Abstract
Babesiosis is a tick-borne parasitic disease of humans and livestock that has dramatically increased in frequency and geographical range over the past few decades. Infection of cattle often causes large economic losses, and human infection can be fatal in immunocompromised patients. Unlike for malaria, another disease caused by hemoprotozoan parasites, limited treatment options exist for Babesia infections. As epigenetic regulation is a promising target for new antiparasitic drugs, we screened 324 epigenetic inhibitors against Babesia divergens blood stages and identified 75 (23%) and 17 (5%) compounds that displayed ≥90% inhibition at 10 and 1 μM, respectively, including over a dozen compounds with activity in the low nanomolar range. We observed differential activity of some inhibitor classes against Babesia divergens and Plasmodium falciparum parasites and identified pairs of compounds with a high difference in activity despite a high similarity in chemical structure, highlighting new insights into the development of epigenetic inhibitors as antiparasitic drugs.
Keywords: Babesia, Plasmodium, epigenetic, small molecule screen, parasites
Graphical Abstract
Babesiosis is an emerging parasitic disease caused by the intraerythrocytic Babesia parasite that has many clinical features similar to malaria infection. Babesia infections in cattle are widespread and lead to economic losses through death, reduction in meat and milk yield, and the cost of control measures. More than 100 Babesia species have been identified, but only a few infect humans. However, human babesiosis is an increasing concern worldwide, as the number of reported cases have increased over the last decades, and the geographical rage of transmission has expanded.1,2 In Europe, Babesia divergens is responsible for most human babesiosis cases.3 In the United States, the majority of human Babesia infections is caused by Babesia microti, although cases of B. divergens-like organisms have been reported as well.4 Transmission occurs through the bite of an infected tick or occasionally through blood transfusion, which has prompted the screening of the blood supply for Babesia parasites in an increasing number of states.5 Symptomatic human babesiosis is manifested by malaria-like symptoms such as fever and general malaise. The current treatment recommendation for human babesiosis is a combination of atovaquone and azithromycin.6 However, for immunocompromised patients, cases of treatment failure have been reported,7,8 and babesiosis can lead to organ failure and death. Asplenic patients, in particular, are at high risk for relapsing infections and require long antimicrobial treatment.9 Recently, tafenoquine, a newly FDA-approved drug for malaria treatment, showed activity against Babesia microti in mice, but further studies are needed.10 As treatment options for relapsing Babesia infection are limited, research into new drugs for babesiosis is critical.
In eukaryotes, epigenetic regulation of gene expression, mediated by small modifications of nucleosomes and on DNA itself, has been found to be critical for cellular homeostasis and differentiation.11 In a recent screen of 324 commercially available epigenetic inhibitors against Plasmodium falciparum,12 we showed that 54 compounds exhibited ≥50% inhibition at 1 μM in vitro, suggesting that the epigenetic machinery could be a promising novel drug target. Because Plasmodium and Babesia are related parasite species with similarly complex life cycles that share much of the epigenetic regulatory machinery, we decided to determine the activity of these epigenetic inhibitors against Babesia divergens, for which an in vitro culture system and drug assays are established.
RESULTS AND DISCUSSION
Evolutionary Conservation of Epigenetic Modifying Enzymes in Piroplasmid Parasites.
Among eukaryotes, the most common epigenetic modifications are acetylation of histone lysine residues, methylation of histone lysine and arginine residues, and methylation of deoxycytidine on DNA. We were able to identify orthologues for most of the enzyme classes that place and remove these marks in the genomes of piroplasmid parasites, which include Babesia and Theileria species (Figure 1, Supplemental Data Set 1). Orthologues to 9 of the 11 Su(var.)3–9/Enhancer of Zeste/Trithorax (SET)-domain-containing lysine-specific methyltransferases (KMT) present in P. falciparum could be identified in at least one piroplasmid genome. Orthologues of PfSET4 and PfSET5 were absent in the entire clade, while an orthologue to PfSET6 could be identified only in B. microti. Orthologues to PfSET9 could be identified in all piroplasmida genomes but are missing key residues within the catalytic SET domain, thus making it unlikely that these have retained methyltransferase activity. Most piroplasmid genomes also retain two additional trithoraxlike SET-domain proteins found in other apicomplexan parasites that were lost in malaria parasites. Histone demethylases were notably reduced compared to P. falciparum, with B. divergens and B. bovis encoding only a single member of the Jumonji and LSD demethylase families.
