Aspirin-exacerbated respiratory disease (AERD) is characterized by severe chronic rhinosinusitis with nasal polyps (CRSwNP) and eosinophilic asthma. The upper and lower respiratory tract symptoms are notoriously difficult to treat, with many patients failing first-line therapies.1 The advent of targeted respiratory biologic medications offers promise for patients with AERD who have had inadequate response to prior standard-of-care approaches. However, there are limited data to guide selection of the proper respiratory biologic for AERD patients. Herein we discuss our understanding of the immunopathology of respiratory tract inflammation in AERD and how that may help guide biologic selection in this patient population.
Pathobiology of respiratory tract inflammation
AERD is predominantly a type 2 inflammatory disease characterized by marked tissue eosinophilia, activated mast cells and dysregulated production of cysteinyl leukotrienes and prostaglandins.1, More recently, a subendotype of AERD defined by type 1 and type 3 cytokines has also been identified.2 We now know the respiratory tract epithelium in nasal polyp tissue is also severely dysregulated, with basal cell hyperplasia and glandular cell reductions.3 Both intrinsic and extrinsic factors likely drive the epithelial dysregulation and overproduction of innate epithelial cell-derived type 2 cytokines, interleukin (IL)-33 and thymic stromal lymphopoietin (TSLP). Both IL-33 and TSLP are elevated in the respiratory tissue of patients with AERD and play a role in promoting eosinophilic inflammation and mast cell activation with consequent overproduction of prostaglandin D2 and leukotrienes.1
Group 2 innate lymphoid cells (ILC2s) and Th2 cells are abundant in polyp tissue, and along with mast cells, produce type 2 cytokines such as IL-4, IL-5, and IL-13 (Figure 1). IL-4 and IL-13 signal through the common IL-4Rα subunit to promote a number of effects, including tissue fibrosis and remodeling, goblet cell hyperplasia and mucus production, mast cell activation and survival, and local IgE class switching.1 Although AERD is not typically considered to be an atopic disease, a recent study identified a relationship between nasal polyp tissue IgE and rapidity of nasal polyp regrowth in patients with AERD, suggesting IgE as a marker of severe disease and perhaps as a driver of ongoing mast cell activation and respiratory tissue inflammation (Figure 1).4
