Abstract
Although many persons with obesity can lose weight by lifestyle (diet and physical activity) therapy, successful long-term weight loss is difficult to achieve, and most people who lose weight regain their lost weight over time. The neurohormonal, physiological, and behavioral factors that promote weight recidivism are unclear and complex. The National Institute of Diabetes and Digestive and Kidney Diseases convened a workshop in June 2019, titled “The Physiology of the Weight-Reduced State,” to explore the mechanisms and integrative physiology of adaptations in appetite, energy expenditure, and thermogenesis that occur in the weight-reduced state and that may oppose weight-loss maintenance. The proceedings from the first session of this workshop are presented here. Drs. Michael Rosenbaum, Kevin Hall, and Rudolph Leibel discussed the physiological factors that contribute to weight regain; Dr. Michael Lowe discussed the biobehavioral issues involved in weight-loss maintenance; Dr. John Jakicic discussed the influence of physical activity on long-term weight-loss maintenance; and Dr. Louis Aronne discussed the ability of drug therapy to maintain weight loss.
Summary
Four presentations were made in a session that focused on describing the potential mechanisms responsible for the high rate of recidivism after weight loss in people with obesity and therapeutic approaches: (1) Drs. Michael Rosenbaum, Kevin Hall, and Rudolph Leibel discussed “Physiology of the Weight-Reduced State: Factors Opposing Maintenance of Reduced Body Weight,” (2) Dr. Michael Lowe discussed “Biobehavioral Dysregulation, Behavioral Phenotypes, and Weight Loss Maintenance,” (3) Dr. John Jakicic discussed “Weight Loss and Weight Loss Maintenance: Response to Behavioral and Lifestyle Interventions, and (4) Dr. Louis Aronne discussed “Pharmacology of Weight Loss Maintenance.”
The factors involved in the regulation of body weight are complex and they involve homeostatic mechanisms that maintain energy intake to prevent the adverse effects of excessive food deprivation; habitual, social, and stress behaviors that lead to “unconscious” or “passive” consumption of food; and hedonic mechanisms driven by cravings, pleasure, and reward. These processes are further influenced by gene–environment interactions. Drs. Rosenbaum, Hall, and Leibel suggested that the physiological and behavioral changes opposing active periods of weight loss differ from those opposing the maintenance of a reduced body weight. This is an important distinction because it implies that the therapy used for initial weight loss in people with obesity should be fundamentally different from the therapy used for people with obesity who have lost weight. Although the effects of active weight loss on substrate and energy metabolism and circulating hormones are different than after weight loss has been achieved and maintained, the major factor responsible for successful weight loss and weight maintenance is a sustained decrease in energy intake. Therefore, it is important to understand the barriers that prevent most people who lose weight from continuing to consume a reduced-calorie diet. The common experience of weight recidivism supports the notion of a predetermined body weight “set point,” which is the concept that each person is biologically and genetically programmed to maintain a certain weight. The set point hypothesis has been expanded to consider a system that defends body fat so that energy stores are returned to baseline after changes caused by periods of energy imbalance (negative or positive). However, this concept does not adequately consider the environmental, behavioral, social, and hedonic influences on food intake that contributed to the development of obesity before weight loss occurred and therefore contribute to weight regain after weight loss.
Dr. Lowe reviewed the concept of active (cravings, hedonic hunger, emotional eating, or food addiction) and passive food consumption that drives appetitive behavior and increases food intake after weight loss. Although this area is critically important in understanding the mechanisms responsible for weight recidivism, very little is known about how the weight-reduced state affects active and passive regulation of food intake. Moreover, the mechanisms responsible for the considerable heterogeneity in weight regain are not known. Dr. Lowe suggested that two novel factors should be considered that might predict long-term weight-loss success and failure: (1) each person’s level of “weight suppression,” which is the relationship between a person’s initial weight at the start of weight-loss therapy and their highest-ever weight, and (2) the degree of week-to-week or month-to-month variability in body weight during the initial 6 months of intentional weight loss.
Dr. Jakicic reviewed data that suggested that physical activity is an important lifestyle behavior that is associated with long-term maintenance of weight loss. However, the design of most studies makes it difficult to determine whether increased physical activity was responsible for, or simply associated with, long-term weight-loss success. Moreover, additional research is needed to determine the most effective use of physical activity in preventing weight regain, including the pattern and volume of activity and when increased physical activity should be introduced into a weight-management program.
Dr. Aronne discussed the importance of anti-obesity pharmacotherapy, particularly combination drug therapy, to enhance initial weight loss and improve long-term weight maintenance. It is important to appreciate individual variability in the response to weight-loss medications, which is not captured by mean group changes in body weight. It is not possible to determine how an individual patient will respond to a specific drug; therefore, it is reasonable to consider a 12-week evaluation of any weight-loss medication and terminating therapy if adequate success is not achieved.
The Physiology of the Weight-Reduced State: Factors Opposing Maintenance of Reduced Body Weight
Introduction
It is widely agreed that prolonged maintenance of nonsurgically reduced body weight is difficult (1,2). This difficulty was comprehensively addressed at a National Institutes of Health workshop titled “Physiology of the Weight Reduced State” in Bethesda, Maryland, on June 3-4, 2019. This manuscript is a summary of two presentations (“The Physiology of the Weight-Reduced State: Factors Opposing Weight Loss Maintenance” [RLL and KDH] and “Weight Loss, Weight Loss Maintenance, and Weight Regain—Distinct Physiologic States or Parts of a Continuum?” [MR and KDH]) regarding distinctions between losing weight and maintaining a weight-reduced state.
Most individuals trying to sustain successful weight loss report a need to consciously restrict food intake below what hunger dictates, and that increased energy expenditure in moderately vigorous to vigorous physical activity (exercise) is generally helpful (3). The relative long-term constancy of body weight in adult humans (both with and without obesity) at an average of approximately 0.3 to 0.5 kg/y (4) (3,000 kcal/y of stored energy) in the setting of more than approximately 800,000 kcal/y ingested (5) is compatible with, but does not prove, the operation of homeostatic mechanisms for body weight regulation. Such mechanisms are consistent with the concurrent operation of socioenvironmental, “psychological,” and other contexts in which the homeostatic mechanisms operate. This type of interaction is well recognized in genetics by the so-called “norm of reaction,” which describes the powerful impact of environment on the salience (penetrance) of gene-mediated phenotypes (6). Periods of active weight loss (negative energy balance) and sustained periods at a reduced body weight (energy balance) are both associated with hypometabolism and hyperphagia, which “conspire” to facilitate weight regain (7,8).
These homeostatic changes are the result, at least in part, of changes in skeletal muscle (increased work efficiency), neuronal signaling related to energy intake (increased food reward and impulsivity, delayed satiation), and neuroendocrine function (decreased circulating concentrations of bioactive thyroid hormones and leptin) (7,9-11). We have studied the responses of people with and without obesity to experimental weight loss by examining their physiology in three weight-stable states: usual (customary) body weight, following a 10% weight loss (12,13), and at that 10% reduced body weight while receiving doses of leptin sufficient to restore circulating concentrations to those present when stable at their usual body weight (14). The coordinate changes in drive to eat (increased) and energy expenditure (decreased) as a result of weight loss create a “perfect physiological storm” for weight regain. An unexpected observation is that most of the reduction in energy expenditure occurs as a result of increased chemo-mechanical contraction efficiency of skeletal muscle (15,16). That is, in an analogy to automobile performance, the weight-reduced individual gets more mileage per calorie of energy expended to a degree beyond that predicted solely by weight loss. This remarkable change in skeletal muscle physiology is consistent with the reduced thyroid hormone and sympathetic nervous activity that characterizes the weight-reduced state (12,15,16). Weight loss, hypothyroidism (17,18) and sympathectomy (19) result in similar skeletal muscle phenotypes.
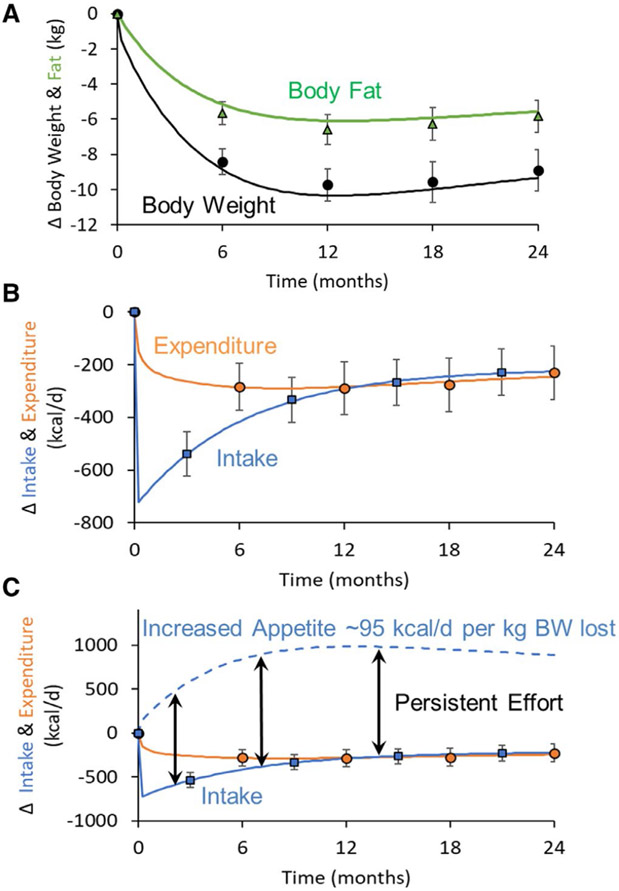
For people with obesity, about 75% of hypocaloric dietary weight reduction is accounted for by loss of body fat (13). Circulating concentrations of leptin decline in proportion to, or slightly more than predicted by, body fat loss during reduced-weight maintenance and substantially more during active weight loss (9). The administration of exogenous leptin in physiological doses restores most of the energy and behavioral physiology to pre-weight-loss status in weight-stable weight-reduced individuals (9,14,20,21). The constellation of changes is summarized in Figure 1.
Figure 1.
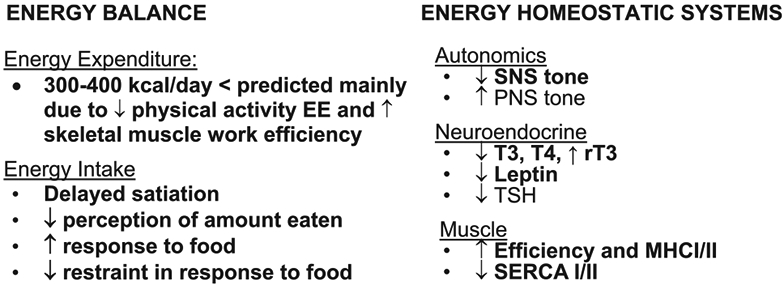
Changes from baseline in energy balance and homeostatic systems during maintenance of a 10% or greater reduced body weight and responsiveness to exogenous leptin in individuals who initially had obesity or never had obesity (9). Energy expenditure owing to physical activity is calculated as the difference between direct measurement of 24-hour energy expenditure and measurement of resting energy expenditure plus diet-induced thermogenesis. Eating behavior, including energy intake, is examined by visual analog scales during a fixed liquid formula meal, kilocalories of the liquid formula consumed to reach satiation, and functional MRI (fMRI) studies of brain responses to food. Assessments of autonomic nervous system activity were made by analyses of heart rate variability during sequential blockade of the PNS and SNS with atropine and esmolol, respectively, and by 24-hour urine catecholamine excretion. Skeletal muscle contractile efficiency was measured by graded bicycle ergometry. MHC and SERCA muscle gene expression studies were done by mRNA quantification in biopsies of vastus lateralis muscle. Entries in bold are at least partially reversed by leptin repletion in weight-reduced individuals. MCH, myosin heavy chain; PNS, parasympathetic nervous system; rT3, reverse T3; SERCA, sarcoplasmic endoplasmic reticulum Ca++-dependent ATPase; SNS, sympathetic nervous system; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone.
