ABSTRACT
Objectives:
The aim of this article is to review the factors that attract Candida albicans to denture base resin (DBR) and to verify the influence of different surface treatments, chemical modification, or structural reinforcements on the properties of DBR.
Materials and Methods:
Searches were carried out in PubMed, Scopus, WOS, Google Scholar, EMBASE, and J-stage databases. The search included articles between 1999 and 2020. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The keywords used during the search were “Candida albicans,” “Denture base,” “PMMA,” “Acrylic resin,” “Surface properties,” “hydrophobicity/hydrophilicity,” “contact angle,” and “surface free energy.” English full-text articles involving in-vitro studies with different acrylic resin modifications were included, whereas abstracts, dissertations, reviews, and articles in languages other than English were excluded. A meta-analysis was performed where appropriate.
Results:
Out of the 287 articles, 21 articles conformed to inclusion criteria. Sixteen articles were subjected to meta-analysis using random-effects model at 95% confidence interval. Results showed that DBR coatings/plasma coatings were effective methods to modify surface properties with estimated contact angle (CA) of 59.37° [95% confidence interval (CI): 53.69, 65.04]/55.87° (95% CI: 50.68, 61.06) and surface roughness (Ra) of 0.55 µm (95% CI: 0.52, 0.58)/0.549 µm (95% CI: 0.5, 0.59), respectively. Antifungal particle incorporation into poly(methylmethacrylate) DBR also produced similar effects with an estimated Ra of 0.16 µm (95% CI: 0.134, 0.187).
Conclusion:
The three properties responsible for C. albicans adhesion to DBR were Ra, CA, and surface free energy in terms of hydrophobicity. Therefore, the correlations between the hydrophobicity of DBR and C. albicans adhesion should be considered during future investigations for Candida-related denture stomatitis.
KEYWORDS: Candidiasis, PMMA denture base, surface properties
INTRODUCTION
Candida-associated denture stomatitis (DS) infection depends mainly on the denture base (DB) properties and the ability of Candida albicans (C. albicans) (the most common pathogen in DS) to adhere to the denture surface.[1,2] Reports have confirmed that Candida adhesion to acrylic is associated with hydrophobic interactions between the two.[2,3] Because C. albicans are hydrophobic, they can easily adhere to the hydrophobic poly(methylmethacrylate) (PMMA) DB.[4] Therefore, hindering this interaction may help prevent various infections including DS. Achieving this would be of extreme benefit for elderly patients with dentures and their caretakers.[2] The most relevant factors of a DB that influence microbial attachment are surface roughness (Ra), hydrophobicity/hydrophilicity, and surface free energy (SFE), in addition to salivary pellicles and the presence of other microorganisms.[5,6]
Higher microbial adhesion is linked to Ra and hydrophobicity of the DB material,[7] in which roughness is capable of providing more surface area and protective hideout spot for microorganisms away from denture cleaning forces.[7] To limit the microbial colonization, Ra of DB should not exceed 0.2 μm.[7,8]
The chemical composition of PMMA which includes carboxylate, methyl ester groups, as well as other additives, cross-linking agents, fillers, and colorants affects the hydrophobicity and SFE of the DB.[9] Studies have reported that SFE and wettability of different denture base resins (DBRs) are related to variations in these additives.[10] In recent years, several nanoparticles such as ZrO2, SiO2, TiO2, and diamond nanoparticles have been incorporated within the PMMA in an attempt to enhance the physio-mechanical properties of the material. These fillers were also found to increase the resistance of the material to microbial adhesion.[10,11]
Researchers have used surface coating, chemical modifications, or synthesized and incorporated fillers with antimicrobial properties within PMMA to solve the issue of Candida adhesion. However, reviews of the effect of these treatment modifications on PMMA properties with correlation to hydrophobicity are not yet available. The aims of this study were to (1) systematically review literature pertaining to the modifications of DBR and (2) to correlate the variables to Candida adhesion/biofilm formation. The null hypothesis of this study was that alteration of the DBR in the form of filler addition, chemical composition modification, or surface coating will not affect the hydrophobicity of the resin surface and therefore will not affect Candida adhesion.
