Abstract
The in vitro activity of daptomycin against 224 current gram-positive clinical isolates including vancomycin-resistant Enterococcus faecium (VREF), methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus spp. (MRSS), and penicillin-resistant Streptococcus pneumoniae (PRSP) was evaluated. The MICs at which 90% of isolates are inhibited for daptomycin and vancomycin, respectively, were as follows: MRSA, 1 and 2 μg/ml; MRSS, 1 and 4 μg/ml; PRSP, 1 and 0.5 μg/ml; and VREF, 2 and >64 μg/ml. Daptomycin was bactericidal against 82% of 17 VREF isolates. The antibacterial activity of daptomycin was strongly dependent on the calcium concentration of the medium. Daptomycin was active against all gram-positive cocci tested.
Daptomycin is a semisynthetic lipopeptide antibiotic derived from the fermentation of Streptomyces roseosporus. Its spectrum of activity, limited to gram-positive bacteria, includes resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus spp. (MRSS), glycopeptide-intermediate Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus spp. (VRE) (1, 3, 8, 9, 13, 14, 16). The characteristics of daptomycin that distinguish it from vancomycin and teicoplanin are its concentration-dependent bactericidal activity against enterococci and staphylococci, its novel mechanism of action, and its requirement for free calcium at physiologic concentrations (4–7). Hence, careful control of the concentration of free ionized Ca2+ in the test medium is needed in order for daptomycin to exert its antibacterial activity (P. Fuchs, A. Barry, and S. Brown, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 350, p. 199, 1999). The object of this study was to compare the antibacterial activity of daptomycin to that of vancomycin against recently isolated resistant gram-positive cocci and to demonstrate its bactericidal effect against VRE and MRSA.
All bacterial isolates were unique, recently isolated, gram-positive cocci that included 54 MRSA isolates, 27 methicillin-susceptible Staphylococcus aureus (MSSA) isolates, 29 MRSS isolates, 61 Streptococcus pneumoniae isolates (16 penicillin-susceptible, 21 penicillin-intermediate, and 24 penicillin-resistant strains), 10 Streptococcus pyogenes isolates, 23 vancomycin-resistant Enterococcus faecium (VREF) isolates, and 20 vancomycin-susceptible Enterococcus faecalis isolates. Of these, 50 MRSA and 40 penicillin-resistant and -intermediate Streptococcus pneumoniae were part of collections from J. R. Lonks and J. M. Boyce, Miriam Hospital, Providence, R.I. All other bacteria were isolated at the clinical microbiology laboratory, New England Medical Center, Boston, Mass. Of the MRSS, isolates, 79% were blood isolates. The VREF were confirmed as different clones by pulsed-field gel electrophoresis (3).
The MICs of the antibiotics for the staphylococci were determined by agar dilution. The MICs for S. pneumoniae, S. pyogenes, and enterococci were determined by broth microdilution with the NCCLS-recommended medium and 0.05-ml inocula. The minimum bactericidal concentrations (MBCs) were determined from microtiter plates (after the MICs were recorded) by plating 0.01 ml from all wells that showed no growth onto blood agar plates (BAPs). All methodologies followed the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (10–12).
Daptomycin was provided by Cubist Pharmaceuticals Inc., Cambridge, Mass.; vancomycin was purchased from Sigma Chemical Co., St. Louis, Mo.
S. aureus ATCC 29213, S. aureus ATCC 43300 (MRSA), E. faecalis ATCC 29212, and S. pneumoniae ATCC 49619 were used as controls.
The effect of the calcium concentration on the antibacterial activity was measured by time-kill studies in media with increasing concentrations of calcium. In addition, the MICs of daptomycin against 23 VREF isolates were determined by microdilution in the following media: base Mueller-Hinton (MH) broth (assumed no calcium supplementation); base MH broth supplemented with 25 mg (broth A), 50 mg (broth B), or 75 mg Ca2+ (broth C) of Ca2+ per liter, or MH II (labeled ∼20 to 25 mg of Ca2+ per liter; (BBL, Cockeysville, Md.) (11). To determine the exact concentration of calcium in the various media, samples were sent for chemical analysis. Chemical analyses of the five MH media resulted in the following total calcium and ionic calcium concentrations, respectively: 23 mg/liter and 0.33 mM for base MH, 21 mg/liter and 0.36 mM for MH II, 45 mg/liter and 0.62 mM for broth A, 66 mg/liter and 0.9 mM for broth B, and 88 mg/liter and 1.12 mM for broth C.