Figure 1.
Evolutionary conservation of epigenetic modifying enzymes in piroplasmid parasites. Comparison of epigenetic modifying enzyme orthologues among P. falciparum, B. microti, T. equi, T. annulata, C. felix, B. bovis, and B. microti with a representation of the evolutionary relationship among these species.
All three of the histone arginine methyltransferases (RMTs) in P. falciparum had orthologues in B. divergens, but the PRTM3 orthologue was lost outside the Babesia sensu stricto clade (represented by B. bovis and B. divergens). Histone acetyltransferases (HATs) and deacetylases (HDACs) were generally conserved between malaria parasites and the piroplasmida. Notable is the absence of Sir2A from all piroplasmid genomes and loss of HAT1 from the Babesia sensu stricto clade. An orthologue of a proposed Cytosine-5 DNA methyltransferase in Plasmodium falciparum13 is present in Theileria equi but could not be identified in the other genomes examined.
Activity of Epigenetic Inhibitors against Babesia divergens.
Given these differences in histone modifying enzymes between P. falciparum and the piroplasms, we tested the susceptibility of B. divergens to a library of epigenetic inhibitors previously screened against P. falciparum.12 Of the 324 compounds tested, 125 (39%) showed ≥50% inhibition at 10 μM against B. divergens blood stages, of which 46 (14%) retained greater than half-maximal activity at 1 μM (Figure 2A, Figure S1 and Table S1, Supplemental Data Set 2). Seventy-five (23%) and 17 (5%) compounds exhibited greater than 90% inhibition at 10 and 1 μM, respectively (Table S2). Dose–response curves were performed for 17 compounds with submicromolar EC90 values (Figure 2B). Of these, the HDAC inhibitors quisinostat and apicidin were the most potent compounds with EC50 values as low as 5–6 nM. These top hits included two FDA-approved drugs mitomycin C and panobinostat with EC50 values of 64 and 27 nM, respectively. Peak plasma concentration of panobinostat dosage indicated for treatment of multiple myeloma only corresponds to EC75 for Babesia, making it an unlikely candidate for treatment.14,15 Mitomycin C is a CpG DNA cross-linking agent indicated for gastric and pancreatic adenocarcinoma treatment and was recently also approved for low-grade upper tract urothelial cancer.16 Intravenous administration during chemotherapy leads to plasma concentration of 5 μM,17 around 20-fold higher than its EC90 value against B. divergens. We previously determined toxicity of selected compounds against human HepG2 cells.12 Several compounds displayed only moderate toxicity against HepG2 cells even at 1 μM (Figure 2B). This drug screen identifies promising compounds for additional SAR studies for possible use as a new class of anti-Babesia drugs.
Figure 2.
Activity of epigenetic inhibitors against Babesia divergens. (A) 125 compounds with ≥50% inhibition at 10 μM. Heat map of mean percent inhibition at 10 and 1 μM compared to solvent-treated controls (n = 3). Compounds are grouped based on the reported epigenetic process affected in higher eukaryotes: Histone deacetylation (HDAC), histone acetylation (HAT), histone methylation (KMT), histone demethylases (HDM), DNA methylation (DNMT), and “Other”. (B) Dose response analysis for 17 compounds with submicromolar EC50 values (n = 2) with corresponding HepG2 inhibition at 1 μM.
Differential Activity of Epigenetic Inhibitors against B. divergens and P. falciparum.
