Abstract
Background:
Irradiative sterilization of clinical specimens prior to chemical laboratory testing provides a way to not only sterilize pathogens and ensure laboratorian safety but also preserve sample volume and maintain compatibility with quantitative chemical diagnostic protocols. Since the compatibility of clinical biomarkers with gamma irradiation is not well characterized, a subset of diagnostic biomarkers ranging in molecular size, concentration, and clinical matrix, was analyzed to determine recovery following gamma irradiation.
Methods:
Sample irradiation of previously characterized quality control materials (QCs) at 5 Mrad was carried out at the Gamma Cell Irradiation Facility at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. Following irradiation, the QCs were analyzed alongside non-irradiated QCs to determine analyte recovery between dosed and control samples.
Results:
Biomarkers for exposure to abrin, ricin, and organophosphorus nerve agents (OPNA) were analyzed for their stability following gamma irradiation. The diagnostic biomarkers included adducts to butyrylcholinesterase, abrine, and ricinine, respectively, and were recovered at over 90 % of their initial concentration.
Conclusions:
The results from this pilot study support the implementation of an irradiative sterilization protocol for possible mixed-exposure samples containing both chemical and biological threat agents (mixed CBTs). Furthermore, irradiative sterilization significantly reduces a laboratorian’s risk of infection from exposure to an infectious agent without compromising chemical diagnostic testing integrity, particularly for diagnostic assays in which the chemical analyte has been shown to be fully conserved following a 5 Mrad irradiative dose.
Keywords: Gamma irradiation, mixed chemical/biological threats, irradiative sterilization, laboratory safety, pathogen reduction, pathogen inactivation, mixed CBTs
Background
Gamma irradiation is the most penetrating of electromagnetic radiation, with energies exceeding 100 kiloelectron volts (keV), wavelengths below 10 picometers (pm), and frequencies greater than 100 exahertz (EHz) (1). Commercially, gamma irradiation has been used for over 60 years for routine sterilization of medical devices, pharmaceuticals, and cosmetics (2). A gamma cell irradiator can be used to expose a sample to high levels of radiation, such as 50 kGy (5 Mrad). The level of radiation required to reduce the concentration of most viruses by an order of magnitude or 90 % (D10 value) is 3–5 kGy(1, 3). Of biological pathogens, viruses generally have the highest radiation resistance, exceeding that of fungi and bacteria (1). Therefore, a 5 Mrad dose of gamma irradiation is expected to reduce any suspected biological pathogen by at least 90 %.
Initial screening for pathogens can delay testing for specific chemical agents. Additionally, if limited sample volume is an issue, these pathogen screens will consume a portion of the specimen and reduce the number of chemical diagnostic assays that can be conducted. Pathogen reduction prior to chemical testing could be carried out using a solvent-detergent method, methylene blue treatments, Mirasol (Terumo BCT, Lakewood, CO, USA), INTERCEPT (Cerus Corporation, Concord, CA, USA), THERAFLEX UV (Macopharma, Mouvax, France), or gamma irradiation (4–7). Of these, gamma irradiation conserves sample volume, allows pathogen reduction under frozen conditions, ensures safe handling under biosafety level 2 (BSL-2) conditions, and is compatible with common quantitative chemical biomarkers and their analysis methods, including liquid chromatography coupled to mass spectrometry (LC-MS) (1, 2, 8).
The CDC’s Division of Laboratory Sciences (DLS) is equipped with Biological Safety Level 2 (BSL-2) laboratories for chemical exposure testing. However, some biological threat agents require handling in a BSL-3 or -4 laboratory. Gamma irradiation ensures the safe handling of mixed chemical/biological threat (CBT) specimens prior to their chemical analysis in a BSL-2 laboratory. It is not well characterized, however, whether high levels of radiation will affect the integrity of storage transport containers for specimens or the recovery of diagnostic biomarkers. In the initial pilot study presented herein, (1) containers and vials were irradiated to test for weaknesses and (2) the recovery of several biomarkers of exposure to chemical threats were evaluated following gamma irradiation at 5 Mrad. These biomarkers included protein adducts to butyrylcholinesterase (BChE) from exposure to organophosphorus nerve agents (OPNAs) as well as small molecule biomarkers of exposure to the ribosome-inactivating proteins abrin and ricin (9–13). Abrine (N-methyl tryptophan) and ricinine (3-cyano-4-methoxy-N-methyl-2-pyridone) share a common plant source with abrin and ricin, respectively. The structures of BChE, abrine, and ricinine are provided in Figure 1 and were evaluated by mass spectrometric detection following irradiation doses of 5 Mrad. These biomarkers ranged in analyte size, sample matrix, and concentration level which are representative of samples tested in a chemical exposure analyses.