MATERIALS AND METHODS
SEARCH STRATEGY
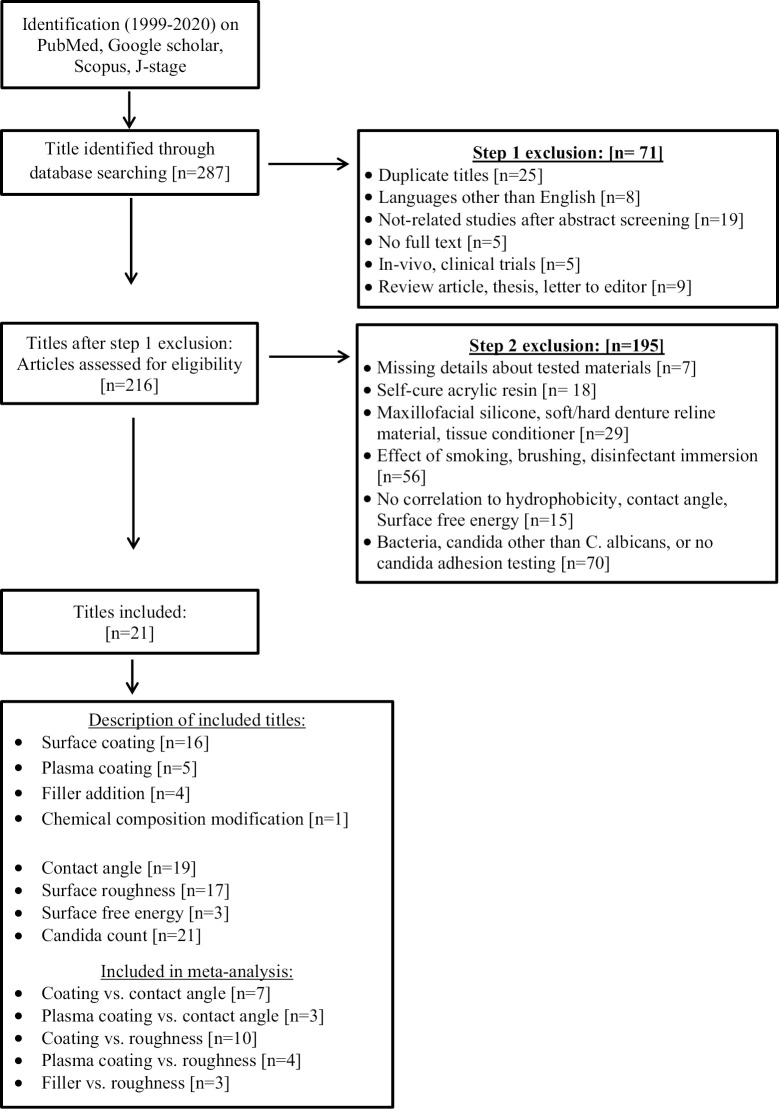
This systematic review was completed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). Focus question was generated through the PICO(S) approach and research strategy [Table 1] to systematically review the available literature. Two PICO questions were formulated as follows: first, do the modifications of DB alter the hydrophobicity and Candida adhesion thereafter? Secondly, what factors will influence the hydrophobicity of modified DBR? An electronic search of English-language dental literature on PubMed, Scopus, WOS, Google Scholar, EMBASE, and J-stage databases was conducted for articles published between January 1999 and March 2020 [Figure 1]. To identify all relevant articles, a list of keywords was used for the search. These included “Denture base,” “PMMA,” “Surface properties,” “hydrophobicity/hydrophilicity,” “contact angle,” “surface energy,” and “C. albicans.” The inclusion criteria included full-text articles in the English language, with in-vitro design, investigating heat-polymerized DBR, C. albicans adhesion, contact angle (CA), surface wettability, Ra, and/or SFE with different DB modifications (antimicrobial additives, surface coating, chemical composition modification). In contrast, papers in languages other than English, in-vivo clinical study, case reports, abstracts, short communication, letters to the editors, reviews, and dissertations and materials other than heat-polymerized acrylic resin or resin not used for DBs were excluded.
Table 1.
Systematic search strategy
| Focus questions | What are the influencing factors of the hydrophobicity of modified denture base resin? |
|---|---|
| PICOS | |
| P: Participant | Modified denture base materials |
| I: Interventions | Incorporating antifungal agents |
| Surface coatings | |
| Chemical composition modification | |
| C: Comparison | Unmodified heat-polymerized/microwave-polymerized acrylic resin |
| Modified heat-polymerized acrylic resin | |
| O: Outcomes | Effectiveness of modifications on surface free energy/hydrophobicity |
| S: Study design | Networking meta-analysis |
Figure 1.