Figure 1:
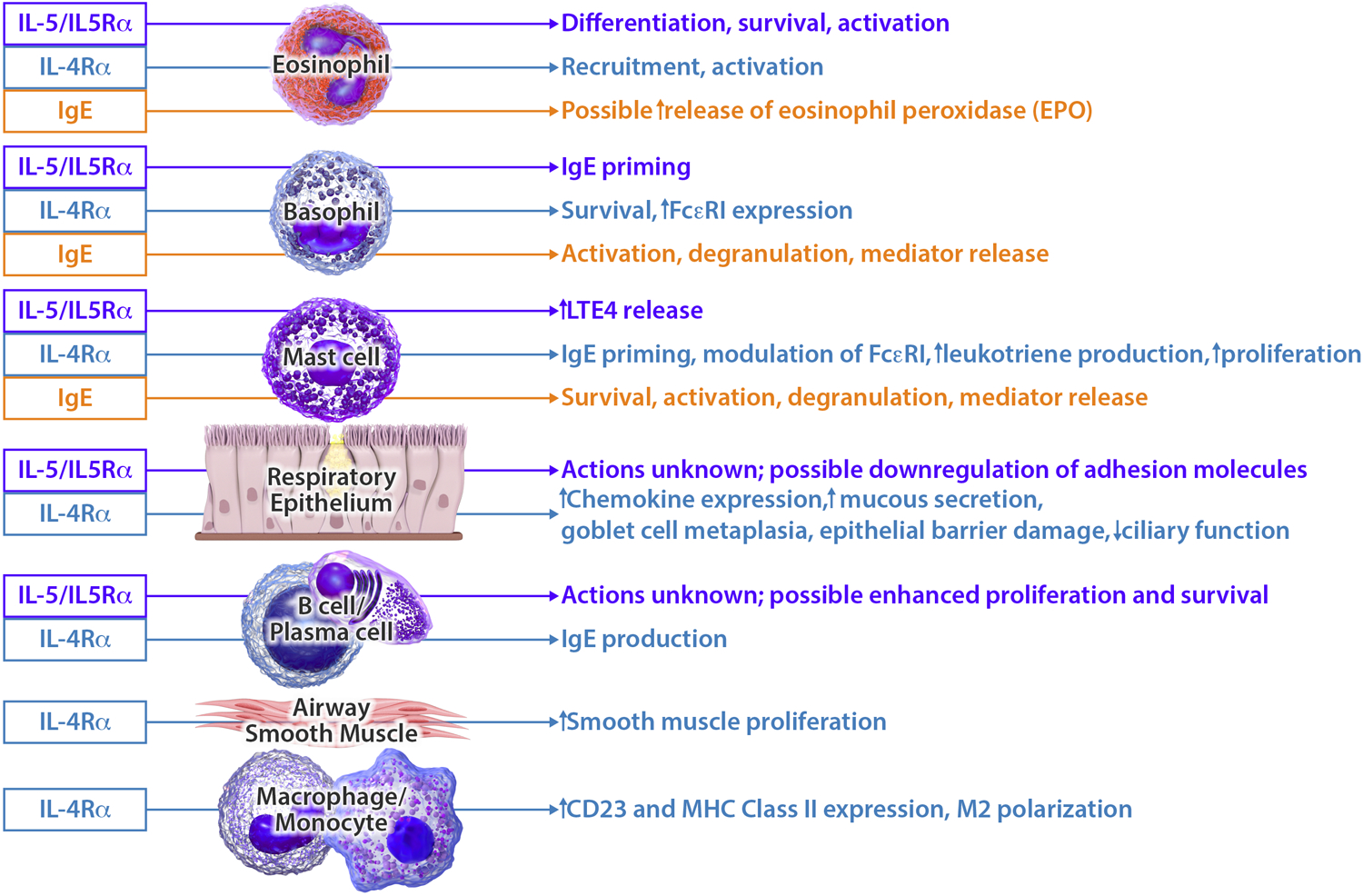
Impact of IgE, IL-5, IL-5Rα, and IL-4Rα on type 2 inflammation in the upper and lower airway. IgE, IL-5, IL-5Rα, and IL-4Rα, which are the targets of currently available respiratory biologic medications, act on multiple cell types in the upper and lower airway including eosinophils, basophils, mast cells, epithelium, plasma cells, macrophages, and smooth muscle.
IL-5 is required for eosinophil survival and activation, but other cell types also express a functional IL-5Rα and recently a possible role for IL-5/IL-5Rα signaling on nasal polyp plasma cells in patients with AERD has been identified.4 While much attention has been focused on the nasal polyp tissue eosinophilia that is prominent in AERD, complete depletion of eosinophils from the respiratory tissue with the small molecule dexpramipexole did not lead to symptomatic improvement or reduction in nasal polyposis, suggesting that eosinophils may not be the main effector cell that drives the chronic inflammation.5
Targeted therapy with respiratory biologic medications in AERD
Increased understanding of the immunobiology of CRSwNP and asthma in AERD has resulted in the identification of several type 2 cytokines and cytokine receptors that may be targeted by available and upcoming biologics. Among these, dupilumab (anti-IL-4Rα and omalizumab (anti-IgE) have been specifically studied in patients with AERD, and both have U.S. Food and Drug Administration indications for the treatment of moderate-to-severe asthma, and as add-on therapy for inadequately controlled CRSwNP.
Dupilumab inhibits signaling of both IL-4 and IL-13, two pivotal drivers of type 2 inflammation (Figure 1). A nested analysis of 19 AERD patients receiving either dupilumab or placebo in a phase 2a, randomized controlled trial (NCT01920893) demonstrated improved upper and lower airway symptom control among those with AERD versus CRSwNP patients without NSAID sensitivity.6 Specific improvement was seen in the objective nasal polyp score (least-squares mean difference −2.51 versus placebo and −0.72 versus CRSwNP without NSAID sensitivity), as well as objective measures of mucosal inflammation and olfaction. Additionally, subjective improvement in asthma control, sinonasal symptoms and sense of smell/taste was found in CRSwNP patients with or without comorbid NSAID sensitivity.