Time-kill studies were performed with antibiotic concentrations equivalent to 1×, 4×, and 8× the MIC and an inoculum density of ∼106 CFU/ml. The bacterial culture in logarithmic growth was added to 25 ml of broth containing the corresponding concentration of antibiotic. The flasks were placed on a 35°C shaker incubator in ambient air. Aliquots for total bacterial counts were withdrawn at time zero, 15 min, and 1, 3, 6, and 24 h and were diluted accordingly. Duplicates of 0.1 ml were plated on BAPs, and the plates were incubated at 35°C for 24 h. Bactericidal activity was defined as a 99.9% (≥3 log10) reduction in the numbers of CFU per milliliter compared to the numbers of CFU per milliliter for the initial inoculum (10). Time-kill studies for daptomycin and vancomycin against S. aureus SSL 50 (MRSA; MICs of daptomycin and vancomycin, 1 μg/ml) were performed in MH broths with calcium concentrations of 25, 50 and 75 mg/liter by the method described above.
Daptomycin was two- to fourfold more active than vancomycin against the staphylococcal isolates (MRSA, MRSS, and MSSA), S. pyogenes, and E. faecalis (Table 1). The MICs of daptomycin were lower than those of vancomycin for penicillin-susceptible S. pneumoniae and comparable to those of vancomycin for the penicillin-resistant or -intermediate strains. The MIC at which 90% of isolates are inhibited (MIC90) for daptomycin was 2 μg/ml, and the MIC range for daptomycin was 0.5 to 2 μg/ml for the 23 VREF strains (vancomycin MICs, >64 μg/ml).
TABLE 1.
Susceptibilities of gram-positive isolates to daptomycin and vancomycin
| Species (no. of isolates tested) | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Daptomycin
|
Vancomycin
|
|||||
| 50% | 90% | Range | 50% | 90% | Range | |
| MRSA (54) | 1 | 1 | 0.5–1 | 2 | 2 | 1–2 |
| MSSA (27) | 0.25 | 1 | 0.06–1 | 0.5 | 1 | 0.5–2 |
| MRSS (29) | 0.5 | 1 | 0.25–2 | 2 | 4 | 2–4 |
| S. pneumoniae (penicillin susceptible; MIC, ≤0.06 μg/ml (16) | 0.03 | 0.125 | 0.015–0.5 | 0.125 | 0.25 | 0.125–0.25 |
| S. pneumoniae (penicillin intermediate; MIC, 0.12 to 1.0 μg/ml) (21) | 0.25 | 1 | 0.008–1.0 | 0.25 | 0.5 | 0.008–1.0 |
| S. pneumoniae (penicillin resistant; MIC, ≥2 μg/ml) (24) | 0.5 | 1 | 0.015–1.0 | 0.25 | 0.5 | 0.125–0.5 |
| S. pyogenes (10) | 0.015 | 0.125 | 0.008–0.5 | 0.25 | 0.5 | 0.25–1 |
| E. faecalis (vancomycin susceptible) (20) | 1 | 1 | 0.25–2 | 1 | 2 | 0.5–2 |
| VREF (23) | 1 | 2 | 0.5–2 | >64 | >64 | >64 |
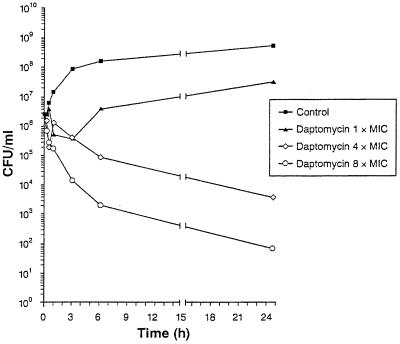
Daptomycin was bactericidal by comparison of the MICs and the MBCs against 82% of the VREF isolates tested. For the three strains against which cidal activity was not demonstrated, the MBCs were 8- to 16-fold higher than the MICs (data not shown). Bactericidal activity was also demonstrated against E. faecium SSL 110 (VREF; daptomycin MIC, 1 μg/ml) (Fig. 1). At a concentration equivalent to 8× the MIC, daptomycin initiated bactericidal activity after 6 h and reduced the initial bacterial inoculum by >4 log10 after 24 h. At 4× the MIC, daptomycin reduced the total bacterial count by 2.82 log10 after 24 h. At 1× the MIC, daptomycin inhibited bacterial growth for 6 h, but the initial inoculum was increased by 1 log10 unit after overnight incubation. Time-kill studies with vancomycin against E. faecium SSL 110 were not done, since the organism showed resistance to vancomycin (MIC, >1,024 μg/ml).
FIG. 1.
Effect of daptomycin concentration on in vitro time-kill kinetics for E. faecium SSL 110 (VRE) in MH II broth (25 μg of Ca2+/ml).
