 not 47 49. 45 not 48 50. clinical trial.pt. 51. exp clinical trials/ 52. (clinic$ adj25 trial$).tw. 53. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. 54. Placebos.sh. 55. placebo$.tw. 56. random$.tw. 57. research design.sh. 58. (latin adj square).tw. 59. 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 60. 59 not 48 61. 60 not 49 62. 38 and 61 * Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. |
Appendix 2. Risk of bias
| Study | At baseline | Randomisation | Allocation concealed | Intention‐to‐treat | Assessor blinding | Losses accounted for |
| Bouche 2002 | similar | yes | not reported | yes | not reported | no losses |
| Ebbeling 2003 | similar | yes | not reported | yes, in addition to analysis leaving out dropouts | not reported | yes |
| Ebbeling 2005 | similar | yes | not reported | no, 68% completed study (n=23) | not reported | yes |
| McMillan‐Price 2006 | similar | yes | not reported | yes | not reported | no losses |
| Slabber 1994 | similar | yes | not reported | yes | not reported | no losses |
| Sloth 2004 | similar | yes | not reported | no | not reported | yes |
Appendix 3. Original data
| data |
| Comparisons and data 01 Low glycaemic diet versus high glycaemic or other diet 01.01 change in body mass (kg) 01.01.01 change in body mass (kg) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 ‐0.30 9.60 11 0.50 8.90 McMillan‐Price 2006 32 ‐5.60 4.00 32 ‐4.30 4.00 Slabber 1994 16 ‐7.42 2.49 16 ‐4.48 4.23 Sloth 2004 23 ‐1.90 2.40 22 ‐1.30 1.40 01.02 change in total fat mass (DXA kg) 01.02.01 change in total fat mass (DXA kg) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 ‐0.52 5.27 11 ‐0.02 5.21 Ebbeling 2003 8 ‐2.60 3.97 8 1.60 2.38 McMillan‐Price 2006 32 ‐4.50 2.80 32 ‐2.80 2.80 Sloth 2004 23 ‐1.00 1.90 22 ‐0.40 1.40 01.03 change in body mass index (BMI units) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Ebbeling 2003 8 ‐1.20 1.85 8 0.60 1.32 Slabber 1994 16 ‐2.73 0.85 16 ‐1.61 1.52 01.04 change in total cholesterol concentration (mmol/L) 01.04.01 change in total cholesterol (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 ‐0.40 1.26 11 ‐0.22 1.29 McMillan‐Price 2006 32 ‐0.18 0.57 32 0.05 0.57 Sloth 2004 23 ‐0.33 0.53 22 ‐0.11 0.52 01.05 change in LDL cholesterol concentration (mmol/L) 01.05.01 change in LDL cholesterol concentration (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 ‐0.36 1.06 11 ‐0.27 0.70 McMillan‐Price 2006 32 ‐0.17 0.57 32 0.04 0.57 Sloth 2004 23 ‐0.24 0.48 22 0.06 0.47 01.06 change in HDL cholesterol concentration (mmol/L) 01.06.01 change in HDL cholesterol concentration (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 0.03 0.26 11 0.00 0.03 McMillan‐Price 2006 32 0.03 0.23 32 0.08 0.23 Sloth 2004 23 ‐0.05 0.24 22 ‐1.00 0.23 01.07 change in triglycerides concentration (mmol/L) and % change (%) 01.07.01 change in triglycerides concentration (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 ‐0.09 0.70 11 0.04 1.30 McMillan‐Price 2006 32 ‐0.05 0.40 32 ‐0.14 0.40 Sloth 2004 23 ‐0.80 4.32 22 ‐0.70 4.22 01.07.02 % change in triglycerides concentration (%) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Ebbeling 2005 11 ‐35.40 14.81 12 ‐7.10 21.56 01.08 change in fat free mass (kg) 01.08.01 change in fat free mass (kg) Study ID Control N Control Mean Control SD low glycemic N low glycemic Mean low glycemic SD McMillan‐Price 2006 32 ‐0.30 1.10 32 ‐0.50 1.10 Sloth 2004 23 ‐0.80 1.44 22 ‐0.80 0.94 01.09 fasting plasma glucose concentration: final concentrations (mmol/L) and change in concentration (mmol/L) 01.09.01 final concentrations (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 5.30 0.36 11 5.26 0.36 01.09.02 change in fasting plasma glucose (mmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD McMillan‐Price 2006 32 ‐0.06 0.57 32 ‐0.04 0.57 Sloth 2004 23 0.14 0.24 22 ‐0.02 0.28 01.10 fasting plasma insulin concentration: final concentrations (pmol/L) and change in concentration (pmol/L) 01.10.01 final concentrations (pmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 93.90 50.40 11 228.00 756.00 01.10.02 change in fasting plasma insulin (pmol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD McMillan‐Price 2006 32 ‐13.30 39.03 32 ‐8.10 39.03 Sloth 2004 23 ‐6.00 14.00 22 ‐6.00 19.00 01.11 free fatty acids: final concentrations (umol/L) and change in concentration (umol/L) 01.11.01 final concentrations (umol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Bouche 2002 11 0.39 0.20 11 0.32 0.13 01.11.02 change in plasma free fatty acids (umol/L) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Sloth 2004 23 0.75 1.90 22 0.90 1.90 01.12 systolic blood pressure: change (mmHg) and % change (%) 01.12.01 change in systolic blood pressure (mmHg) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Sloth 2004 23 ‐4.00 4.80 22 ‐5.00 9.38 01.12.02 % change in systolic blood pressure (%) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Ebbeling 2005 11 ‐0.90 5.00 12 ‐0.50 4.00 01.13 diastolic blood pressure: change (mmHg) and % change (%) 01.13.01 change in diastolic blood pressure (mmHg) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Sloth 2004 23 ‐1.00 4.80 22 ‐2.00 9.38 01.13.02 % change in diastolic blood pressure (%) Study ID Intervention N Intervention Mean Intervention SD Control N Control Mean Control SD Ebbeling 2005 11 ‐2.00 5.00 12 0.30 5.00 |
Data and analyses
Comparison 1. Low glycaemic diet versus high glycaemic or other diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 change in body mass (kg) | 4 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.99, ‐0.18] |
| 1.1 change in body mass (kg) | 4 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.99, ‐0.18] |
| 2 change in total fat mass (DXA kg) | 4 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.89, ‐0.38] |
| 2.1 change in total fat mass (DXA kg) | 4 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.89, ‐0.38] |
| 3 change in body mass index (BMI units) | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐2.02, ‐0.52] |
| 4 change in total cholesterol concentration (mmol/L) | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.43, ‐0.02] |
| 4.1 change in total cholesterol (mmol/L) | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.43, ‐0.02] |
| 5 change in LDL cholesterol concentration (mmol/L) | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.44, ‐0.05] |
| 5.1 change in LDL cholesterol concentration (mmol/L) | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.44, ‐0.05] |
| 6 change in HDL cholesterol concentration (mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 change in HDL cholesterol concentration (mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
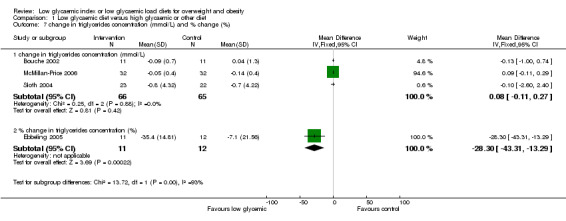
| 7 change in triglycerides concentration (mmol/L) and % change (%) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 change in triglycerides concentration (mmol/L) | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.11, 0.27] |
| 7.2 % change in triglycerides concentration (%) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐28.30 [‐43.31, ‐13.29] |
| 8 change in fat free mass (kg) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.30, 0.56] |
| 8.1 change in fat free mass (kg) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.30, 0.56] |
| 9 fasting plasma glucose concentration: final concentrations (mmol/L) and change in concentration (mmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 final concentrations (mmol/L) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.26, 0.34] |
| 9.2 change in fasting plasma glucose (mmol/L) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.02, 0.25] |
| 10 fasting plasma insulin concentration: final concentrations (pmol/L) and change in concentration (pmol/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 final concentrations (pmol/L) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐134.1 [‐581.85, 313.65] |
| 10.2 change in fasting plasma insulin (pmol/L) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐9.79, 7.63] |
| 11 free fatty acids: final concentrations (umol/L) and change in concentration (umol/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 final concentrations (umol/L) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 11.2 change in plasma free fatty acids (umol/L) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.26, 0.96] |
| 12 systolic blood pressure: change (mmHg) and % change (%) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 change in systolic blood pressure (mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 % change in systolic blood pressure (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 diastolic blood pressure: change (mmHg) and % change (%) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 change in diastolic blood pressure (mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 % change in diastolic blood pressure (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
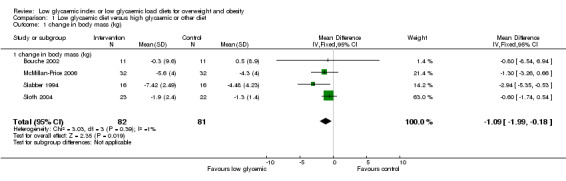
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 1 change in body mass (kg).