These physiological changes that occur with weight loss can account for the most common responses to diet-mediated weight reduction, i.e., early weight loss that plateaus within 1 year. Mathematical models have been developed to help quantitatively understand the energy balance dynamics in response to lifestyle (22,23) and pharmacological (24) interventions for obesity. Such mathematical models were derived from a wealth of data collected over the past century quantifying how energy metabolism and body composition change during controlled human feeding studies, and the models were then validated by comparing predictions with results of human studies that were not used to calibrate the models (25). This paper discusses insights derived from systems regulating energy homeostasis and a mathematical model regarding the similarities and differences between periods of dynamic weight loss and reduced-weight maintenance, with implications for therapies to sustain weight loss.
Mathematical models
Mathematical models of energy intake and expenditure during weight-loss interventions illustrate changes in these parameters over time and as a function of weight loss. Figure 2 shows the simulated body composition and energy balance dynamics in men with overweight during an intensive 2-year intervention aimed at achieving a constant 25% restriction of caloric intake, i.e., a sustained reduction in energy intake of about 700 kcal/d relative to baseline (22). Body weight and body fat decrease rapidly at the beginning of the intervention but reach a plateau within about 1 year. Energy expenditure decreases rapidly by ~200 kcal/d at the onset of the intervention followed by a more gradual reduction as body mass is progressively lost. Despite the reductions in energy expenditure and body mass, energy intake progressively increases after its initial reduction by ~700 kcal/d at the onset of the caloric restriction to only ~300 kcal/d below baseline at 12 months.
Figure 2.
Mathematical model simulations of the mean changes in body composition and energy balance dynamics of 35 men with overweight (mean BMI = 26 kg/m2) with mean age of 40.5 years participating in a 2-year intervention designed to reduce energy intake by 25% (22). (A) Body weight (•) and body fat (Δ) data along with model simulations (curves) showing the rapid early losses followed by a plateau at 12 months. (B) Mean data for changes in energy expenditure (○) and intake (□), along with error bars representing standard errors, were plotted along with the mean model simulations (curves) showing rapid early decreases in both energy expenditure and intake followed by a progressive exponential rise in intake to eventually balance expenditure at 12 months. (C) Model-predicted increases in appetite (dashed curve) were calculated assuming that, for each kilogram of lost weight, appetite increased above baseline by 95 kcal/d. Following the initial reduction in energy intake, its subsequent exponential increase toward baseline (solid blue curve) was an expected response of a persistent and approximately constant effort to adhere to the prescribed diet intervention as quantified by the difference between the dashed appetite and solid intake curves.
Mathematically, the physiological states of active weight loss over the first several months and maintenance of lost weight after about a year are quantitatively different with regard to energy expenditure and intake. Interestingly, and as shown in the energy intake plot in Figure 2B, maintaining lost weight requires a relatively small persistent decrease in energy intake compared with what is required to lose the weight in the first place. Specifically, Figure 2B shows that energy intake was substantially lower over the first 6 months during active weight loss than it was after 12 months during maintenance of lost weight. So why is maintaining lost weight so difficult?
Increased appetite likely plays a quantitatively greater role than decreased energy expenditure because the feedback circuits controlling long-term energy intake have greater overall strength compared with the feedback circuits controlling calorie expenditure. As shown in Figure 2C, appetite increases progressively above baseline (dashed blue curve) as a result of the weight loss and is an approximately constant amount higher than the actual energy intake (solid blue curve) (26). In other words, a relatively persistent intervention intensity is required to counter both increased appetite and decreased energy expenditure even after reaching the reduced body weight plateau. Specifically, it has been estimated that, for each kilogram of lost weight, calorie expenditure decreases by about 25 kcal/d whereas appetite increases by about 95 kcal/d above baseline levels prior to weight loss (26). The greater strength of the negative feedback circuit controlling appetite might explain why high levels of physical activity are often associated with successful maintenance of lost weight (27). Specifically, increased physical activity may allow for increased energy intake at the same level of weight loss (28), thereby mitigating the persistent effort required to counter increased appetite.
The mathematical model simulations illustrate the likely reasons why body weight reaches a plateau during weight loss and indicate the need for a persistent intervention intensity to maintain lost weight over the long term. In contrast with the observed increases in energy intake following the initial decrease at the onset of the intervention, achieving a constant decrease in energy intake from baseline would require people to increase their efforts over time to counter the progressive increases in appetite with weight loss. Such a constant reduction in energy intake would eventually result in a body weight plateau, when energy expenditure decreases to match intake, but this process takes several years (23) and is incompatible with the ubiquitous pattern of weight loss in response to a variety of weight-loss interventions in which the plateau occurs within ~12 months. The model simulations in Figure 2 illustrating a relatively constant effort to adhere to a 25% calorie restriction do not explain the reason for weight regain that is often observed following the body weight plateau (29). One possibility is that intervention intensity wanes after achieving what is often perceived as a disappointing amount of weight loss at the plateau (30). In other words, patients may be unwilling to engage in the same effort to sustain the intervention if they no longer perceive the ongoing benefits of weight loss.
Alternatively, it is possible that the physiological and behavioral factors that oppose long-term maintenance of lost weight and result in weight regain may be distinct from those factors responsible for resisting further weight loss. This distinction relates to an important question about whether the same therapeutic interventions (lifestyle, pharmacotherapy, surgery, etc.) should be effective in producing versus maintaining weight reduction. The physiological similarities and differences between weight loss and reduced-weight maintenance are discussed subsequently.
Predictors
If the metabolic and behavioral factors opposing weight loss and those responsible for weight regain were all part of a physiological continuum, one might predict that genotypic and phenotypic predictors of these processes would be identical. For example, early weight loss in the initial few months of a lifestyle (diet and physical activity) or pharmacological intervention for obesity are predictive of subsequent weight loss and final weight (31,32). However, while early weight loss does predict the magnitude of sustained weight loss (one definition of successful reduced-weight maintenance), it is not significantly correlated with the absolute amount of weight regained after weight loss or the slope of weight regain over time following successful weight loss (31).
As shown in Table 1, there are a greater number of dissimilar [brain-derived neurotrophic factor (BDNF), FTO (encodes an alpha-ketoglutarate dependent dioxygenase), potassium channel tetramerization domain containing 15 (KCTD15), mitochondrial translational initiation factor 3 (MTIF3), transcription initiation factor IIE subunit β (TFA2β), and transmembrane protein 18 (TMEM18)] than similar [neuronal growth regulator 1 (NEGR1), peroxisome proliferator activated receptor γ (PPARγ)] genotypes that are predictive of the rate and/or amount of weight loss and regain (33-35). This incongruence suggests that physiologically distinct mechanisms may be affected by attempts to lose weight versus keeping it off and also that genetic markers could potentially be used to modify therapeutic options for individual patients based on projected difficulties in losing weight versus keeping it off.
TABLE 1.
Correlations of SNPs with weight loss (first 6 mo of intervention) and weight regain (6 mo to ~2 y) in the Look AHEAD and DPP trials
| Weight loss | Weight regain | |
|---|---|---|
| BDNF | ||
| rs6265 (33) | N.S. | 0.55 (0.21) kg/allele/y, P = 0.011 |
| FTO | ||
| rs3751812 (34) | N.S. | 1.56 (0.55) kg/allele/3 y, P < 0.005a |
| rs9939609 (34) | N.S. | 1.03 (0.52) kg/allele/3 y, P < 0,049a |
| rs9922708 (34) | N.S. | 1.38 (0.55) kg/allele/3 y, P < 0.012a |
| KCTD15 | ||
| rs29941 (33) | 0.50 (0.24) kg/allele/y, P = 0.041b | N.S. |
| MTIF3 | ||
| rs1885988 (34,35) | −0.73 (−1.30 to −0.15) kg/allele/3 y, P = 0.009c | N.S. |
| NEGR1 | ||
| rs2815752 (33) | −0.79 (0.29) kg/allele/2 y, P = 0.006b | −0.35 (0.16) kg/allele/y, P = 0.034 |
| PPARγ | ||
| rs1801282 (33) | −0.63 (0.22) kg/allele/0.5 y, P = 0.005 | −0.79 (0.27) kg/allele/y, P = 0.004 |
| TFA2β | ||
| rs2272903 (34) | −0.93 (0.31) kg/allele/2 y, P = 0.003 −0.64 (0.31) kg/allele/y, P = 0.037c | N.S. |
| TMEM18 | ||
| rs6548238 (33) | 0.62 (0.31) kg/allele/y, P = 0.044b | N.S. |
Data refer to the mean (SD) number of kilograms of weight gain that can be attributed to each “dose” of the relevant allele over the specified unit of time (0.5-3 y depending upon study).
BDNF, brain-derived neurotrophic factor; FTO, fat mass and obesity-associated gene; KCTD15, potassium channel tetramerization domain containing 15; MTIF3, mitochondrial translational initiation factor 3; TFA2β, transcription initation factor IIE subunit β; TMEM18, transmembrane protein 18; NEGR1, neuronal growth regulator 1; PPARγ, peroxisome proliferator activated receptor γ; DPP, Diabetes Prevention Program; DSE, Diabetes Support and Education; ILI, intensive Lifestyle Intervention; N.S., not significant; PPARG, XXX; SNP, single-nucleotide polymorphism.
Only significant in the DSE group of the Look AHEAD trial.
Only significant in the metformin-treated group of the DPP trial.
Only significant in the ILI group of the Look AHEAD trial.
Examination of the correlations of some of these phenotypes (Table 2) shows that there are more dissimilar behavioral (hunger, depression, anxiety), physiological (resting metabolic rate), and biochemical (leptin, ghrelin, angiotensin-converting enzyme) phenotypes than similar anatomic (BMI) and behavioral (dietary restraint) phenotypes associated with weight loss and weight regain (36-46). The average effect size for listed genes is about 0.50 kg/allele/y, which is a small effect relative to the average weight loss of ~5 kg in year 1 and to the ~3 kg weight regain between years 2 and 4 (Table 1). R values for weight loss and weight regain based on associated phenotypes (Table 2) suggest that such phenotypes account for 10% to 50% of outcome variance during weight loss (0.5-2.5 kg) or weight regain (0.3-1.5 kg) over the same time periods.
TABLE 2.