Elevated IgE is found in nasal polyp tissues and is associated with increasing airway inflammation in AERD.4 Omalizumab is an anti-IgE biologic which may improve airway inflammation in AERD via direct reductions in eosinophil, basophil and mast cell activation (Figure 1). In a study of 21 AERD patients with comorbid aeroallergen sensitivity treated with omalizumab, Hayashi et al7 demonstrated decreased biomarkers of mast cell activation (leukotriene E4 and a prostaglandin D2 metabolite) following twelve months of therapy. Patient symptoms also improved, with reductions in lower airway exacerbations, corticosteroid utilization, hospitalization and CRSwNP/asthma symptom scores.
Anti-IL-5/IL-5Rα biologics, which reduce local respiratory tissue eosinophilia in patients with CRSwNP and asthma, have shown promise in the treatment of severe asthma, and can be efficacious for upper respiratory symptoms in AERD.8 However, as isolated depletion of eosinophils does not provide symptomatic improvement or the reduction of obstructive nasal polyps,5 the mechanism of anti-IL-5 biologics in AERD patients may not be limited to its effects on eosinophils. Additional cellular mechanisms by which IL-5 blockade may provide benefit include decreased mast cell leukotriene E4 production and improved function of the respiratory epithelial barrier (Figure 1), as shown in a recent case-control study9 of AERD patients treated with mepolizumab. Benralizumab (anti-IL-5Rα) and mepolizumab (anti-IL-5) are currently being evaluated for the treatment of CRSwNP. In a recently completed phase 3 study of mepolizumab for CRSwNP, a subgroup analysis of the subjects with co-morbid AERD showed greater improvement in the co-primary endpoints, nasal obstruction and nasal polyp size, in the mepolizumab-treated AERD subjects compared to placebo.10 Benralizumab has a current indication for severe eosinophilic asthma and its use for CRSwNP is supported by a subgroup analysis of a phase 3b clinical trial that demonstrated improved 22-item Sinonasal Outcome Test (SNOT-22) scores among n=153 subjects with comorbid CRSwNP (NCT03170271). While this field continues to emerge, early findings suggest that only a subset of patients with AERD have satisfactory clinical response to biologics targeting IL-5/IL-5Rα, and dupilumab may improve respiratory symptoms in AERD patients who have had an inadequate response to anti-IL-5/IL-5Rα biologics.8
Monoclonal antibodies targeting IL-33 and TSLP are currently under investigation for treatment of asthma and/or CRSwNP (NCT03469934, NCT03112577, NCT03614923, NCT03706079). We look forward to studies of these biologics in patients with AERD, as both cytokines are significantly elevated in tissue from patients with AERD and likely have an important role in driving mast-cell mediated inflammation.