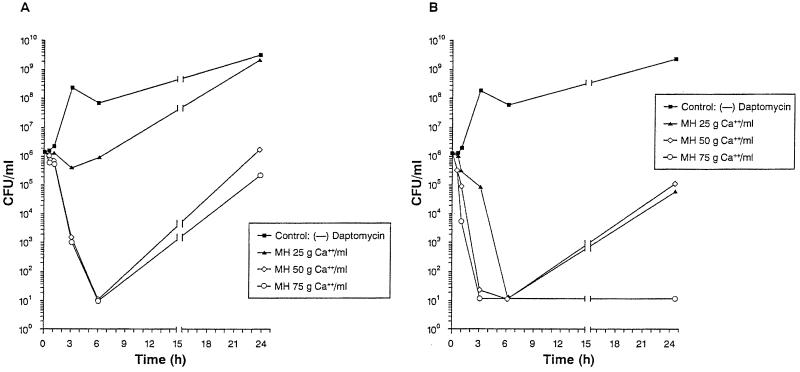
Increasing the concentration of calcium in the medium enhanced the antibacterial activity of daptomycin. Increments of ∼25 mg of calcium per liter lowered the MICs for 23 VREF isolates by at least twofold (data not shown). Figures 2A and B illustrate the effect of calcium on the bactericidal activity of daptomycin against S. aureus SSL 50. Daptomycin at 1× the MIC (Fig. 2A) in media containing 50 and 75 mg of calcium per liter reduced the bacterial inoculum by 4.5 log10 after 5 h, but regrowth occurred after 24 h. In the medium with 25 mg of calcium per liter, inhibition of growth occurred during the first 6 h, with regrowth occurring at 24 h.
FIG. 2.
Effect of calcium concentration in the test medium (MH broth) on activity of daptomycin against S. aureus SSL 50 (MRSA). (A) Daptomycin concentration equivalent to 1× the MIC (1 μg/ml). (B) Daptomycin concentration equivalent to 4× the MIC (4 μg/ml).
In media containing 50 and 75 mg calcium per liter, daptomycin at 4× the MIC (Fig. 2B) reduced to 3 h the time needed to produce the same bactericidal effect. A similar reduction was observed after 6 h in the medium with 25 mg of calcium per liter. In the medium with 75 mg of calcium per liter the killing effect was sustained after overnight incubation. Regrowth of the organisms was observed after 24 h in the media with 25 and 50 mg of calcium per liter.
The most profound bactericidal effect happened at a concentration of daptomycin equivalent to 8× the MIC and a calcium concentration of 75 mg/liter. A reduction of 4 log10 was observed after 1 h of incubation in medium containing 75 mg of calcium per liter, and the effect was sustained for the 24 h. In the media containing 50 and 25 mg of calcium per liter, a bactericidal effect was observed after 3 and 6 h, respectively; however, as with the cultures with daptomycin at 4× the MIC, regrowth occurred after 24 h (data not shown).
The bactericidal effect of vancomycin against S. aureus SSL 50 (MRSA) was not influenced by the calcium concentration of the medium. Vancomycin was bactericidal only at concentrations equivalent to 8× the MIC after 24 h of incubation (data not shown). Compared to vancomycin, daptomycin at a concentration 8× the MIC caused a greater reduction in the numbers of CFU per milliliter in a shorter period of time.
This report confirms the excellent in vitro activity of daptomycin against susceptible and resistant gram-positive bacterial isolates (8, 13, 15). Daptomycin showed activity superior to that of vancomycin against MRSA, MRSS, and VREF. Daptomycin also demonstrated very good activity against vancomycin-susceptible E. faecalis and all streptococci (including 24 penicillin-resistant S. pneumoniae isolates). The MIC90s of daptomycin for all the isolates were well within the levels of daptomycin achievable in human serum (7).
Daptomycin showed excellent bactericidal activity against MRSA and VREF strains. Its bactericidal activity against MRSA was superior to that of vancomycin at all the concentrations of antibiotic tested. The bacterial regrowth observed in time-kill experiments with MRSA at lower concentrations of drug and calcium could be attributed to strain differences or heterogeneous susceptibility to daptomycin, which, as demonstrated by other investigators, is also dependent on the calcium concentration and, possibly, the pH of the test medium (6; N. Oliver, T. Andrew, T. Li, and J. Silverman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-117, p. 262, 1998).