Phenotypes significantly correlated with weight loss (first 6 mo of intervention) and weight regain (6 mo to 2-4 y depending upon study)
| Weight status | Weight loss | Weight regain | |
|---|---|---|---|
| Adiposity (36,37) | Baseline | r = 0.27, P < 0.001 | r = −0.24, P = 0.05 |
| Hunger (38) | Changes with weight loss | r = 0.35, P = 0.002a | |
| Dietary restraint (39,40)b | Initial | r = −0.15, P = 0.03 | |
| Changes with weight loss | r = 0.35, P < 0.01 | r = −0.36, P < 0.01 | |
| Anxiety/neuroticism (41) | Baseline | r = 0.50, P < 0.01 | |
| Depression and antidepressant use (42) | Initial | N.S. | Increased hazard ratio for weight regain of 1.31 (P = 0.03) for depression and 1.72 (P < 0.005) for antidepressant use |
| Leptin (adjusted for FM and age)b (43) | Initial | r = 0.30, P = 0.009 | N.S. |
| Ghrelin (43) | Baseline | r = 0.31, P = 0.014 | N.S. |
| Leptin/ghrelinc (43,44) | Baseline | r = −0.33, P = 0.009 | Baseline ratio of leptin/ghrelin was associated with an increased risk for weight regain (odds ratio 1.051; P = 0.008) |
| RMR (36) | Baseline | N.S. | r = −0.38, P = 0.01 |
| ACE (45) | Baseline | N.S. | r = 0.23, P = 0.012 |
| TSHd (46) | Baseline | r = −0.36, P < 0.001 | N.S. |
| Free T3d (46) | Baseline | r = −0.33, P = 0.002 | N.S. |
| ΔTSH from baselined (46) | Reduced | N.S. | r = −0.20, P = 0.009 |
| ΔFree T3 from baselined (46) | Reduced | N.S. | r = −0.22, P = 0.003 |
Data are correlation coefficients of various anatomic, behavioral, biochemical, and physiological phenotypes (independent variables) with weight loss during lifestyle intervention or weight regain once weight loss was completed (dependent variables) except as otherwise indicated. R values are unadjusted unless otherwise noted.
ACE, angiotensin-converting enzyme; FM, fat mass; N.S., not significant; RMR, resting metabolic rate; T3, triiodothyronine; TSH, thyroid-stimulating hormone.
Corrected for meeting attendance and self-monitoring in a 6-month outpatient weight-loss program.
Dietary restraint prior to weight loss and changes occurring during weight loss have been independently associated with the degree of weight loss and weight regain.
Adjusted for fat mass.
Pediatric studies adjusted for age, sex, and pubertal stage and correlated with BMI-SDS at baseline after a 1-year intervention or change in BMI-SDS from year 1-2 after the intervention.
Circulating leptin concentrations during weight stability are highly positively correlated with fat mass (47,48), and initial adiposity is positively correlated with the amount of weight lost as a result of lifestyle intervention (36,37). However, baseline circulating concentrations of leptin, especially when adjusted for baseline fat mass, are significantly negatively correlated with both absolute and fractional weight loss in some (43) but not all (49) studies. Bobbioni-Harsch et al. (50) found that high plasma leptin concentrations were associated with a lower baseline resting energy expenditure (REE) independent of both fat-free mass and fat mass and a greater reduction in REE in response to a hypocaloric diet. It is possible that negative energy balance associated with active weight loss in individuals with a lower baseline leptin/fat mass invokes less hypometabolism and/or hyperphagia. Further research in this area is clearly warranted.
Mechanisms and responses to interventions
A key physiological question is whether or not the individual metabolic and behavioral energy homeostatic systems that oppose changes in body weight operate along a mechanistic continuum involving the same systems to “resist” weight change. Do weight loss and reduced-weight maintenance invoke similar physiological changes that are the opposite of those changes associated with weight regain after weight loss or intentional weight gain above baseline? Available data suggest that some components may operate along a continuum in which greater dynamic (weight loss or gain) or static (maintenance of elevated or reduced weight) perturbations above or below usual weight are associated with opposition in the same systems “defending” usual body weight (Tables 3 and 4) (9,13,51-58). Nonresting energy expenditure is disproportionately increased during and after weight gain, decreased during and after a 10% weight loss, and further decreased after a 20% weight loss (13,59), and it is consistent with such a model. In contrast, REE is disproportionately increased or decreased during weight gain or loss or maintenance of a 10% reduced body weight but does not appear to be increased above predicted during maintenance of a 10% weight gain, nor is there any additional decline in REE during weight loss from 10% to 20% below usual (13,59). Some (55,60), but not all (61), studies have reported little or no effect of weight regain on weight-loss-induced hypometabolism in contrast with the hypermetabolism that occurs during prolonged (>1 month) overfeeding to promote weight gain above usual (62). Differential responses to leptin repletion during and after weight loss provide further evidence that only some aspects of metabolic and behavioral adaptation may fall along a continuum. As discussed subsequently, circulating concentrations of leptin relative to fat mass are significantly lower during caloric restriction than during reduced-weight maintenance. Leptin repletion during caloric restriction has been reported to reduce hunger and appetite but not to affect energy expenditure, autonomic function (24-h urine catecholamine release), or thyroid function (63,64). In contrast, the effects of weight loss on all these systems are at least partially “reversed” by leptin repletion during reduced-weight maintenance or in states of congenital leptin deficiency (9). These data suggest that there is variability in the continuity of different components of the metabolic adaptations to weight change or maintenance of altered body weight. As shown in Figure 2, these systems, whether continuous or discontinuous, can be incorporated into the generation of predictive models of changes in energy intake, output, or stores over time.
TABLE 3.
Comparison of dynamic weight loss and static reduced-weight maintenance
| Active weight loss | Maintenance of a reduced body weight | |
|---|---|---|
| Prior metabolic state | Usual weight (energy balance) | Weight loss (negative energy balance) |
| Current metabolic state | Negative energy balance | Energy balance |
| Changes compared with weight maintenance at usual body weight | ||
| Energy expenditure | ↓↓ REE (~2× reduced-weight maintenance residual) | ↓ REEa |
| ↓ NREE | ↓ NREEa | |
| ↑ Muscle contraction efficiency | ↑ Muscle contraction efficiencya | |
| Neuroendocrine axes | ↓↓ T3, ↓↓T4, ↓↓TSH, ↑rT3 | ↓ T3a, ↓T4a, ↓TSH, ↑rT3 |
| ↓↓ Leptin/FM by 40%-50% | ↓ Leptin/FM by ~10%a | |
| ↑ Cortisol | Cortisol within normal range | |
| ↑ GH | No change or small ↑ GH | |
| Autonomics | ↑↑ PNS tone and ↓↓ SNS tone | ↑ PNS tone and ↓SNS tonea |
| Energy intake | ↓↓ Satiation | ↓ Satiationa |
| ↑↑ Hunger | ↑ Hungera | |
GH, growth hormone; FM, fat mass; nl, XXX; NREE, nonresting energy expenditure; PNS, parasympathetic nervous system; REE, resting energy expenditure; rT3, reverse T3 (bioinactive enantiomer of T3); SNS, sympathetic nervous system; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
TABLE 4.
Comparison of weight gain relative to weight initial and weight regain following weight loss
| Intentional weight gain from baseline body weight |
Weight regain from reduced body weight |
|
|---|---|---|
| Prior metabolic state | Usual weight (energy balance) | Reduced-weight maintenance (energy balance) |
| Present metabolic state | Positive energy balance | Positive energy balance |
| Changes compared with weight maintenance at usual weight | ||
| Energy expenditure per kg FFM | ↑ REE | ↓ REE |
| Muscle contractile efficiency | ↓ | ↑ |
| Hunger | ↓ | ↑ |
| Thyroid | ↓ T3 and T4 | Usual |
| Leptin per kg FM | ↑ | ↓ |
A second key question is whether the metabolic and behavioral adaptations that occur during and after weight change are primarily responsive to signals regarding energy balance (intake vs. output) or energy stores (adipose tissue) or both. The effects of changes in energy balance and stores are mediated by genetics, physical activity, food availability and hedonics, and other factors (see Tables 1 and 2). As discussed subsequently, it is apparent that energy homeostatic systems and their responses to therapeutic intervention are sensitive to signals reflecting both energy balance and energy stores.
If signal(s) regarding energy balance are the major drivers of the metabolic and behavioral opposition to changes in energy stores, then there should be little or no adaptive thermogenesis during reduced or elevated weight maintenance (energy intake = output) beyond those predicted by changes in weight and body composition. In addition, voluntary weight loss below usual weight and weight loss following voluntary overfeeding should pose the same difficulties, whereas voluntary weight gain should result in the same metabolic and behavioral changes as weight regain following weight loss. This is clearly not the case. There is adaptive thermogenesis before and after weight loss; caloric restriction to promote weight loss from baseline results in hypometabolism, which is not evident during weight loss after voluntary overfeeding; and hypometabolism during weight regain is not mimicked during voluntary weight gain above baseline (13,55).
If signal(s) regarding energy stores (fat mass) per se are the key mediators of homeostatic responses to weight change, then there should be little or no difference between metabolic and behavioral changes, or responses to therapies to alter energy balance, in response to weight loss or weight gain and maintenance at elevated or reduced weight. As discussed subsequently, it is apparent that energy homeostatic systems are highly responsive both to changes in energy balance during dynamic weight change and to changes in energy stores as a result of that weight change even during periods of weight stability.
As noted earlier, the effects of exogenous leptin on energy homeostasis are dependent upon the nutritional context in which it is applied (65,66). Administration of leptin to genetically leptin-deficient rodents and humans in “physiological” doses that restore circulating leptin concentrations to levels appropriate for individuals without obesity increases energy expenditure, increases sympathetic nervous system activity (67,68), decreases food intake, and normalizes hypothalamicpituitary-adrenal, thyroid, and gonadal function (48,69-71). In rodents at usual weight, leptin administration in doses that are up to three times “physiological” result in only transient weight loss followed by weight regain (72-74). At higher doses, there is transient weight loss with less weight regain. In humans at usual weight, leptin does not appear to induce sustained weight loss from usual weight and has only a small effect in promoting weight loss during caloric restriction (despite leptin levels that are lower than those during weight maintenance after weight loss) (48,75). In humans who are hypoleptinemic as a result of caloric restriction, administration of 20 mg/wk (63) or 80 mg/wk (64) of pegylated leptin causes a small reduction of hunger but has no significant effect on energy expenditure or neuroendocrine function (63,64).
In contrast with the limited effects of leptin or pegylated leptin administration on energy homeostasis in humans who are either leptin sufficient or losing weight, leptin has potent effects on the hypometabolic, hyperphagic state that characterizes the weight-stable weight-reduced state (14,20,70). Similarly, thyroid hormone repletion after weight loss reverses much of the increased skeletal muscle contractile efficiency and related decline in nonresting energy expenditure, but the same low dose of thyroid hormone has not been shown to promote weight loss at baseline or during caloric restriction (12). Responses to therapeutic interventions to promote weight loss, whether by lifestyle or pharmacotherapy, are dependent on the status of energy stores and current energy balance and their interaction with other factors such as genetics, food availability/hedonics, and exercise.
Neuromolecular mechanisms of energy homeostasis
Body energy stores, predominantly fat, are regulated by multiple systems that conspire to defend these energy stores against energy imbalance and changes in energy stores. Many of these systems are sensitive to leptin, but disproportionately so during attempts to maintain reduced energy stores versus actually to lose weight, and energy homeostatic responses are stronger to leptin depletion than excess (76). The leptin receptor is highly expressed in the cells of the hypothalamic nuclei that play major roles in homeostatic weight regulation (77) and communicate with other telencephalic and diencephalic neurons that mediate behavioral responses to food. Many neurons outside of the hypothalamus also express the receptor, though their functional roles in these cells remain unclear.