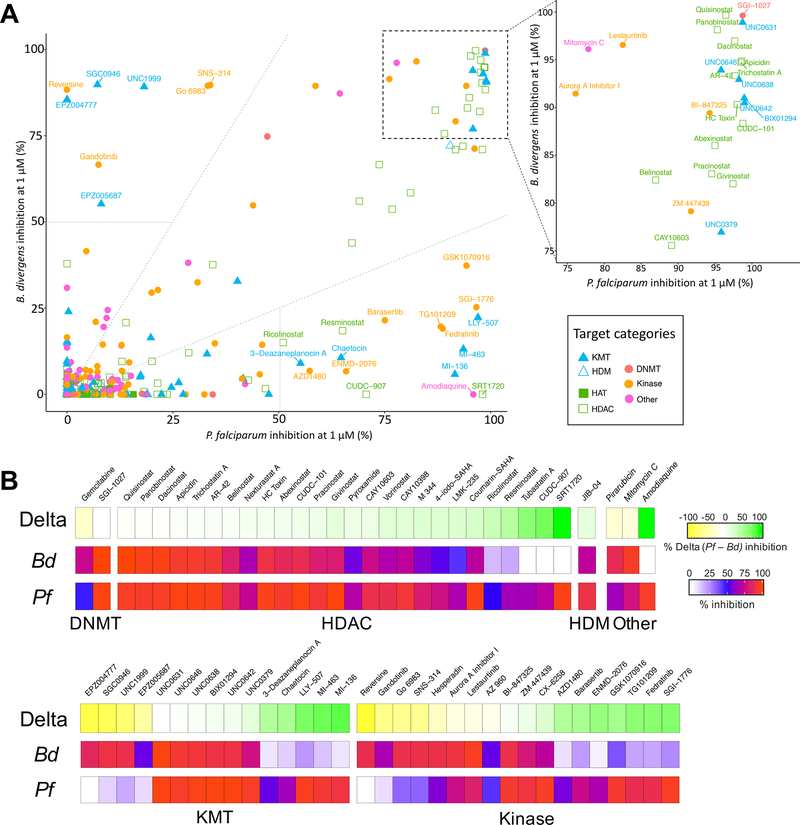
Next, we compared these results to our recently completed screen of this library against P. falciparum blood stages.12 A similar number of compounds had >50% inhibition at 1 μM against both species (46 against B. divergens compared to 54 against P. falciparum). For both species, compounds targeting histone methylation, deacteylation, demethylation, or phosphorylation were the most active, while compounds targeting histone acetylation, PARPylation, histone reader domains, DNA methylation, or other pathways had little to no activity (Table S1).
Twenty-five compounds with >50% inhibition at 1 μM against one species exhibited greater than 2-fold difference in activity in the other (Figure 3). HDAC inhibitors were generally less active against B. divergens than against P. falciparum, with 18% of the 85 HDAC inhibitors in the library showing ≥90% inhibition at 1 μM against P. falciparum versus only 8% for B. divergens (Table S2). Interestingly, several HDAC inhibitors with high differential activity target the human HDAC1 (class I) or HDAC6 (class IIb), suggesting that the HDACs may be more divergent from the human enzymes in B. divergens than in P. falciparum. An additional 25 compounds exhibited greater than 75% inhibition against both P. falciparum and B. divergens. Thirteen of these were HDAC inhibitors, many of which have activity against multiple HDAC classes. Surprisingly, of the 15 KMT inhibitors in Figure 3B, the four compounds with greater activity against B. divergens target either the H3K79 KMT DOT1L or the H3K27 KMT EZH1/2 in humans, orthologues to which are absent from both species. As in our previous study, the DNMT inhibitor SGI-1027 was also among the most active compounds against B. divergens despite no identifiable DNMT orthologue in Babesia species and PfDNMT being dispensable for asexual growth,18 suggesting that SGI-1027 likely has one or more alternative targets.
Figure 3.
Differential activity of epigenetic inhibitors against B. divergens and P. falciparum. (A) Scatterplot comparing percent inhibition at 1 μM against B. divergens and P. falciparum. Compound names are indicated for compounds with more than twofold difference in activity (dotted lines) and more than 50% inhibition at 1 μM against one species (dashed lines). An enlarged scatterplot with labeled compound names is displayed for compounds with ≥75% at 1 μM against both species. (B) Heat map of compounds with at least 50% inhibition at 1 μM against one species, ordered by the delta activity (% Pf inhibition – % Bd inhibition) and grouped by proposed target category.