Figure 1. Pilot study biomarkers to evaluate post-irradiative recovery.
Chemical structures of the biomarkers (A) butyrylcholinesterase, (B) abrine, and (C) ricinine are provided.
Methods
Safety
No pathogens were used in this study as the focus was on container and biomarker stability under commonly used sterilization conditions. Therefore, specimens were handled in BSL-2 laboratories, and BSL-3 or BSL-4 safety levels were not required. Appropriate safety control measures (including engineering, administrative, and personal protective equipment) were used for all gamma irradiation procedures based on a site-specific risk assessment that identified physical, health, and procedural hazards. Ricinine is toxic (mice i.p. LD50 = 0.34 g/kg) (14), and care was taken to avoid inhalation, ingestion, skin, or eye exposure. All blood products used were acquired from a commercial source and did not meet the definition of human subjects as specified in 45-CFR 46.102 (f).
Materials
High-performance liquid chromatography (HPLC) grade acetonitrile, methanol, and water were purchased from Fisher Scientific (St. Louis, MO, USA). Formic acid (98 %) was purchased from Sigma-Aldrich (Pittsburgh, PA, USA). Laboratory deionized water (18 MΩ-cm) was filtered in-house using an Aqua Solutions Water Purification system (Jasper, GA, USA). Tween-20, triethanolamine buffer, phosphate buffered saline, dimethyl pimelimidate dihydrochloride and tris-buffered saline were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dynabeads for immunomagnetic separation of BChE from blood products were obtained from Life Technologies (Carlsbad, CA, USA). BChE monoclonal antibodies from clone 3E8 were purchased from BioPorto (Hellerup, Denmark). King Fisher deep well plates, shallow well plates, tip combs, protein precipitation plates, heat-sealing foil and 96-well autosampler PCR plates were all obtained from Thermo Fisher Scientific (Waltham, MA, USA). Filter plates (96-well 0.45 μm, PVDF) were obtained from Millipore (Billerica, MA, USA). Strata-X SPE plates (96-well 60-mg) were obtained from Phenomenex (Torrance, CA, USA).
The Netherlands Organisation for Applied Scientific Research (TNO; Rijswijk, Netherlands) provided synthetic native and isotopically-labeled BChE and P-BChE peptides. Battelle Memorial Institute (Columbus, OH, USA) provided synthetic native and isotopically-labeled MeP-BChE, ExP-BChE, EtP-BChE, and PrP-BChE peptides. Commercial pooled serum was purchased from Bioreclamation, Inc. (Westbury, NY, USA). GA- (tabun), GB- (sarin), GE- (ethyl sarin), GF- (cyclosarin), PrGB- (propyl sarin), VR- (Russian VX), and VX-inhibited serum were purchased from Battelle Memorial Institute (Columbus, OH, USA). Pooled quality control (QC) materials were prepared by mixing inhibited sera in varying ratios. QC-High (QH) was a 1:1 (v:v) mixture of GB-inhibited serum and aged GB-inhibited serum. QC-Mid (QM) was a 1:1:1 (v:v:v) mixture of VX-inhibited serum, aged GA-inhibited serum, and aged GE-inhibited serum. QC-Low (QL) was a 1:1:1:1:1 (v:v:v:v:v) mixture of uninhibited serum:VR-inhibited serum, GD-inhibited serum, GF-inhibited serum, and PrGB-inhibited serum.