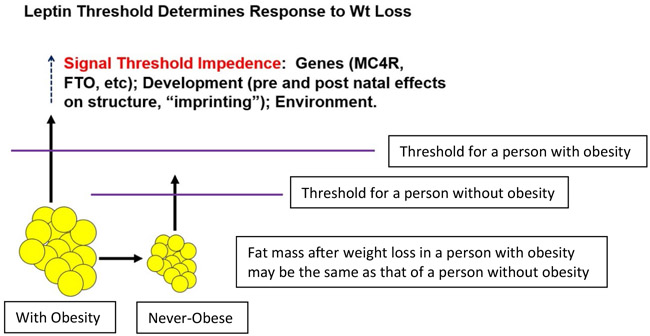
A simple schematic of the physiology is shown in Figure 3. Circulating leptin concentrations in the fed state are determined by fat mass (cell number × size). The “threshold” for sufficiency of leptin action is determined by genetic, developmental, and other factors that influence both the structure of relevant parts of the central nervous system as well as the acute responses of those cells to ambient leptin. The threshold is higher for individuals with obesity than those without obesity, and therefore more leptin (fat mass) is needed to create a state of sufficiency in the central nervous system. Once that level is achieved, further increases have little physiological effect. However, if fat mass is reduced by caloric restriction, once the concentration of leptin falls below the threshold, specific neurons sensing this decline invoke behavioral (hunger) and metabolic (reduced energy expenditure) changes that have the aggregate effect of restoring body fat (leptin). The molecular-cellular threshold does not decrease with weight loss; therefore, the metabolic/behavioral response to declines in leptin does not abate. Such abatement could commit an animal to a state in which reproduction was permanently impaired: an evolutionary dead end. The threshold is an individual molecular-cellular phenotype. Therefore, these responses do not differ qualitatively or quantitatively among individuals with and without obesity. What differs among individuals is the threshold, and therefore leptin signal transduction is dependent upon an individual’s “usual weight” and any ongoing or previous negative energy balance (78).
Figure 3.
A threshold model for leptin action. There are similar responses to declines in leptin beyond individualized “thresholds.” These thresholds are lower in individuals who have never had obesity than in those with obesity. A weight-reduced individual with obesity may thus invoke the potent metabolic and behavioral opposition to sustained weight loss at levels of energy stores and leptin that would not invoke these changes in an individual who has never had obesity.
This model suggests that circulating concentrations of leptin below an individual’s threshold will evoke similar physiological and behavioral changes promoting weight regain regardless of the underlying cause. However, as noted earlier, responses to exogenous leptin are dependent on metabolic context. When leptin concentrations are raised above an individual “desensitization threshold” (which is higher in individuals with obesity), the leptin axis becomes leptin insensitive, perhaps even to the point of facilitating further weight gain owing to impaired leptin signal transduction (65,66). If this is correct, then a small reduction in circulating leptin concentrations, even if they remain above the desensitization threshold, may restore some leptin responsiveness and facilitate weight loss.
A critical question is whether this set point in changeable, particularly in a downward direction. Although there are clearly genetic effects on human adiposity, and although the majority of these genes are expressed in the central nervous system, the precise manner(s) in which these effects are integrated is not yet clear (79). The threshold model is consistent with the phenomenology of the similarity of responses to weight loss among individuals with and without obesity. As indicated in Figure 3, in addition to the effects of alleles of these genes, there are developmental effects on these circuits that can influence an individual’s apparent threshold (“set point”) for minimum body fat (80-83). But can environmental/acquired influences in the adult individual affect the threshold? Lateral hypothalamic ablative lesions in rodents can reduce the apparent set point for body weight maintenance (84). Cancer cachexia and anorexia nervosa are both associated with maintenance of reduced body weight, but both are accompanied by food aversions and/or changes in energy expenditure that suggest that the lower body weight is imposed rather than “accepted” as a new, defended physiological norm. In human patients, the phenotypes associated with increased metabolic efficiency and drive to eat do not abate with time (85-87), suggesting that prolonged maintenance of reduced body weight can only be achieved by indefinite attention to both food intake and exercise (88) (Figure 2). Despite the fact that circulating concentrations of leptin are lower during weight loss than during maintenance at the same weight, individuals sustaining weight loss are clearly more responsive to leptin repletion. These data suggest that the metabolic and behavioral oppositions to sustained weight loss are in response to decreased energy stores rather than a “carryover” effect of caloric restriction.
The other epidemiologically important question is whether prolonged exposure to an environment favoring weight gain by virtue of unlimited access to highly palatable foods can cause irreversible changes in the structure/function of the nervous system resulting in a functional elevation of the threshold that physiologically defends higher levels of fat. Such effects might also be conveyed transplacentally in obese gravidas (89), as evidenced by decreased adiposity of the offspring of women born after versus before bariatric surgery (90). The recent secular increases in adiposity over evolutionarily short periods are consistent with such a mechanism and argue for imposition of vigorous efforts, via environmental manipulation, to prevent obesity throughout the life-span beginning prenatally.
In summary, obesity is a complex phenotype that reflects the interactions of numerous genes that have been “selected” for service for reproductive integrity within modern environments (both prenatal and postnatal) that favor the expression of these genes in a way that is no longer adaptive. The commonality that the mathematical model suggests is that there is a continuum of responses between active weight loss and the plateau of reduced-weight maintenance that are quantitatively different. The molecular physiological bases of weight regain include some of these same factors/mechanisms, but there are likely novel factors and mechanisms as well.
Weight Loss and Weight-Loss Maintenance: Response to Behavioral and Lifestyle Interventions
Introduction
There is global concern regarding the high prevalence rates of overweight (BMI ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) (91). This concern arises because of the association of excess body weight and body fatness with a variety of health-related conditions that include cardiovascular disease, diabetes, some types of cancer, and musculo-skeletal disorders (92,93). The prevalence of overweight is approximately 70% within the United States, with the prevalence of obesity (BMI ≥ 30 kg/m2) approximately 36% and the prevalence of severe obesity (BMI ≥ 35 kg/m2) approximately 16% (94). Thus, while the importance of curtailing excessive weight gain to prevent obesity should be emphasized, there remains a need for effective options to treat obesity and to sustain a reduced-weight state. At the foundation of these approaches to treat overweight and obesity are lifestyle factors, particularly diet and physical activity, that are imbedded within behavior change interventions to elicit initial and then sustained weight loss. For example, at the foundation of effective weight loss are lifestyle factors such as diet and physical activity, and various intervention approaches that use a variety of behavioral strategies should be implemented that focus on achieving the desired change in these important lifestyle factors for weight loss.
Effectiveness of short-term lifestyle and behavioral interventions for weight loss
The two lifestyle factors that are typically the target of weight-loss interventions are dietary approaches that result in reduced energy intake and physical activity to enhance energy expenditure. The combination of these two lifestyle factors for weight loss is consistent with current clinical guidelines for the treatment of obesity (93). Within short-term studies, the combination of these two lifestyle factors typically results in an average weight loss of 8% to 10% of initial body weight (95), which typically represents approximately 8 to 10 kg of weight loss. When examined closely, it appears that in short-term studies (e.g., ≤ 6 months in duration), physical activity contributes approximately 20% (2.0-3.0 kg) of the weight loss achieved (96,97), with the remaining 80% of weight loss resulting from dietary modification that elicits a reduced energy intake (98). For example, this has been shown in studies of adults with overweight through severe obesity (class III obesity) that compared the effects of an energy-restricted diet (ranging from 1,200 to 2,100 kcal/d) with the same diet combined with physical activity, which was prescribed to progress to approximately 60 min/d on 5 d/wk (96,97).
This magnitude of weight loss has been shown to be associated with numerous health benefits (92). The Diabetes Prevention Program demonstrated that this amount of weight lost resulted in reduced onset of type 2 diabetes. More recently, weight loss equivalent to at least 10% of initial body weight was shown to be associated with reduced odds of cardiovascular events (99). However, the ability to sustain many of these health benefits is closely linked to the ability to sustain weight loss, which is challenging for most individuals. Although the ability to sustain weight loss has been improving in response to lifestyle and behavioral interventions, prevention of weight regain remains a challenge, and this was observed even in some of the most successful large studies of weight loss, including the Diabetes Prevention Program and Look AHEAD study (100,101). Thus, it is of public health importance to identify lifestyle factors within the context of behavioral interventions that are associated with long-term weight loss and attenuation of weight regain.
Lifestyle change for long-term weight loss and attenuation of weight regain
While the balance between both energy intake and energy expenditure is important for body weight regulation, it appears that physical activity is a particularly important lifestyle behavior that is consistently associated with long-term weight loss and attenuation of weight regain. There is a substantial body of literature to support that moderate-to-vigorous physical activity at a dose of approximately 200 to 300 min/wk is associated with greater long-term weight loss (e.g., 18-24 months). This evidence is based on both self-reported (102-105) and objectively measured physical activity from prospective studies (106,107). There is also evidence that a combination of both moderate-to-vigorous and light-intensity physical activity that is of a sufficient dose enhances long-term achievement of at least 10% weight loss (108). However, variability is observed in response to lifestyle interventions, and this warrants additional research to determine for whom these interventions may be most effective.
With regard to physical activity, there are factors that may contribute to variability in long-term weight loss and attenuation of weight regain. There may be differences between individuals in the biological response to physical activity that contributes to variability in weight loss (109), and these responses may include metabolic, molecular, and cellular differences between individuals that warrant examination. Moreover, the variability in response to physical activity may be partially explained by the variability in the effects on energy intake (110,111), variability in the effects on REE (112), or other factors that may impact energy expenditure or overall energy balance.
Additional considerations within the context of physical activity and its influence on body weight regulation may be the timing of when one is engaged in physical activity or how the various components of physical activity (e.g., sedentary behavior, light-intensity, moderate-intensity, vigorous-intensity physical activity) interact. For example, a post hoc analysis of data collected within a 10-month intervention showed that physical activity accumulated early in the day (between 7:00 am and 12:00 pm) was associated with greater weight loss than when physical activity was accumulated later in the day (3:00 pm to 7:00 pm) (113). Whether this pattern of physical activity impacts other aspects of energy balance that may influence body weight regulation, such as energy intake, the circadian cycle or sleep, or other biological responses, is unclear and warrants investigation. Moreover, there is a need for research to understand whether the mode (e.g., aerobic, resistance training, yoga) of physical activity has differential effects on these pathways and therefore body weight regulation.
How one accumulates physical activity and the intensity of that physical activity may also have important considerations. For example, replacing sedentary behavior (e.g., nonsleeping behavior performed in a supine, semi-supine, or seated position) with moderate-to-vigorous physical activity may reduce adiposity; however, replacing sedentary behavior with standing or light-intensity physical activity alone may not reduce adiposity (114). Moreover, physical activity of moderate to vigorous intensity may need to be performed in bouts of at least 10 continuous minutes to demonstrate an effect on weight loss within the context of a behavioral intervention (115). These data may suggest the importance of examining the pattern of physical activity in addition to the total volume of physical activity to better understand the influence on body weight regulation.
Intervention approaches for long-term weight loss and attenuation of weight regain
In addition to the components of energy balance (energy intake and energy expenditure), there is also evidence to support that how a behavioral intervention is designed and implemented may impact the weight loss achieved. One approach is to include additional strategies beyond what is typically included in a standard behavioral intervention. For example, a time-based intervention approach in which behavioral enhancements (additional telephone contacts, supervised exercise, physical activity promotion campaigns) were proactively added across an 18-month intervention demonstrated greater weight loss than when these behavioral enhancements were all added at the start of the intervention or when these enhancements were not provided to the intervention (116). Moreover, stepped-care interventions that integrate more intensive behavioral strategies only when less intensive strategies fail to elicit a desired weight-loss response may be a cost-effective approach to delivery of behavioral interventions that focus on lifestyle factors (117). It may also be important for interventions to target specific behavioral domains or constructs that may be predictive of weight-loss success (118), and interventions may need to focus on enhancing initial weight loss because this appears to be predictive of long-term weight-loss success (119).