Similarity and Activity Cliff Analysis of Activity against B. divergens and P. falciparum.
Structural feature analysis of all 324 unique compounds revealed 5 clusters of 4 compounds or more with >80% structural similarity (Figure S2), including 7 HDAC inhibitors with a common hydroxamate-based scaffold, 3 KMT inhibitors with shared an 1H-indazole-4-carboxamide scaffold, and 7 KMT inhibitors sharing a common diaminoquinazoline backbone. Activity cliff analysis identifies pairs with high differential activity despite high structural similarity. Delta activity and SALI values are plotted for all pairs of the library in Figure S3. Compound pairs of interest have >50% delta activity and >80% structural similarity (Figure 4A). Twelve pairs were activity cliffs in both P. falciparum and B. divergens, while 3 only had more than 50% delta activity in B. divergens and 4 only in P. falciparum.
Figure 4.
Activity cliff analysis. (A) Scatterplot of 19 activity cliff pairs with >50% delta activity and >80% structural similarity, grouped by species. Compound pairs that display an activity cliff in both species are indicated in matching colors. (B, C) Examples of activity cliff pairs with respective chemical structures and in vitro activity at 1 μM.
The KMT inhibitor UNC1999 displays an activity cliff for activity against B. divergens when paired with GK343 and GSK503, despite the former having 91% structural similarity (Figure 4B). Interestingly, the IC50 values for mammalian EZH2 enzyme inhibition are similar for all three compounds, while UNC1999 is the only compound with potent EZH1 inhibition as well. Kinase inhibitors belinostat and oxamflatin show activity cliffs for both species (Figure 4C). Additional structural comparisons of the remaining activity cliff pairs can be found in Figure S4. These activity cliff pairs provide insight into the structural features that confer activity against P. falciparum and B. divergens.
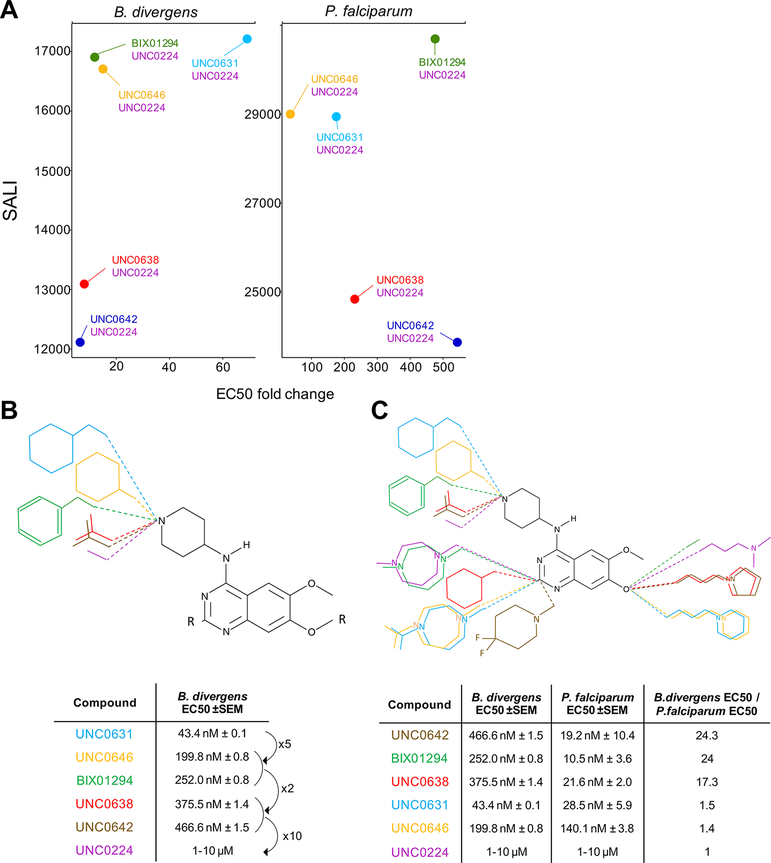
Differential KMT Inhibitors with a Diaminoquinazoline Backbone.