Stock solutions of ricinine (100 μg/mL in acetonitrile), abrine (100 μg/mL in water), 13C6-ricinine (275 ng/mL in water), and 13CD3-abrine (4125 ng/mL in water) were obtained from Cerilliant (Round Rock, TX, USA). Calibrators and QCs were prepared by diluting stock solutions of abrine and ricinine in filtered pooled plasma (0.22 μm PES filter) from Tennessee Blood Services (Memphis, TN, USA). Calibrators and QCs for ricinine and abrine in urine were obtained from Cerilliant (Round Rock, TX, USA). Calibrators were diluted 1:25 with filtered pooled urine (0.22 μm PES filter) obtained from Tennessee Blood Services. QC concentrations in urine and plasma were 200, 20.0, and 0.800 ng/mL (1.22, 0.122, and 0.00487 nM) ricinine and 350, 80.0, and 8.00 ng/mL (1.60, 0.367, and 0.0367 nM) abrine for QH, QM, and QL, respectively.
Sample Preparation for OP-BChE
For immunomagnetic separation of BChE from blood products, the BChE antibody was conjugated to Protein G-coated DynaBeads. Preparation of the beads began by aliquoting 2 mL of beads washing them with two-4 mL volumes of phosphate buffered saline containing Tween 20. Beads were next suspended in 8 mL of PBS-tween and 400 μg of BChE antibody was added. Beads were rotated overnight (16 hours) at room temperature. After incubation, the PBS-tween/antibody mixture was removed and beads were washed with two volumes of triethanolamine (TEA) buffer (Sigma-Aldrich; St. Louis, IL, USA). Beads were then incubated for 30 min in a 5.4 mg/mL solution of dimethyl pimelimidate in TEA buffer at room temperature. Supernatant was removed, and the beads were washed with 4 mL of PBS-tween. Beads were incubated again with 4 mL of PBS-Tween at room temperature for 15 min. Beads were washed two additional times in 2 mL volumes of PBS-Tween and re-suspended in 1.9 mL of PBS-tween. Beads were stored in this solution at 4 °C until they were used.
For QC and sample analysis, 125 μL of each plasma QC was thawed and filtered through a 96-well 0.45 μm PVDF filter plate (Millipore; Billerica, MA, USA) and centrifuged at 3000 g for 5 min. After this time, samples were transferred to a 96-deep-well plate and magnetic beads were added using a Thermo Fisher Scientific (Waltham, MA, USA) KingFisher liquid handling system. The bead-plasma suspension was shaken at 1400 rpm for 45 min. While shaking, a digestion plate was prepared by adding 75 μL of 0.6 % formic acid to a 96-well plate followed by 10 μL of 2 mg/mL solution of pepsin, also in 0.6 % formic acid. A 10 μL volume of a 250 ppb solution of isotopically-labeled synthetic peptides was added for quantification. After incubation was complete, beads bound to BChE were washed three times with PBS-tween and transferred to the digestion solution via the KingFisher liquid handling system, incubated at 37 °C for 30 min, and shaken at 1000 rpm. After incubation, the KingFisher liquid handling system was used to remove the beads from the digestion solution. The remaining digestion solution was mixed with 285 μL of acetonitrile to precipitate proteins and filtered through a protein precipitation plate (Thermo Fisher Scientific, Waltham, MA, USA) under vacuum. The eluent was then dried under a stream of nitrogen at 60 °C for 45 min. Samples were re-suspended in 0.6 % formic acid and transferred to an Eppendorf (Hauppauge, NY, USA) 96-well plate for loading into the LC autosampler.
Sample Preparation for Abrine and Ricinine
For urine calibrators, QCs, matrix blanks (MB), and samples, a 30 μL aliquot of internal standard (ISTD) mix was dispensed into each well of a 96-well deep well plate. For plasma, a 15 μL aliquot of ISTD mix was used. A 200 μL aliquot of each urine calibrator, QC, MB or sample was added to their respective wells. A 100 μL aliquot was used for plasma. For plasma, 85 μL of water was added to each well. The plate was covered with adhesive foil and shaken at 1000 rpm for 1 min. Solid-phase extraction (SPE) was then carried out on a vacuum manifold using a 96-well 60-mg Strata-X SPE plate. The sorbent was conditioned with 1000 μL of methanol and equilibrated with 1000 μL of water. The entire sample (~200 μL) was loaded onto the sorbent bed. The sorbent was washed with 1000 μL of 5 % methanol in water and then the analytes were eluted to a 96-well deep well with 800 μL of acetonitrile. For plasma, the eluent from each well was transferred to a 96-well protein precipitation plate and eluted to a deep 96-well plate. The samples were evaporated to dryness (~50 min) under nitrogen at 60 °C using a Porvair UltraVap96 (Porvair Sciences, Norfolk, UK). The samples were reconstituted in 100 μL of water, shaken at 1000 rpm for 0.5 min, and transferred to a 96-well PCR plate. The PCR plate was heat-sealed and then analyzed by HPLC coupled to tandem mass spectrometry (HPLC-MS/MS).