1.2. Analysis.
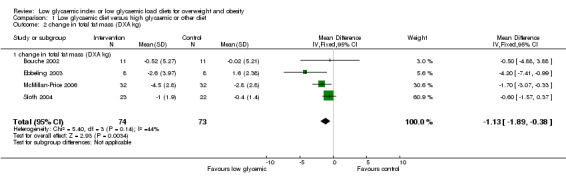
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 2 change in total fat mass (DXA kg).
1.3. Analysis.
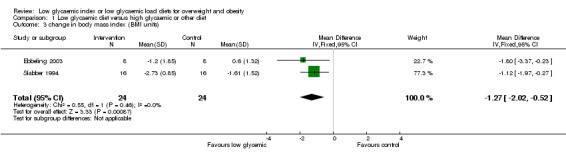
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 3 change in body mass index (BMI units).
1.4. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 4 change in total cholesterol concentration (mmol/L).
1.5. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 5 change in LDL cholesterol concentration (mmol/L).
1.6. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 6 change in HDL cholesterol concentration (mmol/L).
1.7. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 7 change in triglycerides concentration (mmol/L) and % change (%).
1.8. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 8 change in fat free mass (kg).
1.9. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 9 fasting plasma glucose concentration: final concentrations (mmol/L) and change in concentration (mmol/L).
1.10. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 10 fasting plasma insulin concentration: final concentrations (pmol/L) and change in concentration (pmol/L).
1.11. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 11 free fatty acids: final concentrations (umol/L) and change in concentration (umol/L).
1.12. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 12 systolic blood pressure: change (mmHg) and % change (%).
1.13. Analysis.
Comparison 1 Low glycaemic diet versus high glycaemic or other diet, Outcome 13 diastolic blood pressure: change (mmHg) and % change (%).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bouche 2002.
| Methods | Trial design: RCT crossover design with 5‐week washout period Randomisation procedure: Not stated. Allocation concealment: Not stated Blinding: Unclear Intention to treat analysis: Yes Difference in glycaemic index of diets = 30.3 (Intervention low glycaemic index diet 41.0 +/‐ 1%; Comparator high GI diet: 71.3 +/‐ 1.3%) | |
| Participants | Country: France Setting: Community Number: 11 Age: 46 +/‐ 3 (SE) years Sex: Male Other characteristics: Overweight | |
| Interventions | Trial Intervention: 5‐week low glycaemic index diet (foods with a glycaemic index < 45 were recommended) Comparison intervention: 5 weeks of a high glycaemic index diet (foods with a GI > 60 were recommended.) Compliance was assessed using food diaries on the last 7 days of each trial period. Total energy and macronutrient intakes: Reported | |
| Outcomes | Main outcome measures: Total fat mass, trunk fat, glycaemia, fructosamine, Other outcomes: Insulinaemia, total cholesterol, HDL cholesterol, LDL cholesterol, triacylglycerols, free fatty acids, Apo‐lipoprotein A, Apo‐lipoprotein B, gene expression of ob, PPAR‐delta2, LPL, HSL | |
| Notes | Source of funding: INSERM, the Pierre and Marie Curie University, Danone Vitapole, Nestle, the Institution Benjamin Delessert, and the Foundation for Medical Research, France. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ebbeling 2003.