Summary
Lifestyle factors (diet and physical activity) are key contributors to body weight regulation. However, there is variability in response to interventions focused on modifying these lifestyle factors, and an important challenge is the ability of these interventions to result in long-term weight-loss success. Physical activity appears to be one of the consistent predictors of enhanced long-term weight loss and attenuation of weight regain; however, the specific pathways by which this occurs are not clearly understood, and this warrants further investigation. Moreover, how behavioral interventions are designed and implemented may also influence long-term effectiveness, and it will be important to determine for whom these interventions are most effective. Therefore, appropriately designed studies focused on understanding independent and combined biological and behavioral pathways of both short-term and long-term weight loss, and attenuation of weight regain following weight loss, are needed to inform more effective interventions for the treatment of overweight and obesity.
Pharmacology of Weight-Loss Maintenance
Introduction
The complexity required to treat patients with obesity is emphasized by the significant failure rate of dietary and behavioral interventions and medical therapies and weight regain following bariatric surgery. Bariatric surgery has been considered the gold standard treatment for obesity and the most effective option, but there are concerns about weight regain over the long term, with data demonstrating that more than 20% of patients experience weight regain with recurrence of comorbidities (120).
A concept crucial to understanding why failure rates are so high in the treatment of obesity is that body weight is under homeostatic control by complex mechanisms, and in particular hypothalamic neurons, which may be damaged in diet-induced obesity (121). In the disease of obesity, there is a disruption of this homeostasis owing to impaired neurohormonal signaling, which effectively shifts the body’s weight set point higher. The weight-reduced state is characterized by hormonal changes that favor weight regain toward one’s higher set point weight (122). Thus, there are neurohormonal reasons beyond lack of dietary adherence that may impact this set point.
In order to counteract the neurohormonal changes in those with reduced obesity in order to produce better long-term weight loss, the use of pharmacotherapy would make sense if weight loss can be maintained and the health of those treated can be improved. The addition of anti-obesity pharmacotherapy in patients with inadequate weight loss from behavioral interventions or weight regain following bariatric surgery appears to produce better efficacy. The use of anti-obesity pharmacotherapy can both enhance initial weight loss and improve longer-term weight maintenance. Currently, only a few medications are Food and Drug Administration approved for long-term use for obesity management. Concerns over their potential adverse effects and costs, due to lack of insurance coverage, have limited their availability and use by practitioners, and less than 1% of eligible patients are being treated according to guidelines. When combined with a behavioral program, mean weight loss generally ranges from 5% to 10% of initial body weight, but there is considerable variability in response, with the proportion of patients achieving ≥5% or ≥10% weight loss approximately twice that of placebo for all approved drugs (123). A consistent predictor of later weight loss is initial weight loss within the first 3 months of treatment; therefore, if the patient has not lost at least 5% of initial weight after 3 months at the full medication dose, it is recommended that the medication be discontinued for lack of efficacy and the patient reevaluated (124).
This section highlights the use of pharmacotherapy to achieve weight loss to achieve and mitigate weight regain. Addition of pharmacotherapy after bariatric surgery, as well as the use of multiple agents with different mechanisms of action to treat obesity and prevent weight regain, are more effective than behavioral intervention and single-agent therapy alone, emphasizing the complex nature of the weight-regulating system and the need to address multiple aspects of the body-weight regulating in order to achieve long-term success. In order to illustrate the fact that weight can be reduced in the long term (>1 y) using medication, four trials 2 years or longer will be reviewed as examples. This is by no means a comprehensive review of the literature in this area but was undertaken to complement the mechanistic and basic science discussions at the conference.
Trials of long-term pharmacologic management of obesity
Long-term studies of anti-obesity drugs include a 3-year-long trial of liraglutide 3.0 mg daily versus placebo in patients with prediabetes (126), a cardiovascular outcome trial of lorcaserin with follow-up up to 5 years (mean = 3.3 y) (127), and 10-year follow-up data on the use of metformin in the Diabetes Prevention Program (125).
Liraglutide.
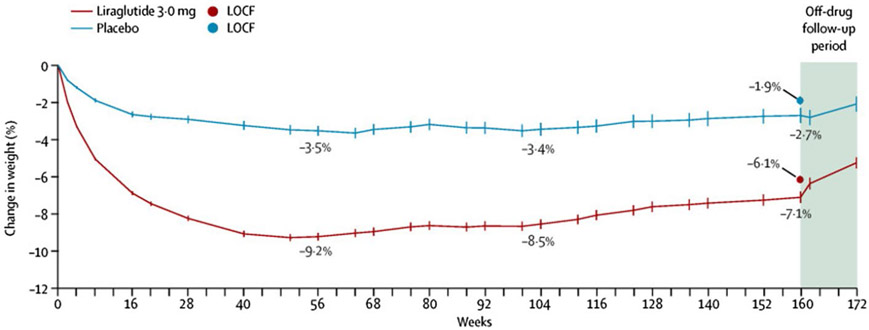
The SCALE Obesity and Prediabetes trial assigned 2,254 patients to receive liraglutide, a glucagon like peptide-1 (GLP-1) agonist (n = 1,505), or placebo (n = 749). Among its effects, GLP-1 has been shown to enhance satiety via central mechanisms. This randomized, double-blind, placebo-controlled trial in adults with prediabetes and a BMI of at least 30 kg/m2, or at least 27 kg/m2 with comorbidities, examined once-daily subcutaneous liraglutide 3.0 mg compared with placebo. Mean age of participants was 47.5 years, mean BMI was 38.8 kg/m2, and 76% were women. Fifty percent of participants completed the 3-year study. After 56 weeks, liraglutide induced greater weight loss than placebo, −9.2 versus −3.5% (difference 5.7%). After 160 weeks, the difference was well maintained in the liraglutide-treated group compared with placebo, −6.1% versus −1.9% (difference −4.3%) (Figure 4). By study end, 26 (1.7%) of 1,472 individuals in the liraglutide group versus 46 (6.2%) of 738 in the placebo group were diagnosed with diabetes while on treatment, a reduction of 73%. While on treatment, 66% of individuals in the liraglutide 3.0-mg group regressed from prediabetes to normoglycemia by week 160 compared with 36% in the placebo group (126).
Figure 4.
Three years’ treatment with liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes. Both arms had dietary intervention.
Lorcaserin.
The CAMELLIA-TIMI 61 trial was the first long-term cardiovascular outcome trial involving a medication used specifically for obesity, lorcaserin, a selective serotonin 2c receptor agonist that suppresses appetite (127). A multinational, randomized, double-blind, placebo-controlled trial recruited 12,000 patients with overweight or obesity (median age 64 years, 64% women, median BMI 35 kg/m2) with or at high risk for atherosclerotic vascular disease. Eligible patients were aged 40 years or older; patients at high risk for atherosclerotic vascular disease had to be aged 50 years or older with diabetes and at least one other risk factor.
After 1 year, weight loss of 5% or greater was found in 38.7% of the group receiving lorcaserin 10 mg twice daily, as compared with 17.4% of the placebo group (P < 0.001). After a median follow-up of 3.3 years, the primary safety outcome (composite of cardiovascular disease death, myocardial infarction, or stroke) was 2% per year in the lorcaserin group and 2.1% per year in the placebo group, demonstrating equivalent cardiovascular disease safety of lorcaserin compared with placebo (P < 0.001). Although several cardiovascular disease parameters, including blood pressure, heart rate, glycemic control, and lipid markers, were slightly more favorable in the lorcaserin group, the primary cardiovascular disease efficacy outcome (composite of major cardiovascular disease events, heart failure, hospitalization for unstable angina, or coronary revascularization) was not found to be superior in the treatment group compared with placebo. This study supports the safety of using lorcaserin in patients with cardiovascular disease (127). Compared with placebo, lorcaserin reduced the risk of incident diabetes by 19% in patients with prediabetes and by 23% in patients without diabetes. However, concern about a 9% increased risk of cancer in the treated group prompted the Food and Drug Administration to ask for lorcaserin to be withdrawn from the market (128).
Combination therapy can produce additive weight loss: phentermine/topiramate
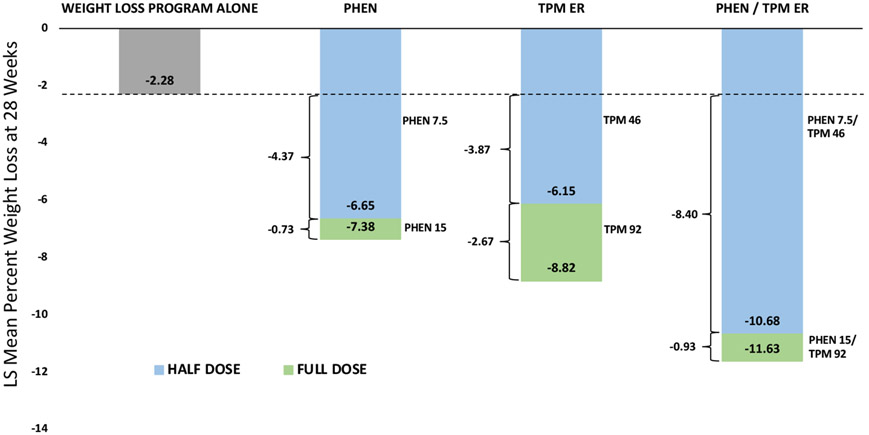
Combination pharmacotherapy is more effective than continued upward titration of a single agent (Figure 5). Targeting several body-weight-regulating pathways simultaneously is likely the most effective approach for mitigating weight regain. This treatment approach is common for other chronic cardiometabolic diseases, such as type 2 diabetes and hypertension. This has been demonstrated by adding topiramate to phentermine as well as by adding phentermine to lorcaserin (129,130).
Figure 5.
Combination therapy can produce greater weight loss: doubling the dose of either phentermine or topiramate does not double the weight loss, whereas adding phentermine to topiramate does give additive weight loss.
Phentermine is a norepinephrine-releasing agent, whereas topiramate has gamma aminobutyric acid stimulating effects which reduce inhibitory tone on pro-opiomelanocortin neurons (131). The combination has been shown to have additive effects on weight loss. In the CONQUER trial, 2,487 participants with a mean age of 51 years, 70% women and with BMI 27–45 kg/m2 (mean 36.6 kg/m2) and two or more comorbidities (hypertension, dyslipidemia, diabetes or prediabetes, or abdominal obesity) were randomized to phentermine/topiramate extended release (ER) 15/92 mg, phentermine/topiramate ER 7.5/46 mg, or placebo (132). The weight loss in patients with and without type 2 diabetes after 56 weeks was 9.8% with phentermine/topiramate ER 15/92 mg, 7.8% with phentermine/topiramate ER 7.5/46 mg, and 1.2% with placebo. There was also a significant decrease in hemoglobin A1c in individuals withtype 2 diabetes mellitus or impaired glucose tolerance who received phentermine/topiramate ER.
The SEQUEL trial (133), a 2-year extension of the CONQUER trial, demonstrated ongoing weight maintenance with phentermine/topiramate ER. In patients who completed 108 weeks, the mean weight loss was 9.3% with phentermine/topiramate ER 7.5/46, and 10.5% with 15/92 mg, compared with 1.8% with placebo. The study also reported a 76% reduction in the progression to diabetes in patients receiving 15/92 mg and a 54% reduction in patients receiving 7.5/46 mg compared with placebo.