Gamma irradiation at 5 Mrad
Irradiation of QC materials was carried out at the Gamma Cell Irradiation Facility at the CDC in Atlanta, GA (Scheme S1). Cryogenic vials and 5×5 vial containers (Fisher Scientific, Hampton, NH, USA) were irradiated without samples to evaluate post-irradiative aging in common storage containers for urine and blood specimens. The same containers were used with the urine and blood matrices in the pilot study. Vials in the 5×5 containers were packed in a secondary container within a re-sealable zip bag (SEVA Technical Services, Newport News, VA, USA) with absorbent pads (SAF-T-PAK an Inmark Company, Austell, GA, USA). Samples remained frozen during shipment and irradiation. The irradiation time needed to reach 5 Mrad was calculated each day based on the dose-rate. Note that no internal standard addition or any other sample preparation was performed prior to gamma irradiation.
Calculations
The recovery of analytes after gamma irradiation at 5 Mrad was calculated by , where Concpost is the concentration of the analyte after irradiation with 5 Mrad, and Concpre is the concentration of the analyte in the same QC material before irradiation. Pooled QC materials that were previously characterized were used to evaluate control of the assay following gamma irradiation. The Microsoft Excel 2016 t-test function was used to calculate p-values for population comparisons.
Results
Post-irradiative aging of sample containers has been reported to cause stress and cracking of some plastics (2). Therefore, empty sample vials were dosed at 5 Mrad prior to pilot sample testing to ensure container integrity at maximum dose levels. The storage boxes used to hold sample vials were observed to yellow following irradiation, but no cracking in the boxes or sample vials was observed (Figure S1). Re-sealable zip bags with absorbent pads were packed with each set of pilot samples to mitigate potential spills from vial cracks should they occur.
The recoveries for each analyte subjected to 5 Mrad gamma irradiation are provided in Table 1. The recovery of biomarkers of exposure to abrin and ricin was evaluated using QC materials spiked with abrine and ricinine. Low, middle, and high concentration QCs (n=3 each) in both urine and plasma were irradiated. The average recovery for all QCs in both matrices was 100 ± 12 % for abrine and 112 ± 19 % for ricinine. Similarly, QCs (n=3) in serum for OP-BChE adducts were dosed with 5 Mrad gamma irradiation. All three QC materials contained unadducted BChE above the lowest reportable limit (LRL) for the method (2 ng/mL), two contained MeP-BChE above the LRL, and the remainder of analytes were only present in one of the QC materials. The recovery for unadducted BChE was 108 ± 31 %. The recoveries of unaged biomarkers of exposure to GB, GD, GF, PrGB, VR, and VX ranged from 96 ± 8 % to 107 ± 2 %. The unaged biomarkers are BChE adducts GB-BChE, GD-BChE, GF-BChE, PrGB-BChE, VR-BChE, and VX-BChE, respectively. The aged biomarker recoveries ranged from 98 ± 13 % to 136 ± 12 %. The aged BChE adducts are EtP-BChE, MeP-BChE, P-BChE, and PrP-BChE, and their respective agents of exposure are listed in Table 1. It should be noted that two of the aged biomarkers had recoveries over 125%. These aged biomarker are products of hydrolysis and may benefit from the presence of radiation stabilizers
Table 1.