| Methods | Trial design: RCT Randomisation procedure: Not stated Allocation concealment: Not stated Blinding: Not stated Dropouts described and reasons given. Intention to treat analysis: Yes, in addition to analysis leaving out the dropouts | |
| Participants | Country: USA Setting: Out‐patients. Number: 30 assessed for eligibility, 14 excluded (n=9 did not meet inclusion criteria, n=5 refused to participate) Number randomised: 16. Intervention group: n=8 Comparator group: n=8. Age: 16.9+‐1.3 vs 15.3 +‐0.9 years Health status: Obese (body mass index exceeding sex‐and age‐ specific 95th percentiles, free of major medical illness. Sex: 5 male, 11 female. Other characteristics: 13 white and 3 non‐white. At baseline, no differences in age, body mass, height, BMI and HOMA estimation of insulin resistance between intervention and comparator groups. However, fat mass was lower for the experimental group compared to the comparator (P < 0.05). Loss to follow‐up: Intervention n=1, comparator n=1 | |
| Interventions | Trial intervention: Ad libitum reduced glycaemic load diet. Comparison intervention: Energy‐restricted, reduced‐fat diet. 6‐month intervention with 6 month follow‐up | |
| Outcomes | Main outcome measures: Body mass index, change in fat mass Other outcomes: Insulin resistance | |
| Notes | Source of funding: National Institute of Diabetes and Digestive Kidney Diseases, Charles H Hood Foundation, Boston Obesity and Nutrition Research Center, NIH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ebbeling 2005.
| Methods | Trial design: RCT Randomisation procedure: Not stated Allocation concealment: Not stated Blinding: Not stated Intention to treat: No, 68 % of the participants completed the study (n=23) | |
| Participants | Country: USA Setting: Community Number: 34 Age: Intervention group 29.8 +/‐ 1.7 years; control group 27.2 +/‐1.3 years Sex: 30 female, 4 male Other characteristics: Body mass index > 27 kg/m2, body weight <136 kg, absence of major medical illness | |
| Interventions | Trial intervention: Ad libitum low glycaemic index diet for 6 months (intensive) with 6 month follow‐up Comparison intervention: Conventional diet recommended for weight loss and cardiovascular disease risk reduction, with emphasis on restricting energy intake by reducing dietary fat | |
| Outcomes | Main outcome measures: Body mass Other outcomes: Total cholesterol, LDL cholesterol, HDL cholesterol, triacylglycerols, PAI‐1, systolic and diastolic blood pressure, insulin sensitivity index | |
| Notes | Source of funding: US National Institute of Diabetes and Digestive Kidney Diseases, Charles H Hood Foundation and US NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McMillan‐Price 2006.
| Methods | Trial design: RCT Randomisation procedure: Participants were stratified by weight and sex, then randomly assigned to 1 of 4 diets Intention to treat: Yes Difference in glycaemic index of diets = 25 (intervention low glycaemic index diet 45 +/‐ 1%; comparator high GI diet: 70 +/‐ 1%) | |
| Participants | Country: Australia Setting: Community Number: 64 Age: Intervention group 31.8 +/‐ 1.7 years; control group 30.5 +/‐ 1.4 years Sex: 48 female Other characteristics: Body mass index of 25 or more with a body weight of less than 150 kg | |
| Interventions | Trial intervention: Low glycaemic index diet. Comparison intervention: High glycaemic index diet. 12 week parallel trial of weight loss diets of defined glycaemic load | |
| Outcomes | Main outcome measures: Change in body mass, fat mass, lean mass Other outcomes: Change in waist circumference, total, HDL and LDL cholesterol, triglycerides, free fatty acids, plasma glucose and insulin, HOMA, leptin | |
| Notes | Source of funding: Supported in part by National Heart Foundation of Australia and Meat and Livestock Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Slabber 1994.