Weight loss observed with metformin in the Diabetes Prevention Program
Based on the 10-year follow-up trial of the Diabetes Prevention Program, the use of metformin, the most commonly prescribed oral agent for the treatment of type 2 diabetes, promotes modest weight loss that can be maintained for that period of time in individuals who are compliant with taking metformin. This appears to occur by multiple mechanisms that may counteract some of the hormonal adaptations to weight loss. These include reduction in hepatic glucose production and intestinal absorption of glucose, as well as changes in hypothalamic physiology leading to improved leptin and insulin sensitivity. There is also evidence that metformin decreases ghrelin levels and increases GLP-1 levels, which could further contribute to its anorectic effect (134). Recently, metformin has been shown to increase levels of growth/differentiation factor 15 (GDF-15), which appears to be necessary for the weight-loss effect (135).
Treatment of weight regain following bariatric surgery
Weight regain following bariatric surgery is a well-documented problem that limits efficacy. Current evidence suggests that additional weight loss can be achieved by starting medication at a weight plateau rather than waiting for weight regain. A multicenter review of treated patients looking at addition of anti-obesity pharmacotherapy to bariatric surgery showed that total-body weight loss was 32.3% (SD = 11.4) (8.3%-56.3%) from surgery plus anti-obesity pharmacotherapy in patients who started medications at their weight plateau after bariatric surgery (P = 0.486) versus 26.8%’ (SD = 10.5) (4.3%-60.2%) in patients who started pharmacotherapy after weight regain (136).
Long-term weight loss reported in a clinical setting
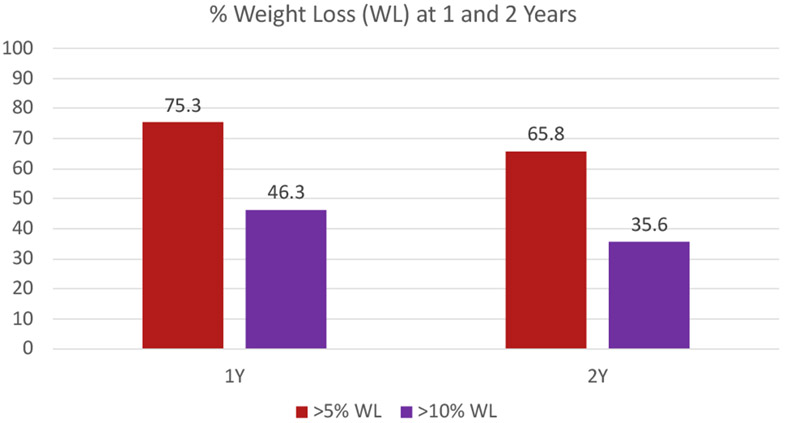
The efficacy of antiobesity medications (AOMs) in combination with lifestyle modification is robustly supported by randomized clinical trials, but there is a paucity of data on their effectiveness for weight-loss maintenance in clinical practice. Data on polypharmacotherapy are also poorly reported. A retrospective analysis of patient data from our own center at Weill Cornell included patients aged 18-75 who were followed for 2 years. At 1 year, 75.3% of patients achieved ≥5% weight loss and 46.3% achieved ≥10% weight loss (Figure 6). Of these cohorts, 87.4% maintained ≥ 5% weight loss (total 65.8% of initial cohort), and 76.9% (total 35.6% of initial cohort) maintained ≥10% weight loss at 2 years. At 2 years, 96.2% of patients were taking ≥1 weight-loss medication, and 79.3% of patients were taking two or more AOMs, with an average of 2.5 ± 1.2 AOMs per patient (137). This study demonstrates that clinically significant weight loss is achievable and maintainable over 2 years with the use of combined AOMs in an academic weight-management center.
Figure 6.
Categorical analysis of >5% or >10% weight loss and maintenance in patients treated with anti-obesity medications at an academic center.
Summary
Medical treatment for obesity continues to develop. The few agents that are currently available produce and maintain weight loss that is greater than a lifestyle modification program alone can achieve over a 2-year period. Addition of pharmacotherapy after bariatric surgery can aid with maintenance as well as further weight loss in many patients who do not have adequate weight loss. The use of multiple agents with different mechanisms of action is more effective than behavioral intervention and single-agent therapy alone, emphasizing the complex nature of the weight-regulating system and the need to address multiple mechanisms in order to achieve long-term success.
Understanding Weight Regain Following Weight Loss: Insights From Four Domains of Behavioral Research
Introduction
The theme of the National Institutes of Health workshop upon which this special series is based was “The Physiology of the Weight-Reduced State.” Historically, the focus of the literature on this topic has been the metabolic, rather than the appetitive, consequences of the weight-reduced state. Nonetheless, whatever the impact of reductions in metabolism following weight loss, post-weight-loss increases in energy intake have the potential to affect weight regain in a rapid and profound fashion (26). By the same token, making even small improvements in control of energy intake following weight reduction could substantially improve people’s ability to sustain weight losses.
Lifestyle or behavioral treatments of obesity have been researched intensively for over 50 years, but improvements in weight-loss maintenance have been minor relative to the amount of time and resources devoted to this objective. The goal of this paper is to briefly review four domains of behavioral research that may shed new light on vulnerability to weight regain. These domains involve eating and/or weight regulation and are included because of their potential relevance to understanding why weight-loss maintenance is so difficult. These four domains are loss-of-control eating, passive versus active sources of overconsumption, the impact of weight suppression (the difference between highest past weight at adult height and current weight), and weight variability (the level of within-person variation in weight around the best-fitting regression line).
Loss-of-control eating
The concept of loss-of-control eating (feeling unable to prevent eating or what or how much is eaten) is central to the study of eating disorders but is less often applied to the study of obesity. Loss of control applies both to objective binge eating (in which a genuinely large amount of food is consumed) and to so-called subjective binge eating (in which an individual feels out of control but eats a relatively normal amount of food). Interestingly, among those with eating disorders, the sense of losing control is a much more reliable reflection of co-occurring psychopathology than the amount eaten during eating bouts (138). It is well known that individuals exhibiting loss of control are also more likely to demonstrate a variety of abnormalities in metabolism and body composition. For example, a recent study (139) found that scores on a measure of loss-of-control eating (the Power of Food Scale, which measures hedonic hunger, or the intensity of desire to consume delicious foods when not hungry) (140) are associated with abnormalities in metabolic hormones in individuals with obesity, independently of BMI. Tanofsky-Kraff and colleagues (141) found that binge eating in children predicted later development of some components of metabolic syndrome, effects that were only partially accounted for by contemporaneous weight gain. Although binge eating disorder per se affects only a small fraction of individuals with obesity (142), the subjective sense of loss of control (independently of amount eaten) is more common and may itself reflect significant metabolic and psychopathological dysfunction (143). These findings indicate that the metabolic and appetitive consequences of weight loss among individuals with obesity and loss-of-control eating may differ from those without loss of control. Although the study of eating addiction is a step in this direction, many individuals without so-called eating addiction will still experience loss-of-control eating.
“Passive” versus “active” sources of overconsumption
The appetitive component of positive energy balance leading to weight gain after weight loss (like that leading to weight gain in the first place) can be subdivided into what might be called passive and active overconsumption. Blundell and MacDiarmid (144) long ago noted that weight gain leading to obesity can occur in an entirely passive, unconscious manner. This can occur when negligible increases in energy intake (e.g., from small increases in the energy density or portion size of familiar foods) creates small, daily, positive energy balances (on the order of a few percent of total daily intake) that, over several years, produces substantial weight gain. The same passive overconsumption presumably contributes to weight regain following weight loss. This type of eating can be differentiated from active or “driven” overconsumption associated with phenomena like cravings, hedonic hunger, emotional eating, or food addiction. Little is known about how the weight-reduced state affects passive and active sources of overconsumption that contribute to weight regain. Research examining this distinction could inform new treatment approaches to weight-loss maintenance.
Weight suppression
A third relevant topic involves viewing the energy balance status of individuals with obesity undergoing weight loss from a longer-term perspective. There is evidence that overweight individuals’ potential for weight loss is affected by the relationship of their initial weight to their highest-ever weight. The discrepancy between these two is called weight suppression (145). (When highest past weight is reached before age 21, this discrepancy is better quantified in terms of z-BMI; see S. Singh et al., unpublished data, YEAR). Call et al. (146) found that individuals high (≥2.04 kg) versus low (<2.04 kg) in weight suppression at the outset of a weight-loss trial lost significantly less weight 1 year later (7.8 vs. 12 kg). This difference is clinically, not just statistically, significant. As is the case in those with eating disorders, where weight suppression has been identified as a robust predictor of future weight gain (147), this finding supports the conclusion that an individual’s past highest weight is interpreted by the body as the “new normal” weight for that individual (148). Future weight-loss studies should consider including weight suppression when analyzing their results because higher starting weight suppression may put a “brake” on an individual’s potential weight loss. Furthermore, this result suggests that weight-loss maintenance may be even more challenging than previously believed because the strength of the forces fueling regain may be based on individuals’ highest past weight, not on the lower weight at which they undertake weight loss.
Weight variability during weight loss
A final topic related to the physiology of the weight-reduced state is an individual’s capacity for weight regulation. One way of assessing this capacity is to determine how much an individual’s weight varies from week to week. Relative to total energy intake and expenditure over long periods of time, people maintain a remarkable degree of weight stability over months or years (149). Nonetheless, the degree to which people’s weight varies over short periods of time can be quantified by collecting a series of body weights, fitting a regression line to these weights and determining the degree of variation of these weights from the regression line (called the “root mean squared error,” or RMSE). In an initial study, we showed in college students prone to weight gain that higher RMSE measured over 6 months predicted greater weight gain from 6 to 24 months (150). In a second study, we showed that weight variability over 3 years was related to weight gain over those 3 years, to probability of having two overweight parents, and to the pattern of brain activation in reward and inhibitory areas in response to the taste of a milkshake (151). In a third study, we showed that RMSE during intentional weight loss predicts smaller subsequent weight losses (152). Greater weight variability scores may reflect a reduced capacity for homeostatic weight regulation per se. Such a deficiency might also contribute to reduced ability to maintain a weight loss. If this is perspective is accurate, and if increased weight variability is causally related to poorer weight-loss outcomes, teaching individuals to stabilize their food and energy intake patterns might improve weight-loss and/or weight-maintenance outcomes (153).
To sum up this brief review, several behavioral domains related to eating or weight regulation may be pertinent to the physiology of the weight-reduced state. First, the subjective sense of loss-of-control eating may interact with the physiology of the weight-reduced state both appetitively and metabolically. Second, the obesity field has researched the metabolic consequences of weight loss far more than the appetitive consequences, even though increased appetite may be far more influential than slowed metabolism in weight regain (26). Studying the role of enhanced appetite might also benefit by differentiating between so-called passive and active overconsumption. Third, it is well known that there is tremendous individual variation in the amount of weight lost in weight-loss studies. A partial explanation for this variability is that weight-loss outcomes are in part shaped by each participant’s level of weight suppression (146) at the outset of a trial. Fourth, a further novel explanation for wide differences in weight-loss outcomes is the degree of weight variability experienced as participants lose weight (152,154). These findings could suggest new treatment avenues for improving weight-loss maintenance (153).
Acknowledgments
Dr. Aronne gratefully acknowledges the assistance of Dr. Rekha Kumar (manuscript development).