The need for inclusion in the test medium of higher concentrations of free calcium that mimic physiological levels in order to maximize the antibacterial activity of daptomycin is an issue that merits further investigation. Our study as well as others show that the current NCCLS guidelines for calcium supplementation (∼25 mg/liter) of MH broth for routine susceptibility testing is insufficient for the expression of daptomycin's full antibacterial activity (11; Fuchs et al., 39th ICAAC). Fuchs et al. (39th ICAAC) observed that enterococci categorized as resistant or intermediate when tested in medium containing 25 mg of calcium per liter were susceptible when tested in medium containing calcium at 50 mg/liter. On the basis of these results they recommended that medium supplemented with 50 mg of calcium per liter be used to test daptomycin's susceptibility during clinical trials (Fuchs et al., 39th ICAAC). This concentration approximates the physiological levels of free calcium (45 to 55 μg/ml) in human serum and simulates the environment of the pathogen in human infection (2). Additional studies should be performed in order to establish adequate recommendations for calcium supplementation of the medium for the routine testing of the antibacterial activity of daptomycin.
These findings underscore the role of daptomycin as an alternative agent for the treatment of infections with resistant gram-positive bacteria. Clinical trials, in progress, will determine the clinical potential of this new bactericidal antibiotic.
(This study was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 26 September 1998.)
Acknowledgments
This study was supported by a grant from Cubist Pharmaceuticals.
We thank the personnel of the Clinical Pathology Laboratories for referral of isolates and for chemical assays of calcium. We also thank Barbara Rapino, June Cox St. Pierre, and Jessica Ross for technical assistance, as well as Roselia Martinez for assistance with manuscript preparation.
REFERENCES
- 1.Benson C A, Beaudette F, Trenholm G. Comparative in vitro activity of LY146032, a new peptide, with vancomycin and eight other agents against gram-positive organisms. J Antimicrob Chemother. 1987;20:191–196. doi: 10.1093/jac/20.2.191. [DOI] [PubMed] [Google Scholar]
- 2.Eliopoulos G M, Willey S, Reiszner E, Spitzer P G, Caputo G, Moellering R C. In vitro and in vivo activity of LY146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986;30:532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goering R V, Winters M A. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol. 1992;30:557–580. doi: 10.1128/jcm.30.3.577-580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanberger H, Nilsson L E, Maller R, Isaksson B. Pharmacodynamics of daptomycin and vancomycin on E. faecalis and S. aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob Agents Chemother. 1991;35:1710–1716. doi: 10.1128/aac.35.9.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakey J H, Ptak M. Fluorescence indicates a calcium-dependent interaction between the lipopeptide antibiotic LY 146032 and phospholipid membranes. Biochemistry. 1988;27:4639–4645. doi: 10.1021/bi00413a009. [DOI] [PubMed] [Google Scholar]
- 6.Lamp K C, Rybak M J, Bailey E M, Kaatz G W. In vitro pharmacodynamic effects of concentration, pH and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother. 1992;36:2709–2714. doi: 10.1128/aac.36.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamp K C, Rybak M J. Teicoplanin and daptomycin bactericidal activities in the presence of albumin or serum under controlled conditions of pH and ionized calcium. Antimicrob Agents Chemother. 1993;37:605–609. doi: 10.1128/aac.37.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low D E, McGeer A, Poon R. Activities of daptomycin and teicoplanin against Staphylococcus haemolyticus and Staphylococcus epidermidis, including evaluation of susceptibility testing recommendations. Antimicrob Agents Chemother. 1989;33:585–588. doi: 10.1128/aac.33.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobarakai N, Quale J M, Landman D. Bactericidal activities of peptide antibiotics against multidrug-resistant E. faecium. Antimicrob Agents Chemother. 1994;38:385–387. doi: 10.1128/aac.38.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. NCCLS document M26-T. 12, no. 19. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. NCCLS document M7-A4, vol. 17, no. 2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Ninth informational supplement. NCCLS document M100-S9. 19, no. 1. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 13.Niu W W, Neu H C. Activity of mersacidin, a novel peptide, compared with that of vancomycin, teicoplanin, and daptomycin. Antimicrob Agents Chemother. 1991;35:998–1000. doi: 10.1128/aac.35.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak M J, Hershberger E, Moldovan T, Grucz R G. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob Agents Chemother. 2000;44:1062–1066. doi: 10.1128/aac.44.4.1062-1066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shonekan D, Mildvan D, Handwerger S. Comparative in vitro activities of teicoplanin, daptomycin, ramoplanin, vancomycin, and PD127,391 against blood isolates of gram-positive cocci. Antimicrob Agents Chemother. 1992;36:1570–1572. doi: 10.1128/aac.36.7.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva M, Jacobus N V, Gorbach S L. In vitro activity of LY146032 against gram-positive bacteria. Diagn Microbiol Infect Dis. 1988;9:79–85. doi: 10.1016/0732-8893(88)90100-9. [DOI] [PubMed] [Google Scholar]