| Methods | Trial design: RCT Randomisation procedure: Assigned by minimisation to 2 groups Allocation concealment: Not stated Blinding: Unclear Intention to treat analysis: Yes | |
| Participants | Country: South Africa Setting: Community Number: 32 Age: 35 +/‐ 6 years Sex: Female Other characteristics: Obese, hyperinsulinaemic compared to healthy females | |
| Interventions | Trial intervention: Low insulin response, energy‐restricted diet (low glycaemic index) Comparison: Normal diet. 12 week crossover study with 12 week washout in between (stage 1 parallel study also reported) | |
| Outcomes | Main outcome measures: Weight loss and plasma insulin concentrations Other outcome measures: Body mass index, plasma glucose, C‐peptide and insulin | |
| Notes | Source of funding: Supported by Central Research Fund of the University of the Orange Free State | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sloth 2004.
| Methods | Trial design: RCT Randomisation procedure: Randomly assigned and matched for age, body weight, height, body mass index, blood pressure, heart rate, estimated energy expenditure and alcohol intake. Allocation concealment: Not stated. Participants were informed of the purpose of the study, but not about the type of test foods they received. Blinding: Not stated. Intention‐to‐treat analysis: No Weighted glycaemic index of diets: Intervention 78.6; Comparator 102.8 | |
| Participants | Country: Denmark Setting: Community Number: 55 Age: Low glycaemic index diet intervention, 28.9 +/‐ 1.3 years; comparison group, 30.8 +/‐1.3 Sex: All female Other characteristics: Healthy overweight women with body mass index 27.6 +/‐ 0.2. | |
| Interventions | Trial intervention: Low glycaemic index diet. Comparison intervention: High glycaemic index diet. 10 week parallel trial. Ad libitum | |
| Outcomes | Main outcome measures: Body weight, fat mass, fat‐free mass, waist‐to‐hip‐ratio, Other outcomes: Total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triacylglycerols, non‐esterified fatty acids,diastolic and systolic blood pressure, heart rate, fasting plasma glucose and serum insulin concentrations | |
| Notes | Source of funding: Danone Vitapole, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GI = glycaemic index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agus 2000 | Intervention duration (8 days) less than 2 weeks. |
| Ball 2003 | Intervention duration (24 hours) less than 2 weeks. |
| Clapp 1998 | Intervention duration (7 ‐ 10 days) less than 2 weeks in first study and the intervention included a co‐intervention (exercise) in second study. Participants were not obese. |
| Dumesnil 2001 | Intervention duration (6 days) less than 2 weeks. |
| Hanai 1997 | Not a randomised controlled trial. |
| Jenkins 1985 | Not randomised. Overweight or obesity was not a criterion (hyperlipidaemia was) for participants. |
| Jenkins 1987 | Participants were not obese. |
| Pereira 2005 | Weight loss was not an outcome. The defined end‐point for both arms of the study was 10% weight loss, and hence the time duration was different for the intervention and comparator groups. |
| Piatti 1993 | Glycaemic index was not reported. |
| Spieth 2000 | Not a randomised controlled trial. |
| Van Horn 1986 | Participants were not obese. Glycaemic index was not reported. |
| Wolever 1992 | Participants had non‐insulin dependent diabetes mellitus, which was an exclusion factor for this review. |
| Wolever 2002 | Overweight or obesity, was not an essential inclusion factor for the population. Weight loss was not an outcome, as both the intervention and comparator diets were, by design, 'weight maintaining'. |
Contributions of authors
DIANA THOMAS: co‐ordinated the review, developed the protocol, searched for trials, screened search results, assessed trials for quality, extracted and entered data, analysed and interpreted data, developed the review.
ELIZABETH ELLIOTT: screened search results, assessed trials for quality, analysed and interpreted data, provided clinical perspective, developed the review.
LOUISE BAUR: searched for trials, screened search results, assessed trials for quality, extracted and entered data, provided clinical perspective.
Sources of support
Internal sources
The Children's Hospital at Westmead, NSW, Australia.
The University of Sydney, Australia.