Recovery of diagnostic analytes following irradiation with 5 Mrad.
| Diagnostic Analyte | Agent of Exposure | % Recovery | n* |
|---|---|---|---|
|
| |||
| Abrine | Abrin | 100 ± 12 | 18 |
| Ricinine | Ricin | 112 ± 19 | 18 |
| BChE | N/A | 108 ± 31 | 9 |
| EtP-BChE | Ethyl Sarin (GE) | 98 ± 13 | 3 |
| GB-BChE | Sarin (GB) | 96 ± 8 | 3 |
| GD-BChE | Soman (GD) | 100 ± 9 | 3 |
| GF-BChE | Cyclosarin (GF) | 96 ± 6 | 3 |
| MeP-BChE | Sarin (GB), Soman (GD), Cyclosarin (GF), Russian VX (VR), VX | 127 ± 18 | 6 |
| P-BChE | Tabun (GA) | 104 ± 26 | 3 |
| PrGB-BChE | Propyl Sarin (PrGB) | 107 ± 2 | 3 |
| PrP-BChE | Propyl Sarin (PrGB) | 136 ± 12 | 3 |
| VR-BChE | Russian VX (VR) | 104 ± 10 | 3 |
| VX-BChE | VX | 98 ± 15 | 3 |
n is dependent on the number of QCs that include each analyte
Since abrine and ricinine were irradiated in both urine and plasma, a direct comparison was made between their recoveries in the different matrices. The average recovery of abrine and ricinine in urine was 111 ± 14 %, and the analyte recovery in plasma was 101 ± 18 %. When the two population data sets were compared, a p-value of 0.0957 was obtained, indicating that at a confidence level of 95 %, there is no difference between the analyte recovery in urine and plasma matrices.
The molecular size of the biomarker of interest was subjected to a similar comparison. In this comparison, abrine and ricinine were labeled micromolecules, while unadducted and adducted BChE recoveries comprised the population of macromolecules. The average recovery of abrine and ricinine in plasma was 101 ± 18 %, while the average recovery of the BChE macromolecules in serum was 109 ± 21 %. When the two population data sets were compared, a p-value of 0.196 was obtained, indicating that at a confidence level of 95 %, there is no difference between the analyte recovery between the micro- and macromolecule populations.
Finally, the concentration of the biomarker was taken into account by comparing the recoveries obtained at the two populations of low and high quality control levels (QL and QH) for abrine and ricinine. Recoveries of both analytes in urine and plasma comprised the population data sets used for this comparison. The recovery of abrine and ricinine for QL (5.00 ng/mL abrine and 0.3 ng/mL ricinine) was 117 ± 16 %, and the recovery for QH (350 ng/mL abrine and 200 ng/mL ricinine) was 103 ± 16 %. When the