Conclusion
Targeted respiratory biologics represent a clinically significant advancement in our ability to improve treatment outcomes for recalcitrant, type 2 inflammatory diseases such as AERD. However, these immunomodulatory medications are very costly and many do not yet have long-term safety or outcome data available, and therefore are not required by nor appropriate for all AERD patients. We therefore use the treatment pathway shown in Figure 2 to aid in decision making, and typically offer biologics only following comprehensive sinus surgery and a trial of aspirin desensitization. Future head-to-head studies and real-world evidence is needed to support personalized treatment strategies and the identification of the most efficacious biologic for a given patient.
Figure 2:
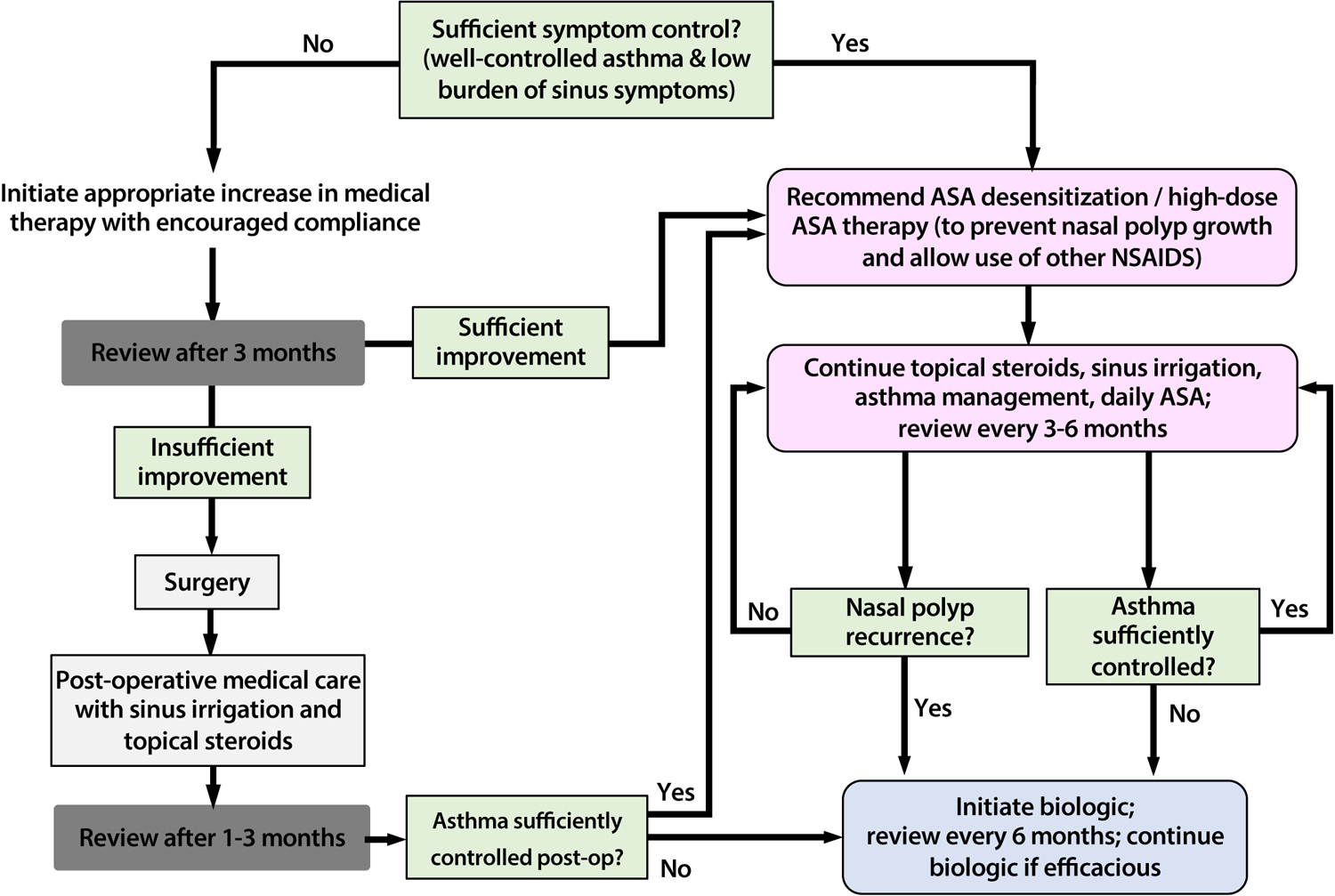
Proposed treatment algorithm for aspirin desensitization and targeted respiratory biologics for patients with AERD.
Funding:
This work was supported by the National Institutes of Health (NIH grants U19AI095219, K23AI139352, R01HL128241), and by generous contributions from the Vinik and Kaye Families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Sanofi-Genzyme, Optinose, AstraZeneca and Regeneron. K Buchheit has served on scientific advisory boards for AstraZeneca and GlaxoSmithKline. J Levy has served on scientific advisory boards for Regeneron Pharmaceuticals and GlaxoSmithKline.
References
- 1.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med. 2018;379(11):1060–70. [DOI] [PubMed] [Google Scholar]
- 2.Scott WC, Cahill KN, Milne GL, Li P, Sheng Q, Huang LC, et al. Inflammatory heterogeneity in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2021;147(4):1318–28 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, et al. IL-5Ralpha marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2020;145(6):1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–E6. [DOI] [PubMed] [Google Scholar]
- 6.Laidlaw TM, Mullol J, Fan C, Zhang D, Amin N, Khan A, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7(7):2462–5 e1. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi Hiroaki CMENYFKKKWKSTTKAYHMT. Omalizumab reduces cysteinyl leukotriene and 9α,11β-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immun. 2016;137:1585 7.e4. [DOI] [PubMed] [Google Scholar]
- 8.Bavaro N, Gakpo D, Mittal A, Bensko J, Laidlaw TM, Buchheit KM. Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Ralpha in a real-world setting. J Allergy Clin Immunol Pract. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchheit KM, Lewis E, Gakpo D, Hacker J, Sohail A, Taliaferro F, et al. Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2021;In Press (JACI-D-21-00366R1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021. [DOI] [PubMed] [Google Scholar]