External sources
Elizabeth Elliott is supported by a National Health and Medical Research Fellowship (457084), Australia.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bouche 2002 {published data only}
- Bouche C, Rizkalla SW, Luo J, Vidal H, Veronese A, Pacher N, et al. Five‐week, low‐glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care 2002;25(5):822‐8. [DOI] [PubMed] [Google Scholar]
Ebbeling 2003 {published data only}
- Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced‐glycemic load diet in the treatment of adolescent obesity. Archives of Pediatrics & Adolescent Medicine 2003;157(8):773‐9. [DOI] [PubMed] [Google Scholar]
Ebbeling 2005 {published data only}
- Ebbeling CB, Leidig MM, Sinclair KB, Seger‐Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low‐glycemic load diet on cardiovascular disease risk factors in obese young adults. American Journal of Clinical Nutrition 2005;81(5):976‐82. [DOI] [PubMed] [Google Scholar]
McMillan‐Price 2006 {published data only}
- McMillan‐Price J, Petocz P, Atkinson F, O'neill K, Samman S, Steinbeck K, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Archives of internal medicine 2007;166 (14):1466‐75. [DOI] [PubMed] [Google Scholar]
Slabber 1994 {published data only}
- Slabber M, Barnard HC, Kuyl JM, Dannhauser A, Schall R. Effects of a low‐insulin‐response, energy‐restricted diet on weight loss and plasma insulin concentrations in hyperinsulinemic obese females. American Journal of Clinical Nutrition 1994;60(1):48‐53. [DOI] [PubMed] [Google Scholar]
Sloth 2004 {published data only}
- Sloth B, Krog‐Mikkelsen I, Flint A, Tetens I, Bjorck I, Vinoy S, et al. No difference in body weight decrease between a low‐glycemic‐index and a high‐glycemic‐index diet but reduced LDL cholesterol after 10‐wk ad libitum intake of the low‐glycemic‐index diet. American Journal of Clinical Nutrition 2004;80(2):337‐47. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agus 2000 {published data only}
- Agus MS, Swain JF, Larson CL, Eckert EA, Ludwig DS. Dietary composition and physiologic adaptations to energy restriction. American Journal of Clinical Nutrition 2000;71(4):901‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ball 2003 {published data only}
- Ball SD, Keller KR, Moyer‐Mileur LJ, Ding Y‐W, Donaldson D, Jackson WD. Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics 2003;111(3):488‐94. [DOI] [PubMed] [Google Scholar]
Clapp 1998 {published data only}
- Clapp J. Diet, exercise and feto‐placental growth. Archives of gynecology and obstetrics 1997;261:101‐7. [Google Scholar]
Dumesnil 2001 {published data only}
- Dumesnil JG, Turgeon J, Tremblay A, Poirier P, Gilbert M, Gagnon L, et al. Effect of a low‐glycaemic index‐‐low‐fat‐‐high protein diet on the atherogenic metabolic risk profile of abdominally obese men.[comment]. British Journal of Nutrition 2001;86(5):557‐68. [DOI] [PubMed] [Google Scholar]
Hanai 1997 {published data only}
- Hanai H, Ikuma M, Sato Y, Iida T, Hosoda Y, Matsushita I, et al. Long‐term effects of water‐soluble corn bran hemicellulose on glucose tolerance in obese and non‐obese patients: improved insulin sensitivity and glucose metabolism in obese subjects. Bioscience, Biotechnology & Biochemistry 1997;61(8):1358‐61. [DOI] [PubMed] [Google Scholar]
Jenkins 1985 {published data only}
- Jenkins DJ, Wolever TM, Kalmusky J, Giudici S, Giordano C, Wong GS, et al. Low glycemic index carbohydrate foods in the management of hyperlipidemia. American Journal of Clinical Nutrition 1985;42(4):604‐17. [DOI] [PubMed] [Google Scholar]
Jenkins 1987 {published data only}
- Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G, et al. Metabolic effects of a low‐glycemic‐index diet. American Journal of Clinical Nutrition 1987;46(6):968‐75. [DOI] [PubMed] [Google Scholar]
Pereira 2005 {published data only}
- Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low‐glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292(20):2482‐90. [DOI] [PubMed] [Google Scholar]
Piatti 1993 {published data only}
- Piatti PM, Pontiroli AE, Saibene A, Santambrogio G, Paroni R, Magni F et aö. Insulin sensitivity and lipid levels in obese subjects after slimming diets with different complex and simple carbohydrate content. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 1993;17(7):375‐81. [PubMed] [Google Scholar]