Funding agencies:
This work was also supported, in part, by the Intramural Research Program of the National Institutes of Health, NIH UL1 TR00040 (Columbia CTSA [MR]) and DK 52431.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Wing R, Phelan S. Long-term weight maintenance. Amer J Clin Nutr 2005;82:222S–225S. [DOI] [PubMed] [Google Scholar]
- 2.Phelan S, Wing R. Prevalance of successful weight loss. Arch Intern Med 2005;165:2430. [DOI] [PubMed] [Google Scholar]
- 3.Thomas J, Bond D, Phelan S, Hill J, Wing R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 2014;46:17–23. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford E, Dietz W. Trends in energy intake among adults in the United States: Findings from NHANES. Amer J Clin Nutr 2013;97:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewontin RC. The analysis of variance and the analysis of causes. Int J Epidemiol 2006;35:520–525. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum M, Leibel R. Metabolic responses to weight perturbation. In: Christen Y, Clément K, eds. Novel Insights into Adipose Cell Functions, Research and Perspectives in Endocrine Interactions. Berlin Heidelberg: Springer-Verlag; 2010:121–133. [Google Scholar]
- 8.Hall K, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am 2018;102:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Leibel R. 20 years of leptin: Role of leptin in energy homeostasis in humans. J Endocrinol 2014;223:T83–T96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulloo A, Schutz Y. Adaptive thermogenesis in resistance to obesity therapies: Issues in quantifying thrifty energy expenditure phenotypes in humans. Curr Obes Rep 2015;4:230–240. [DOI] [PubMed] [Google Scholar]
- 11.Larrouy D, Barbe P, Valle C, et al. Gene expression profiling of human skeletal muscle in response to stabilized weight loss. Am J Clin Nutr 2008;86:125–132. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum M, Goldsmith RL, Haddad F, et al. Triiodothyronine and leptin repletion in humans similarly reverse weight-loss induced changes in skeletal muscle. Am J Physiol Endocrinol Metab 2018;315:E771–E779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Eng J Med 1995;332:621–628. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin KM, Joanisse DR, Haddad F, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Endocrinol Metab 2011;301:R1259–R1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Endocrinol Metab 2003;285:R183–R192. [DOI] [PubMed] [Google Scholar]
- 17.Taylor D, Rajagopalan B, Radda G. Cellular energetics in hypothyroid muscle. Eur J Clin Invest 1992;22:358–365. [DOI] [PubMed] [Google Scholar]
- 18.Gosselin L, Zhan W, Sieck G. Hypothyroid-mediated changes in adult rat diaphragm muscle contractile properties and MHC isoform expression. J Appl Physiol 1996;80:1934–1939. [DOI] [PubMed] [Google Scholar]
- 19.Kardos A, Taylor DJ, Thompson C, et al. Sympathetic denervation of the upper limb improves forearm exercise performance and skeletal muscle bioenergetics. Circulation 2000;13:2716–2720. [DOI] [PubMed] [Google Scholar]
- 20.Kissileff H, Thornton J, Torres M, Mayer L, Kalari V, Rosenbaum RLM. Leptin reverses decline in satiation in weight-reduced obese individuals. Am J Clin Nutr 2012;95:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Sy M, Pavlovich K, Leibel R, Hirsch J. Leptin reverses weight loss–induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Brager D, Hall K. Simulating long-term human weight-loss dynamics in response to caloric restriction. Am J Clin Nutr 2018;107:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall K, Sacks G, Chandramohan G, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall K, Sanghvi A, Gobel B. Proportional feedback control of energy intake during obesity pharmacotherapy. Obesity (Silver Spring) 2017;25:2088–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall K Modeling metabolic adaptations and energy regulation in humans. Annu Rev Nutr 2012;32:35–54. [DOI] [PubMed] [Google Scholar]
- 26.Polidori D, Sanghvi A, Seeley R, Hall K. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring) 2016;24:2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paixão C, Dias CM, Jorge R, et al. Successful weight loss maintenance: A systematic review of weight control registries. Obes Rev 2020;21:e13003. doi: 10.1111/obr.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melby C, Paris H, Sayer R, Bell C, Hill J. Increasing energy flux to maintain diet-induced weight loss. Nutrients 2019;11:2533. doi: 10.3390/nu11102533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz M, VanWormer J, Crain A, et al. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755–1767. [DOI] [PubMed] [Google Scholar]
- 30.Foster G, Wadden T, Vogt R, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85. [DOI] [PubMed] [Google Scholar]
- 31.Unick JL, Neiberg RH, Hogan PE, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23:1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tronieri S, Wadden T, Chao A, Pearl R, Alamuddin N, Berkowitz R. Early weight loss in behavioral treatment predicts later rate of weight loss and response to pharmacotherapy. Ann Behav Med 2019;53:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delahanty LM, Pan Q, Jablonski KA, et al. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diab Care 2012;35:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffery JM, Papandonatos GD, Huggins GS, et al. FTO predicts weight regain in the Look AHEAD clinical trial. Int J Obes (Lond) 2013;37:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papandonatos GD, Pan Q, Pajewski NM, et al. Genetic predisposition to weight loss and regain with lifestyle intervention: analyses from the Diabetes Prevention Program and the Look AHEAD randomized controlled trials. Diabetes 2015;64:4312–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogels N, Diepvens K, Westerterp-Plantenga M. Predictors of long-term weight maintenance. Obes Res 2005;13:2162–2168. [DOI] [PubMed] [Google Scholar]
- 37.Hansen DL, Astrup A, Toubro S, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi-centre STORM trial. Sibutramine Trial of Obesity Reduction and Maintenance. Int J Obes Relat Metab Disord 2001;25:496–501. [DOI] [PubMed] [Google Scholar]
- 38.Batra P, Das SK, Salinardi T, et al. Eating behaviors as predictors of weight loss in a 6 month weight loss intervention. Obesity (Silver Springs) 2013;21:2256–2263. [DOI] [PubMed] [Google Scholar]
- 39.Foster G, Wadden T, Swain R, Stunkard A, Platte P, Vogt R. The Eating Inventory in obese women: Clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord 1998;22:778–785. [DOI] [PubMed] [Google Scholar]
- 40.Vogels N, Westerterp-Plangenga MS. Successful long-rerm weight maintenance: A 2-year follow-up. Obesity (Silver Spring) 2007;15:1258–1266. [DOI] [PubMed] [Google Scholar]
- 41.Munro IA, Bore MR, Nunro D, Garg ML. Using personality as a predictor of diet induced weight loss and weight management. Int J Behav Nutr Phys Act 2011;8:129. doi: 10.1186/1479-5868-8-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price DW, Ma Y, Rubin RR, et al. Depression as a predictor of weight regain among successful weight losers in the Diabetes Prevention Program. Diabetes Care 2013;36:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labayen I, Ortega F, Ruiz J, Lasa A, Simon E, Margareto J. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab 2011;96:E996–E1000. [DOI] [PubMed] [Google Scholar]
- 44.Crujeiras AB, Campion J, Díaz-Lagares A, et al. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept 2013;186:1–6. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Holst C, Wodzig W, et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int J Obes (Lond) 2012;36:1545–1551. [DOI] [PubMed] [Google Scholar]
- 46.Wolters B, Lass N, Reinehr T. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol 2013;168:323–329. [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Nicolson M, Hirsch J, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 1996;81:3424–3427. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel R. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 1997;82:3647–3654. [DOI] [PubMed] [Google Scholar]
- 49.Niskanen L, Haffner S, Karhunen L, Turpeinen A, Miettinen H, Uusitupa M. Serum leptin in obesity is related to gender and body fat topography but does not predict successful weight loss. Eur J Endocinol 1997;137:61–67. [DOI] [PubMed] [Google Scholar]
- 50.Bobbioni-Harsch E, Assimacopoulos-Jeannet F, Lehman T, Munger R, Allaz A, Golay A. Leptin plasma levels as a marker of sparing-energy mechanisms in obese women. Int J Obes Relat Metab Disord 1999;23:470–475. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum M, Hirsch J, Murphy E, Leibel R. The effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 2000;71:1421–1432. [DOI] [PubMed] [Google Scholar]
- 52.Sumithran P, Proietto J. The defence of body weight: A physiological basis for weight regain after weight loss. Clin Sci (Lond) 2013;124:231–241. [DOI] [PubMed] [Google Scholar]
- 53.Sumithran P, Prendergast L, Delbridge E, et al. Long-term persistance of hormonal adaptations to weight loss. N Eng J Med 2011;365:1597–1604. [DOI] [PubMed] [Google Scholar]
- 54.Karschin J, Lagerpusch M, Enderle J, Eggeling B, Müller MJ, Bosy-Westphal A. Endocrine determinants of changes in insulin sensitivity and insulin secretion during a weight cycle in healthy men. PLoS One. 2015;10:e0117865. doi: 10.1371/journal.pone.0117865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring) 2016;24:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Andrade PBM, Neff LA, Strosova MK, et al. Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding. Front Physiol 2015;6:254. doi: 10.3389/fphys.2015.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolaczynski JW, Considine RV, Ohannesian J, et al. Responses of leptin to short-term fasting and refeeding in humans: A link with ketogenesis but not ketones themselves. Diabetes 1996;45:1511–1515. [DOI] [PubMed] [Google Scholar]
- 58.Kolaczynski J, Ohannesian J, Considine R, Marco C, Caro J. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab 1996;81:4162–4165. [DOI] [PubMed] [Google Scholar]
- 59.Rosenbaum M, Leibel RL. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring) 2016;24:1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Müller MJ, Enderele J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosy-Westphal A, Schautz B, Lagerpusch M, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond) 2013;37:1371–1377. [DOI] [PubMed] [Google Scholar]
- 62.Bray GA, Bouchard C. The biology of human overfeeding: A systematic review. Obes Rev 2020;21:e13040. doi: 10.1111/obr.13040 [DOI] [PubMed] [Google Scholar]
- 63.Westerterp-Plantenga M, Saris W, Hukshorn C, Campfield L. Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am J Clin Nutr 2001;74:426–434. [DOI] [PubMed] [Google Scholar]
- 64.Hukshorn C, Meneheere P, Westerterp-Plantenga M, Saris W. The effect of pegylated human recombinant leptin (PEG-OB) on neuroendocrine adaptations to semi-starvation in overweight men. Eur J Endocrinol 2003;148:649–655. [DOI] [PubMed] [Google Scholar]
- 65.Zhao S, Zhu YI, Schultz RD, et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab 2019;30:706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LeDuc C, Leibel R. Auto-regulation of leptin neurobiology. Cell Metab 2019;30:614–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solinas G, Summermatter S, Mainieri D, et al. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett 2004;577:539–544. [DOI] [PubMed] [Google Scholar]
- 68.Satoh N, Ogawa Y, Katsuura G, et al. Sympathetic activation of leptin via the ventro-medial hypothalamus: Leptin-induced increase in catecholamine secretion. Diabetes 1999;48:1787–1793. [DOI] [PubMed] [Google Scholar]
- 69.Wardlaw S Clinical review 127: Obesity as a neuroendocrine disease: lessons to be learned from proopiomelanocortin and melanocortin receptor mutations in mice and men. J Clin Endocrinol Metab 2001;86:1442–1446. [DOI] [PubMed] [Google Scholar]
- 70.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Eng J Med 1999;341:879–884. [DOI] [PubMed] [Google Scholar]
- 71.Leibel RL, Chua SC, Rosenbaum M. Obesity. In: Scriver C, Beaudet A, Sly W, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; 2001:3965–4028. [Google Scholar]
- 72.Pelleymounter M, Cullen M, Healy D, Hecht R, Winters D, McCaleb M. Efficacy of exogenous recombinant murine leptin in lean and obese 10- to 12-mo-old female CD-1 mice. Am J Physiol 1998;275:R950–R959. [DOI] [PubMed] [Google Scholar]
- 73.Campfield L, Smith F, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–548. [DOI] [PubMed] [Google Scholar]