Seven KMT inhibitors of the SET3 KMT G9a share a diaminoquinazoline backbone.19–24 Figure 5A shows pairs with >60% similarity and >50% delta activity at 1 μM in both species. For Babesia divergens, the side group on position 4 of the diaminoquinazoline scaffold seemed to have the most effect on compound activity (Figure 5B). A cyclohexylmethyl-4-piperidylamine side group (UNC0631) showed the highest activity, while the EC50 value increased 5–6 times when changing to a cyclohexyl-4-piperidylamine (UNC0646) or 1-benzyl-4-piperidylamine (BIX01294) side group. Substituting the ring structure with an isopropyl group (UNC0638 and UNC0642) further decreased the remaining activity by half. Activity was completely lost when the side group consisted of a lone 4-piperidylamine (UNC0224).
Figure 5.
Structure–activity relationships of diaminoquinazoline KMT inhibitors. (A) Compound pairs with >60% similarity and >50% delta activity at 1 μM. (B) Structure–activity relationship against Babesia divergens. (C) Changes in chemical structure that confer differential activity against both species.
Previous SAR studies of diaminoquinazoline methyltransferase inhibitors have been performed for P. falciparum,.25,26 We confirmed that substituting the 1-benzyl-4-piperidylamine of BIX01294 on position 4 of the diaminoquinazoline scaffold with a cyclohexylmethyl-4-piperidylamine (UNC0631) or a cyclohexyl-4-piperidylamine (UNC0646) reduced the activity against P. falciparum (Figure S5). However, in disagreement with previous findings, the substitution with a 1-isopropyl-4-piperidylamine (UNC0642 and UNC0638) did not impact the activity against P. falciparum.
It was previously reported that a lysine mimetic side group on position 7 of the diaminoquinazoline scaffold lacks activity against P. falciparum despite exhibiting potent activity against G9a due to interactions in the lysine binding channel of this enzyme. We confirmed that a dimethylpropylamine side group (UNC0224) loses activity as previously reported, but interestingly, we found the lysine mimetic side groups 1-propylpyrrolidine (UNC0642 and UNC0638) and 1-propylpiperidine (UNC0631 and UNC0646) retained most of their activity. This suggests that compounds with a lysine mimetic side group on position 7 of the diaminoquinazoline scaffold can inhibit P. falciparum only if combined with certain side groups on position 2 and 4 of the scaffold.
Three of the diaminoquinazoline compounds have a high differential activity against both species, showing 17–24 times more activity against P. falciparum (Figure 5C). As two of these compounds (UNC0642 and UNC0638) share a 1-isopropyl-4-piperidylamine group on position 4 and a 1-propylpyrrolidine group on position 7, these might contribute to this difference in activity. As the closest related P. falciparum KMT to HsG9a is PfSET3, it is possible that the binding site on the KMT enzyme for these three compounds is more divergent from HsG9a in BdSET3 than in PfSET3.
Overall, we show that epigenetic enzymes may be a promising novel target in Babesia divergens. Our library of 324 epigenetic inhibitors includes 19 pairs of compounds with high delta activity despite high structural similarity, which provide insight into the structural features that confer activity against P. falciparum and B. divergens. Multiple diaminoquinazoline backbone KMT inhibitors are highly active against both species tested with UNC0631 displaying a low nanomolar range EC50 value against both P. falciparum and B. divergens, while UNC0224 is inactive against both species despite minor structural differences with the active diaminoquinazoline compounds. Given the paucity of treatment options for human babesiosis, advancing several of these hits for evaluation in animal models seems warranted. This is especially the case for testing these compounds for activity against B. microti, the most common cause of human babesiosis in North America, for which no readily available tissue culture system exists. Despite a shared genus, B. microti falls outside of the Babesia sensu stricto clade containing B. divergens and may well exhibit differential sensitivity to compounds active against B. divergens.