two population data sets were compared, a p-value of 0.0329 was obtained, indicating that at a confidence level of 95 %, there is an observed difference between the analyte recovery in low and high concentrations. Of note, the error bars for the two populations do overlap in Figure 2, and the p-value would suggest no difference at a higher confidence level of 98.5 %. Future work in this area will likely provide more information on the effects of analyte concentration on recovery following irradiative sterilization.
Figure 2. Comparison of the effects of matrix, molecular size, and concentration on percent recovery following gamma irradiation at 5 Mrads.
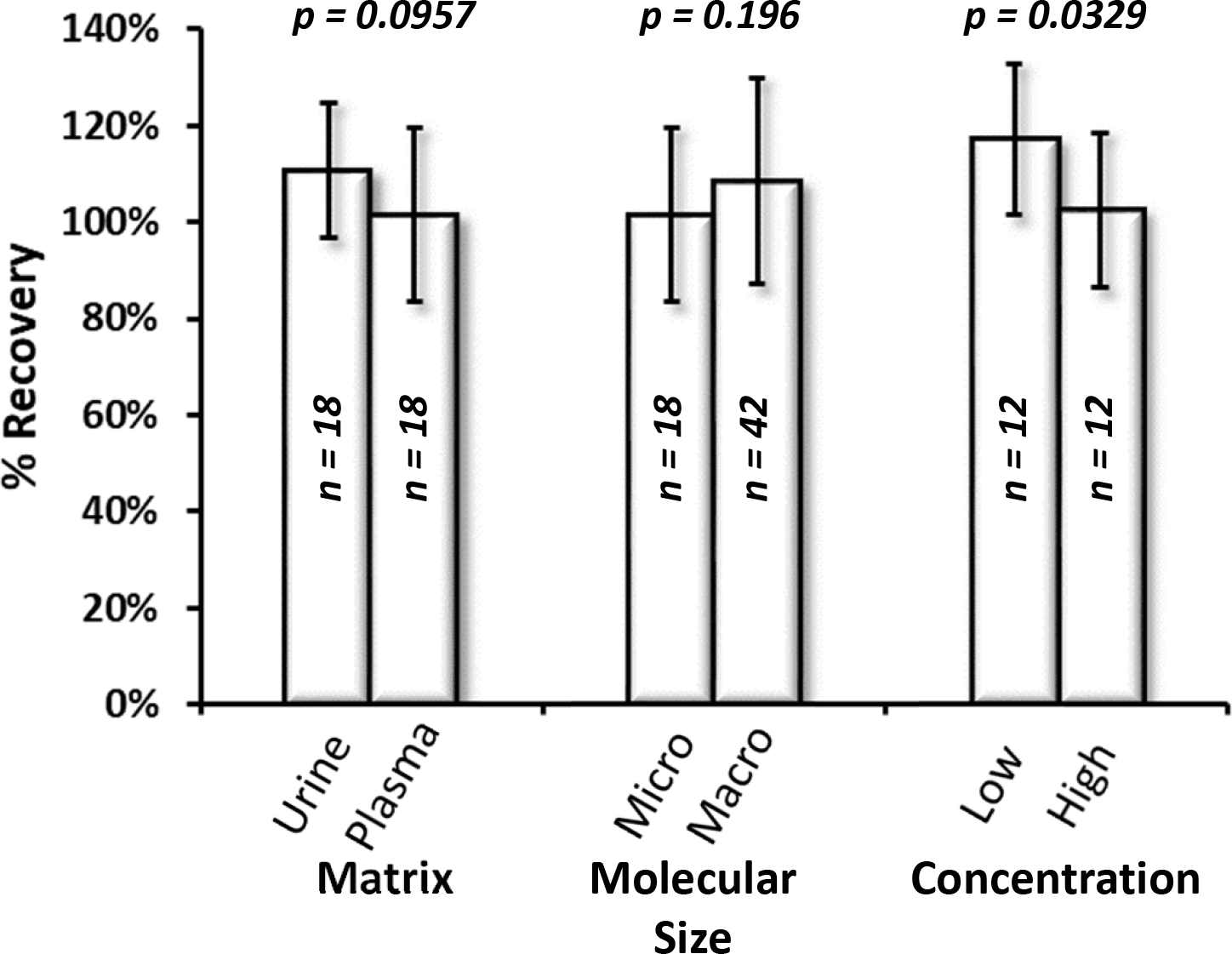
Matrix comparison includes abrine and ricinine in urine (Urine, n=18) compared to plasma (Plasma, n=18). Molecular size comparison includes abrine and ricinine in plasma (Micro, n=18) and unadducted and adducted BChE (Macro, n=42). Concentration comparison includes abrine and ricinine QL in urine and plasma (Low, n=12) and abrine and ricinine QH in urine and plasma (High, n=12).
Conclusions
The high biomarker recoveries observed in this pilot study support the use of gamma irradiation for the sterilization of mixed CBT exposure specimens prior to their analyses in BSL-2 laboratories. In this study, the majority of chemical biomarkers were retained at over 90 % recovery following irradiative sterilization at 5 Mrad, a dose that has been shown in the literature to inactivate most biological pathogens. The results reported from this pilot study are the first of their kind, and future testing of additional diagnostic biomarkers in clinical matrices will be used to determine whether gamma irradiation can be implemented universally or whether alternative sterilization techniques may be needed for chemical laboratories. This innovative and translational approach of merging biological safety protocols and chemical diagnostic workflows introduces a novel strategy for safeguarding laboratorians performing chemical analyses on mixed CBT exposure specimens.
Supplementary Material
Scheme S1. Workflow for gamma cell irradiation of clinical specimens. The process of preparing, irradiating, and returning clinical specimens.
Figure S1. Container tolerance following irradiation with 5 Mrad dose. Containers on the right were not irradiated while containers on the left were dosed with 5 Mrad.