- 74.Burnett L, Skowronski A, Rausch R, LeDuc C, Leibel R. Determination of the half-life of circulating leptin in the mouse. Int J Obes (Lond) 2017;41:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999;292:1568–1575. [DOI] [PubMed] [Google Scholar]
- 76.Leibel R The role of leptin in the control of body weight. Nutr Rev 2002;60:S15–S19. [DOI] [PubMed] [Google Scholar]
- 77.Pan WW, Myers MG Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 2018;19:95–105. [DOI] [PubMed] [Google Scholar]
- 78.Morabito MV, Ravussin Y, Mueller BR, et al. Weight perturbation alters leptin signal transduction in a region-specific manner throughout the brain. PLoS One. 2017;12:e0168226. doi: 10.1371/journal.pone.0168226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loos R The genetics of adiposity. Curr Opin Genet Dev 2018;50:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leibel R Is obesity due to a heritable difference in "set-point" for adiposity? West Med J 1990;153:429–431. [PMC free article] [PubMed] [Google Scholar]
- 81.Speakman JR, Levitsky DA, Allison DB, et al. Set points, settling points, and some alternative models: Theoretic options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech 2011;4:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller MJ, Bosy-Westphal A, Heymsfield SB. Is there evidence for a set point that regulates human body weight? F1000 Med Rep 2010;2:59. doi: 10.3410/M3412-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levin B, Keesey R. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol 1998;274:R412–R419. [DOI] [PubMed] [Google Scholar]
- 84.Keesey R, Boyle P, Kemnitz J, Mitchel J. The role of the lateral hypothalamus in determining the body weight set-point. In: Novin D, Wyrwicka W, Bray G, eds. Hunger Basic Mechanisms and Clinical Implications. Raven Press; 1975:243–255. [Google Scholar]
- 85.Rosenbaum M, Hirsch J, Gallagher D, Leibel R. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 2008;88:906–912. [DOI] [PubMed] [Google Scholar]
- 86.Shick S, Wing R, Klem M, McGuire M, Hill J, Seagle H. Persons successful at long-term weight loss and maintenance continue to consume a low-energy, low-fat diet. J Am Diet Assoc 1998;98:408–413. [DOI] [PubMed] [Google Scholar]
- 87.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–448. [DOI] [PubMed] [Google Scholar]
- 88.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001;21:323–341. [DOI] [PubMed] [Google Scholar]
- 89.Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–e1649. [DOI] [PubMed] [Google Scholar]
- 90.Guénard F, Deshaies Y, Cianflone K, Kral J, Marceau P, Vohl M. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Nat Acad Sci USA 2013;110:11439–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 93.National Institutes of Health National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Institutes of Health; 1998. NIH Publication 98–4083. Accessed January 4, 2021. https://www.nhlbi.nih.gov/files/docs/guidelines/ob_gdlns.pdf [Google Scholar]
- 94.National Center for Health Statistics. Health, United States, 2016: with chartbook on long-term trends in health. National Center for Health Statistics; 2017. Report No. 2017–1232. [PubMed] [Google Scholar]
- 95.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, eds. Handbook of Obesity Treatment. The Guilford Press; 2002:301–316. [Google Scholar]
- 96.Goodpaster BH, DeLany JP, Otto AD, et al. Effects of diet and physical actvity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010;304:1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wing RR, Venditti EM, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350–359. [DOI] [PubMed] [Google Scholar]
- 98.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: systematic review. Int J Obes (Lond) 2005;29:1168–1174. [DOI] [PubMed] [Google Scholar]
- 99.The Look AHEAD Research Group; Gregg E, Jakicic J, Blackburn G, et al. Association of the magniture of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors with type 2 diabetes: four year results of the Look AHEAD Trial. Arch Intern Med 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss in overweight women. Arch Intern Med 2008;168:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized trial. JAMA 1999;282:1554–1560. [DOI] [PubMed] [Google Scholar]
- 104.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: Does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684–689. [DOI] [PubMed] [Google Scholar]
- 105.Unick JL, Jakicic JM, Marcus BH. Contribution of behavior intervention components to 24 month weight loss. Med Sci Sports Exerc 2010;42:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Catenacci VA, Grunwald GK, Ingebrigtsen JP, et al. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring) 2011;19:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jakicic JM, Tate DF, Lang W, et al. Objective physical activity and weight loss in adults: The Step-Up randomized clinical trial. Obesity (Silver Spring) 2014;22:2284–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Creasy SA, Lang W, Tate DF, Davis KK, Jakicic JM. Pattern of daily steps is associated with weight loss: Secondary analysis from the Step-Up randomized trial. Obesity (Silver Spring) 2018;26:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bouchard C, Tremblay A, Després J-P, et al. The response to exercise with constant energy intake in identical twins. Obes Res 1994;2:400–410. [DOI] [PubMed] [Google Scholar]
- 110.Finlayson G, Bryant E, Blundell JE, King NA. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol Behav 2009;97:62–67. [DOI] [PubMed] [Google Scholar]
- 111.Unick JL, Otto AD, Helsel D, Dutton C, Goodpaster BH, Jakicic JM. The acute effect of exercise on energy intake in overweight/obese women. Appetite 2010;55:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tremblay A, Fontaine E, Poehlman ET, Mitchell D, Perron L, Bouchard C. The effect of exercise-training on resting metabolic rate in lean and moderately obese individuals. Int J Obes 1986;10:511–517. [PubMed] [Google Scholar]
- 113.Willis EA, Creasy SA, Honas JJ, Melanson EL, Donnelly JE. The effects of exercise session timing on weight loss and components of energy balance: Midwest Exercise Trial. Int J Obes 2020;44:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buman MP, Winkler EAH, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: Associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol 2014;179:323–334. [DOI] [PubMed] [Google Scholar]
- 115.Jakicic JM, King WC, Marcus MD, et al. Short-term weight loss with diet and physical activity in young adults: The IDEA Study. Obesity (Silver Spring) 2015;23:2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jakicic JM, Rickman AD, Lang W, et al. Time-based physical activity interventions for weight loss: A randomized trial. Med Sci Sports Exerc 2015;47:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped-care intervention approach on weight loss in adults: The Step-Up Study randomized trial. JAMA 2012;307:2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teixeira PJ, Going SB, Houtkooper LK, et al. Psychosocial predictors of success for behavioral weight reduction. J Behav Med 2002;25:499–523. [DOI] [PubMed] [Google Scholar]
- 119.Unick JL, Hogan PE, Neiberg RH, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring) 2014;22:1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: A 5-year prospective study. Obes Surg 2008;18:648–651. [DOI] [PubMed] [Google Scholar]
- 121.Kumar RB, Aronne LJ. Hypothalamic inflammation: Is there evidence for human obesity? Curr Obes Rep 2014;3:242–247. [DOI] [PubMed] [Google Scholar]
- 122.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604. [DOI] [PubMed] [Google Scholar]
- 123.Kumar RB, Aronne LJ. Pharmacologic treatment of obesity. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. MDText. com, Inc.; 2017. https://www.ncbi.nlm.nih.gov/books/NBK279038/ [Google Scholar]
- 124.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017;389:1399–1409. [DOI] [PubMed] [Google Scholar]
- 127.Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med 2018;379:1107–1117. [DOI] [PubMed] [Google Scholar]
- 128.Sharretts J, Galescu O, Gomatam S, et al. Cancer risk associated with lorcaserin – the FDA’s review of the CAMELLIA-TIMI 61 trial. N Engl J Med 2020;383:1000–1002. [DOI] [PubMed] [Google Scholar]
- 129.Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171. [DOI] [PubMed] [Google Scholar]
- 130.Smith SR, Garvey WT, Greenway FL, et al. Coadministration of lorcaserin and phentermine for weight management: A 12-week, randomized, pilot safety study. Obesity (Silver Spring) 2017;25:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71:142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–1352. [DOI] [PubMed] [Google Scholar]
- 133.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malin SK, Kashyap SR. Effects of metformin on weight loss: Potential mechanisms. Curr Opin Endocrinol Diabetes Obes 2014;21:323–329. [DOI] [PubMed] [Google Scholar]
- 135.Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020;578:444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stanford FC, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg Obes Relat Dis 2017;13:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tchang BG, Aras M, Mandel L, et al. 2097-P: Weight loss maintenance in medically managed patients with obesity. Diabetes 2019;68. doi: 10.2337/db19-2097-P [DOI] [Google Scholar]
- 138.Vannucci A, Theim KR, Kass AE, et al. What constitutes clinically significant binge eating? Association between binge features and clinical validators in college-age women. Int J Eat Disord 2013;46:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aliasghari F, Yaghin NL, Mahdavi R. Relationship between hedonic hunger and serum levels of insulin, leptin and BDNF in the Iranian population. Physiol Behav 2019;199:84–87. [DOI] [PubMed] [Google Scholar]
- 140.Lowe MR, Butryn ML, Didie ER, et al. The power of food scale. A new measure of the psychological influence of the food environment. Appetite 2009;53:114–118. [DOI] [PubMed] [Google Scholar]
- 141.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. Children’s binge eating and development of metabolic syndrome. Int J Obes (Lond) 2012;36:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grucza RA, Przybeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry 2007;48:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shomaker LB, Tanofsky-Kraff M, Elliott C, et al. Salience of loss of control for pediatric binge episodes: Does size really matter? Int J Eat Disord 2010;43:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blundell JE, Macdiarmid JI. Passive overconsumption fat intake and short-term energy balance. Ann N Y Acad Sci 1997;827:392–407. [DOI] [PubMed] [Google Scholar]
- 145.Lowe MR. The effects of dieting on eating behavior: A three-factor model. Psychol Bull 1993;114:100–121. [DOI] [PubMed] [Google Scholar]
- 146.Call CC, Piers AD, Wyckoff EP, Lowe MR, Forman EM, Butryn ML. The relationship of weight suppression to treatment outcomes during behavioral weight loss. J Behav Med 2019;42:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lowe MR, Piers AD, Benson L. Weight suppression in eating disorders: A research and conceptual update. Curr Psychiatry Rep 2018;20:80. doi: 10.1007/s11920-018-0955-2 [DOI] [PubMed] [Google Scholar]
- 148.Ferrannini E, Rosenbaum M, Leibel RL. The threshold shift paradigm of obesity: Evidence from surgically induced weight loss. Am J Clin Nutr 2014;100:996–1002. [DOI] [PubMed] [Google Scholar]
- 149.Lowe MR, Benson L, Singh S. Individual differences in within-subject weight variability: There’s a signal in the noise. Physiol Behav 2020;226:113112. doi: 10.1016/j.physbeh.2020.113112 [DOI] [PubMed] [Google Scholar]
- 150.Lowe MR, Feig EH, Winter SR, Stice E. Short-term variability in body weight predicts long-term weight gain. Am J Clin Nutr 2015;102:995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Winter SR, Yokum S, Stice E, Osipowicz K, Lowe MR. Elevated reward response to receipt of palatable food predicts future weight variability in healthy-weight adolescents. Am J Clin Nutr 2017;105:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Benson L, Zhang F, Espel-Huynh H, Wilkinson L, Lowe MR. Weight variability during self-monitored weight loss predicts future weight loss outcome. Int J Obes (Lond) 2020;44:1360–1367. [DOI] [PubMed] [Google Scholar]
- 153.Kiernan M, Brown SD, Schoffman DE, et al. Promoting healthy weight with "stability skills first": A randomized trial. J Consult Clin Psychol 2013;81:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feig EH, Lowe MR. Variability in weight change early in behavioral weight loss treatment: Theoretical and clinical implications. Obesity (Silver Spring) 2017;25:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]