METHODS
Commercially available libraries of 324 epigenetic inhibitors from Selleckchem (Houston, TX) and Cayman Chemicals (Ann Arbor, MI) were purchased. Libraries were aliquoted and diluted in DMSO to 2 and 0.2 mM in V-bottom 96-well plate and stored at −80 °C.
Babesia divergens (Bd Rouen 1987 strain27) was grown in vitro in human A+ RBC at low parasitemia. Cultures were flushed with 90% nitrogen, 5% oxygen, and 5% carbon dioxide and cultured at 37 °C. SYBR Green-based growth assays were used to determine in vitro activity of the epigenetic inhibitors against B. divergens.28,29 Briefly, flat-bottom 96-well plates at a total of 200 μL per well and 0.5% DMSO, at a final 5% hematocrit, 0.5% parasitemia for B. divergens were incubated at 37 °C for 72 h and thereafter frozen at −80 °C. Upon thawing, 100 μL of SYBR Green (ThermoFisher) diluted in lysis buffer (0.2 μL 10 000× SYBR Green per mL lysis buffer) was added to each well, and plates were shaken in the dark at room temperature for 1 h. Fluorescence was then measured using a Molecular Devices SpectraMax ID5 plate reader. Values were normalized to solvent-treated controls (included in triplicate on each plate). EC50 values were calculated using the nlmLS function of the minpack.lm package (v1.2-1) of the R statistical package (v3.6.0).
Protein sequences of histone modifying enzymes were retrieved from PlasmoDB and PiroplasmaDB.30,31 Orthologues were identified using annotated orthologue groups as well as reciprocal BLAST searches. NCBI Conserved Domain Search32 was used to identify conserved protein domains. Protein sequences were aligned using MUSCLE33 and visualized using MView.34 In cases where two consecutive genes were identified as orthologues, the geneID for the orthologue containing the catalytic domain was indicated as the orthologue.
Structural feature (SkelSphere) analysis and activity cliff analysis were performed using Osiris DataWarrior v5.2.1 at 80% chemical structure similarity cutoff. Structure–activity landscape index (SALI) values35 were calculated as
in which Ai and Aj are the activities of compounds i and j, and sim(I,j) is the similarity coefficient between the two molecules.
Supplementary Material
ACKNOWLEDGMENTS
We thank the High Throughput and Spectroscopy Resource Center at Rockefeller University for technical assistance and Elisabeth Martinez (UT Southwestern) for additional JIB-04 inhibitor. We also thank Laura Kirkman for providing the B. divergens strain and valuable feedback on the manuscript. This work was supported by a Bohmfalk Charitable Trust Research Grant and NIH 1R01AI141965 and 1R01AI138499 to B.K. and a Belgian American Educational Foundation postdoctoral fellowship to L.V.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00853.
Figure S1, percent inhibition of all 324 compounds against B. divergens at 10 and 1 μM; Figure S2, structural feature similarity landscape; Figure S3, activity cliff analysis for Babesia divergens at 1 μM; Figure S4, structural representation of the remaining activity cliff pairs in Figure 4A; Figure S5, activity of diaminoquinazoline KMT inhibitors against P. falciparum; Table S1, EC50 activity of epigenetic inhibitors tested grouped by target category; Table S2, EC90 activity of epigenetic inhibitors tested grouped by target category (PDF)
Supplemental Data Set 1, gene identifiers of histone modifying enzymes in Figure 1 (XLSX)
Supplemental Data Set 2, compound table (XLSX)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsinfecdis.0c00853
Contributor Information
Leen N. Vanheer, Department of Microbiology & Immunology, Weill Cornell Medicine, New York 10065, United States
Björn F.C. Kafsack, Department of Microbiology & Immunology, Weill Cornell Medicine, New York 10065, United States.