Impact:
The Centers for Disease Control and Prevention (CDC) maintains readiness to detect human exposure to chemical and/or biological threat (CBT) agents. Conventionally, suspected mixed CBT specimens are screened for pathogen testing prior to chemical testing, which delays the results for chemical testing and depletes sample volume. Our work tests irradiative sterilization as a new protocol for mixed CBT specimens prior to assessing exposure to chemical threats. Herein, we investigate the stability of chemical biomarkers following sterilization by gamma radiation. If successful, this new protocol will both ensure safety of laboratorians and expedite results of chemical testing.
Acknowledgements
This work was supported by the Centers for Disease Control and Prevention, specifically the Center for Preparedness and Response and the Epidemiology Elective Program. The authors would like to acknowledge the continued support of Dr. Joanne Andreadis and Mr. John Kools. The authors would also like to thank Ms. Chariety Sapp, Ms. Stephanie Negrete, and Ms. Tonya Tuberville of the CDC’s Incidence Response Laboratory for sample handling, processing, and transport.
Footnotes
Conflict of Interest Disclosure
The authors declare no competing financial interest.
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
References
- 1.da Silva Aquino KA. Sterilization by gamma irradiation, gamma radiation. In: Adrovic F, editor Gamma radiation Rijeka, Croatia: InTech; 2012. p. 171–206. [Google Scholar]
- 2.IAEA. Trends in radiation sterilization of health care products. Vienna: International Atomic Energy Agency; 2008. p. 1,12,124 [Google Scholar]
- 3.Department of agriculture (2014), gamma irradiation as a treatment to address pathogens of animal biosecurity concern, department of agriculture, canberra, australia. Vol., 2014. [Google Scholar]
- 4.Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply - time to raise the bar. New England Journal of Medicine 2015;372:1882–4. [DOI] [PubMed] [Google Scholar]
- 5.Schlenke P Pathogen inactivation technologies for cellular blood components: An update. Transfusion Medicine and Hemotherapy 2014;41:309–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picker SM. Current methods for the reduction of blood-borne pathogens: A comprehensive literature review. Blood Transfusion 2013;11:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundt JM, Rouse L, Van den Bossche J, Goodrich RP. Chemical and biological mechanisms of pathogen reduction technologies. Photochemistry and Photobiology 2014;90:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IAEA. Effects of ionizing radiation on blood and blood components: A survey. Vienna: International Atomic Energy Agency; 1997 [Google Scholar]
- 9.Johnson RC, Zhou Y, Jain R, Lemire SW, Fox S, Sabourin P, Barr JR. Quantification of l-abrine in human and rat urine: A biomarker for the toxin abrin. Journal of Analytical Toxicology 2009;33:77–84. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg SL, Carter MD, Miller MA, Noras AI, Mojica MA, Carlsen ST, et al. Quantification of ricinine and abrine in human plasma by hplc–ms-ms: Biomarkers of exposure to ricin and abrin. Journal of Analytical Toxicology 2018:bky040–bky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RC, Lemire SW, Woolfitt AR, Ospina M, Preston KP, Olson CT, Barr JR. Quantification of ricinine in rat and human urine: A biomarker for ricin exposure. Journal of Analytical Toxicology 2005;29:149–55. [DOI] [PubMed] [Google Scholar]
- 12.Pantazides BG, Watson CM, Carter MD, Crow BS, Perez JW, Blake TA, et al. An enhanced butyrylcholinesterase method to measure organophosphorus nerve agent exposure in humans. Analytical and Bioanalytical Chemistry 2014;406:5187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham LA, Johnson D, Carter MD, Stout EG, Erol HA, Isenberg SL, et al. A high-throughput uhplc–ms/ms method for the quantification of five aged butyrylcholinesterase biomarkers from human exposure to organophosphorus nerve agents. Biomedical Chromatography 2017;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraz AC, Angelucci ME, Da Costa ML, Batista IR, De Oliveira BH, Da Cunha C. Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from ricinus communis. Pharmacology, biochemistry, and behavior 1999;63:367–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme S1. Workflow for gamma cell irradiation of clinical specimens. The process of preparing, irradiating, and returning clinical specimens.
Figure S1. Container tolerance following irradiation with 5 Mrad dose. Containers on the right were not irradiated while containers on the left were dosed with 5 Mrad.