Flowchart of the study design
ELIGIBILITY CRITERIA
Two investigators (MMAG and RA) reviewed the articles independently according to the same parameters. Studies that (1) measured the effect of incorporated antifungal agents, surface coating, or chemical composition modifications of heat-polymerized PMMA, (2) evaluated the C. albicans adhesion and one of the following properties: CA, SFE, Ra, or hydrophobicity/hydrophilicity, (3) reported sample size, mean, and standard deviation values, and (4) included brand names and specifications of tested materials were included in this review. DB modifications were categorized as follows: control (unmodified PMMA), antifungal additive, surface coatings, and chemical composition modifications.
DATA MANAGEMENT, SCREENING, AND SELECTION
Two independent investigators (MMG and RA) used a standardized Excel sheet to extract the data of the studies. The search was conducted in three steps. First, the titles were reviewed according to the inclusion/exclusion criteria. Secondly, the abstracts of the selected titles were screened to select those of interest for full-text analysis. At the third step, all full-text articles were analyzed. At all stages, any discrepancies between investigators were resolved by discussion. The extracted data included: the authors’ names, year of publication, materials of the study, processing method, Candida species, tests employed, presence of control group, number and dimensions of specimens, type of resin modification, results, statistical analysis and significance, and conclusions. Studies with similar methodology were selected to undergo meta-analysis. Among the scopes of this systematic review is to conduct a meta-analysis taking into consideration the diverse designs (resin modifications) of the studies and the various properties tested and to assess their effect qualitatively (surface properties) and quantitatively (number of Candida colony-forming units) [Tables 2 and 3].
Table 2.
Included studies
| Author, year | Acrylic brand, composition, Candida species | Processing method/sample dimensions | Modification | Tested properties | Results | Conclusions |
|---|---|---|---|---|---|---|
| Yildirim et al., 2005[16] | Denture acrylic, Meliodent (Bayer Dental, Newbury Berkshire, UK) | *Heat-cured | *One surface polished, the other was not (ground with 500 grid sandpaper) |
*Contact angle | *O2 surface modification sig. improved wettability (lowered contact angle) compared with control | *O2 gas is effective in increasing wettability of PMMA even with salivary pellicle. |
| *Control *102 discs (17×1 mm) |
*Plasma surface treatment for 15 min at the O2 level of 0, 50, or 100 W. n=34 | *Candida adhesion | *The reduction in contact angle is directly related to plasma power | *Candida adherence increased as hydrophilicity increased | ||
| *n=60 for wettability *n=30 for Candida adhesion *n=12 for surface analysis |
*Saliva contact | *Saliva reduced contact angle of control, and increased it for plasma-treated | ||||
| *Candida adhesion to control was less than surface treated → increased surface wettability, increased Candida adhesion | ||||||
| *Increase in O/C ratio → more hydrophilic | ||||||
| Nevzatoğlu et al., 2007[17] | ACRON Shade No. 3, GC | *Heat-cured | *Polishing up to 1000 grit *Buff polished |
*Candida adherence | *Candida count was lowest in the coated specimens < buff polished < control | *Straight silicone coating is capable of improving surface properties of denture base material so that it becomes difficult for C. albicans to adhere |
| C. albicans (JCM 1542) | *Discs (20×1 mm) | *20% straight silicon coating for 5, 30 min | *Contact angle | *Contact angle of the coated specimens was larger than control or buff polished | ||
| *Control (uncoated) | ||||||
| Zamperini et al., 2010[18] | Vipi Wave; VIPI | *Microwave-cured | *Processing technique (against glass or against stone) | *Surface roughness | *Surface roughness of specimens processed against glass was lower. No difference between all groups regarding surface roughness in each investigation. | *Adherence of C. albicans was sig. reduced by ArO2/70 W and ArSF6/70 W plasma, regardless of the presence or absence of saliva and surface roughness (smooth or rough). |