REFERENCES
- (1).Gray EB, and Herwaldt BL (2019) Babesiosis Surveillance — United States, 2011–2015. MMWR Surveill Summ 68, 1–11. [DOI] [PubMed] [Google Scholar]
- (2).Hildebrandt A, Gray JS, and Hunfeld KP (2013) Human Babesiosis in Europe: what clinicians need to know. Infection 41, 1057–1072. [DOI] [PubMed] [Google Scholar]
- (3).Krause PJ (2019) Human babesiosis. Int. J. Parasitol 49, 165–174. [DOI] [PubMed] [Google Scholar]
- (4).Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, Fritsche TR, Persing DH, and Limaye AP (2004) Babesia divergens-like infection, Washington State. Emerging Infect. Dis 10,1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).US Federal Drug Administration, Center for Biologics Evaluation and Research. (2019) Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis Guidance for Industry. https://www.fda.gov/regulatory-information/search-fdaguidance-documents/recommendations-reducing-risk-transfusion-transmitted-babesiosis.
- (6).Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, Meissner HC, Osani MC, Rips JG, Sood SK, Vannier E, Vaysbrot EE, and Wormser GP (2021) Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clin. Infect. Dis 65,1. [DOI] [PubMed] [Google Scholar]
- (7).Simon MS, Westblade LF, Dziedziech A, Visone JE, Furman RR, Jenkins SG, Schuetz AN, and Kirkman LA (2017) Clinical and Molecular Evidence of Atovaquone and Azithromycin Resistance in Relapsed Babesia microti Infection Associated With Rituximab and Chronic Lymphocytic Leukemia. Clin. Infect. Dis 65, 1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, Mittleman A, Aguero Rosenfeld M, Topal J, and Krause PJ (2010) Emergence of Resistance to Azithromycin-Atovaquone in Immunocompromised Patients with Babesia microtiInfection. Clin. Infect. Dis 50, 381–386. [DOI] [PubMed] [Google Scholar]
- (9).Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR, Christianson D, Dardick K, Coleman M, Girotto JE, and Spielman A (2008) Persistent and Relapsing Babesiosis in Immunocompromised Patients. Clin. Infect. Dis 46, 370–376. [DOI] [PubMed] [Google Scholar]
- (10).Mordue DG, and Wormser GP (2019) Could the Drug Tafenoquine Revolutionize Treatment of Babesia microti Infection? J. Infect. Dis 220, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Gibney E, and Nolan C (2010) Epigenetics and gene expression. Heredity 105,4–13. [DOI] [PubMed] [Google Scholar]
- (12).Vanheer LN, Zhang H, Lin G, and Kafsack BFC (2020) Activity of Epigenetic Inhibitors against Plasmodium falciparum Asexual and Sexual Blood Stages. Antimicrob. Agents Chemother 64, a02523–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ponts N, Fu L, Harris EY, Zhang J, Chung D-WD, Cervantes MC, Prudhomme J, Atanasova-Penichon V, Zehraoui E, Bunnik EM, Rodrigues EM, Lonardi S, Hicks GR, Wang Y, and Le Roch KG (2013) Genome-wide Mapping of DNA Methylation in the Human Malaria Parasite Plasmodium falciparum. Cell Host Microbe 14, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Anne M, de Sammartino D, Barginear M, and Budman D (2013) Profile of panobinostat and its potential for treatment in solid tumors: an update. OncoTargets Ther, 1613–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T, Giles FJ, Scott JW, Parker K, Liu A, Woo M, Atadja P, Mishra KK, and Ottmann OG (2019) Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia 27,1–9. [DOI] [PubMed] [Google Scholar]
- (16).Macdonald J, Schein P, Woolley P, Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R, and Lagarde C (1980) 5-Fluorouracll, Doxorubicin, and Mitomycin (FAM) Combination Chemotherapy for Advanced Gastric Cancer. Ann. Int. Med 93,1–4. [DOI] [PubMed] [Google Scholar]
- (17).Bedford Laboratories. MITOMYCIN - mitomycin injection, powder, lyophilized, for solution. https://www.drugsdb.eu/drug.php?d=Mitomycin&m=Bedford%20Laboratories&id=729a1d0a-4a03-41c7-abdd-62062a63643b.xml (accessed 02/16/2021).