Spieth 2000 {published data only}
- Spieth LE, Harnish JD, Lenders CM, Raezer LB, Perira MA, Hangen SJ, et al. A low‐glycemic index diet in the treatment of pediatric obesity. Archives of Pediatric Adolescent Medicine 2000 Sep;154(9):947‐51. [DOI] [PubMed] [Google Scholar]
Van Horn 1986 {published data only}
- Horn LV, Liu K, Parker D, Emidy L, Liao Y, Pan WH et aö. Serum lipid response to oat product intake with a fat‐modified diet. Journal of The American Dietetic Association 1986;86(6):759‐64. [PubMed] [Google Scholar]
Wolever 1992 {published data only}
- Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Wong GS, Josse RG. Beneficial effect of low‐glycemic index diet in overweight NIDDM subjects. Diabetes Care 1992;15(4):562‐4. [DOI] [PubMed] [Google Scholar]
Wolever 2002 {published data only}
- Wolever TM, Mehling C. High‐carbohydrate‐low‐glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. British Journal of Nutrition 2002;87(5):477‐87. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Mehling C. Long‐term effect of varying the source or amount of dietary carbohydrate on postprandial plasma glucose, insulin, triacylglycerol, and free fatty acid concentrations in subjects with impaired glucose tolerance. American Journal of Clinical Nutrition 2003;77(3):612‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Brand‐Miller 2002
- Brand‐Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. American Journal of Clinical Nutrition 2002;76(1):281S‐5S. [DOI] [PubMed] [Google Scholar]
Bravata 2003
- Bravata ODM, Sanders L, Huang J, Krumholz H M, Olkin I, Gardner CD, et al. Efficacy and safety of low‐carbohydrate diets: a systematic review. JAMA 2003;289(14):1837‐50. [DOI] [PubMed] [Google Scholar]
Campbell 2002
- Campbell K, Waters E, O'Meara S, Kelly S, Summerbell C. Interventions for preventing obesity in children (Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960:37‐46.
Cooper 1994
- Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Safe Foundation, 1994. [Google Scholar]
Dietz 1998
- Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101(3 Pt 2):518‐25. [PubMed] [Google Scholar]
Fleiss 1981
- Fleiss. Statistical Methods for Rates and Proportions. 2nd Edition. Wiley, New York, 1981. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd, [updated March 2005]. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jenkins 1981
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. American Journal of Clinical Nutrition 1981;34(3):362‐6. [DOI] [PubMed] [Google Scholar]
Kiens 1996
- Kiens B, Richter EA. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans. American Journal of Clinical Nutrition 1996;63(1):47‐53. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Mokdad 2001
- Mokdad OAH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA 2003;289(1):76‐9. [DOI] [PubMed] [Google Scholar]
Parliament 2003
- Parliament (U.K.) Committee of Public Accounts. Tackling Obesity in England. www.publications.parliament.uk/pa/cm200102/cmselect/cmpubacc/421/42103.htm (accessed 16 June 2003).
Pawlak 2002
- Pawlak DB, Ebbeling CB, Ludwig DS. Should obese patients be counselled to follow a low‐glycaemic index diet? Yes. Obesity Reviews 2002;3(4):235‐43. [DOI] [PubMed] [Google Scholar]
Raben 2002
- Raben A. Should obese patients be counselled to follow a low‐glycaemic index diet? No. Obesity Reviews 2002;3(4):245‐56. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Silink 2002
- Silink M. Childhood diabetes: a global perspective. Hormone Research 2002;57 Suppl 1:1‐5. [DOI] [PubMed] [Google Scholar]
Strauss 2001
- Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986‐1998. JAMA 2001;286(22):2845‐8. [DOI] [PubMed] [Google Scholar]
Summerbell 2003
- Summerbell C, Waters E, Edmunds. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001872] [DOI] [PubMed] [Google Scholar]
Tang 2000
- Tang JL, Liu JL. Misleading funnel plot for detection of bias in meta‐analysis. Journal of Clinical Epidemiology 2000;53(5):477‐84. [DOI] [PubMed] [Google Scholar]
WHO 1997
- World Health Organization. Obesity, preventing and managing the global epidemic: Report of the WHO consultation of obesity. Geneva: World Health Organization, 1997. [PubMed] [Google Scholar]