- (18).Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, and Adams JH (2018) Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360, eaap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, and Jenuwein T (2007) Reversal of H3K9me2 by a Small-Molecule Inhibitor for the G9a Histone Methyltransferase. Mol. Cell 25, 473–481. [DOI] [PubMed] [Google Scholar]
- (20).Liu F, Allali-Hassani A, Siarheyeva A, and Jadhav A (2009) Discovery of a 2,4-Diamino-7-aminoalkoxyquinazoline as a Potent and Selective Inhibitor of Histone Lysine Methyltransferase G9a,1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu F, Barsyte-Lovejoy D, Allali-Hassani A, Frye S, and Brown P (2011) Optimization of Cellular Activity of G9a Inhibitors 7-Aminoalkoxy-quinazolines. J. Med. Chem,1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Vedadi M, Barsyte-Lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, Wigle TJ, DiMaggio PA, Wasney GA, Siarheyeva A, Dong A, Tempel W, Wang S-C, Chen X, Chau I, Mangano TJ, Huang X-P, Simpson CD, Pattenden SG, Norris JL, Kireev DB, Tripathy A, Edwards A, Roth BL, Janzen WP, Garcia BA, Petronis A, Ellis J, Brown PJ, Frye SV, Arrowsmith CH, and Jin J (2011) A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat. Chem. Biol 7, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Liu F, Barsyte-Lovejoy D, Li F, Xiong Y, Korboukh V, Allali-Hassani A, Janzen W, Roth B, Frye S, Arrowsmith C, Brown P, Vedadi M, and Jin J (2013) Discovery of an in Vivo Chemical Probe of the Lysine Methyltransferases G9a and GLP. J. Med. Chem 56,1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ma A, Yu W, Li F, Bleich RM, Herold JM, Butler KV, Norris JL, Korboukh V, Tripathy A, Janzen WP, Arrowsmith CH, Frye SV, Vedadi M, Brown PJ, and Jin J (2014) Discovery of a selective, substrate-competitive inhibitor of the lysine methyltransferase SETD8. J. Med. Chem 57, 6822–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sundriyal S, Malmquist NA, Caron J, Blundell S, Liu F, Chen X, Srimongkolpithak N, Jin J, Charman SA, Scherf A, and Fuchter MJ (2014) Development of Diaminoquinazoline Histone Lysine Methyltransferase Inhibitors as Potent Blood-Stage Antimalarial Compounds. ChemMedChem 9, 2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sundriyal S, Chen PB, Lubin AS, Lueg GA, Li F, White AJP, Malmquist NA, Vedadi M, Scherf A, and Fuchter MJ (2017) Histone lysine methyltransferase structure activity relationships that allow for segregation of G9a inhibition and anti-Plasmodium activity. MedChemComm 8, 1069–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gorenflot A, Brasseur P, Precigout EML, Marchand A, and Schrevel J (1991) Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res, 1–10. [DOI] [PubMed] [Google Scholar]
- (28).Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, and Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother 48, 1803–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Rizk MA, El-Sayed SAE-S, AbouLaila M, Tuvshintulga B, Yokoyama N, and Igarashi I (2016) Large-scale drug screening against Babesia divergens parasite using a fluorescence-based high-throughput screening assay. Vet. Parasitol 227,93–97. [DOI] [PubMed] [Google Scholar]
- (30).Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ, Treatman C, and Wang H (2009) PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res 37, D539–D543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Aurrecoechea C, Barreto A, Brestelli J, Brunk BP, Cade S, Doherty R, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Hu S, Iodice J, Kissinger JC, Kraemer ET, Li W, Pinney DF, Pitts B, Roos DS, Srinivasamoorthy G, Stoeckert CJ, Wang H, and Warrenfeltz S (2013) EuPathDB: The Eukaryotic Pathogen database. Nucleic Acids Res 41, D684–D691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Marchler-Bauer A, and Bryant SH (2004) CD-Search: protein domain annotations on the fly. Nucleic Acids Res 32, W327–W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Brown N, Leroy C, and Sander C (1998) MView: a webcompatible database search or multiple alignment viewer. Bioinformatics 14,1–2. [DOI] [PubMed] [Google Scholar]
- (35).Guha R, and Van Drie JH (2008) Structure-Activity Landscape Index: Identifying and Quantifying Activity Cliffs. J. Chem. Inf. Model 48, 646–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.