| Industria e Comercio Exportacao e Importacao de Produtos Odontologicos Ltda Pirassununga, SP, Brazil | *Control *180 discs (13.8×2 mm) in 10 groups *n=18 |
*Plasma treatment for 5 min: | *Contact angle | *Groups 2 and 4 were not sig. different from each other and showed sig. lower absorbance reading. | *Hydrophobicity was altered by the plasma treatments and water immersion | |
| C. albicans (ATCC 90028) | *Processed against glass or stone | 1: argon atmosphere at 50 W | *Candida adhesion | *Contact angle was altered by plasma tx and water immersion for all groups except controls. | *No sig. effect of surface roughness and saliva on adherence of C. albicans | |
| 2: argon/oxygen atmosphere at 70 W 3: atmospheric air at 130 W | *For control, contact angle was sig. different b/w rough and smooth. | |||||
| 4: argon atmosphere, followed by sulfur hexafluoride atmosphere at 70 W *Saliva exposure (30 min unstimulated whole human saliva) |
*XPS showed incorporation of fluorine into the surface of group 4 | |||||
| Zamperini et al., 2010[19] | Vipi Wave; VIPI | *Microwave-cured | *Processing technique (against glass or against stone) | *Surface roughness | *Surface roughness of specimens processed against glass was lower. | *Contact angle was altered by the plasma treatments. However, mean contact angles of treated specimens were similar to those of control specimens, after 48 h of immersion in water. |
| Industria e Comercio Exportacao e Importacao de | *Control *180 discs (13.8×2 mm) in five groups |
*Plasma treatment for 5 min: 1: argon atmosphere at 50 W |
*Contact angle | *Contact angle for all groups changed after water immersion except control. All test groups showed an increase in contact angle after water immersion except group 4 which showed a reduction. | *Adherence of C. albicans was not sig. reduced by plasma treatments, surface roughness, or presence of saliva | |
| Produtos Odontologicos Ltda Pirassununga, SP, Brazil | *n=18 *Processed against glass or stone |
2: argon/oxygen atmosphere at 70 W 3: atmospheric air at 130 W |
*Candida adhesion | *No sig. difference between all groups regarding Candida adhesion irrespective of ± saliva, surface roughness, treatment | ||
|
C. albicans (ATCC 90028) |
4: argon atmosphere, followed by sulfur hexafluoride atmosphere at 70 W | |||||
| *Saliva exposure | ||||||
| Wady et al., 2012[20] | Vipi Wave; VIPI | *Microwave-cured | *AgNPs solution mixed with 75g acrylic powder at concentrations of (1000, 750, 500, 250, 30, 0 ppm), dried, sieved, ball milled | *Surface roughness | *No sig. difference in contact angle between 0 and 7 days or 90- and 180-day storage periods. | *AgNPs had no effect on C. albicans adherence and biofilm formation regardless of concentrations |
| Industria e Comercio Exportacao e Importacao de Produtos | *Control *72 discs (13.8×2 mm) *n=18 |
*Different storage periods (0, 7, 90, 180 days) (n=18) | *Contact angle | *After 90 and 180 days, contact angles were sig. higher than that at 0 and 7 days | ||
| Odontologicos Ltda Pirassununga, SP, Brazil | *Adherence biofilm formation | *Contact angles were lower than control for all experimental groups | ||||
| C. albicans (ATCC 90028) | *No sig. difference b/w 0–7, 90–180 days regarding Candida adhesion and biofilm | |||||
| *Significant absorbance value noted for 90 and 180 days | ||||||
| Lazarin et al., 2013[21] | Vipi Wave; VIPI | *Microwave-cured | *Processed against glass (smooth) or against stone (rough) | *Surface roughness | *Sig. increase in surface roughness for all rough specimens. | *Experimental S and HP coatings showed sig. reduction of short-term attachment (90 min) of C. albicans to PMMA |
| Industria e Comercio Exportacao e Importacao de Produtos Odontologicos Ltda Pirassununga, SP, Brazil | *468 discs (13.8×2 mm) in 13 groups | *Photopolymerized coatings: 1. 2-hydroxyethyl methacrylate (HE) (HEMA) (cured for 4 min) |
*Surface free energy through contact angle measurement | *Total surface free energy was generally higher in all experimental groups compared with controls | ||
|
C. albicans (ATCC 90028) |
*n=36 *Control |
2. hydroxypropyl methacrylate (HP) (HPMA) (cured for 4 min) | *Candida adhesion | *Generally, no sig. difference of surface free energy b/w saliva-coated and uncoated specimens | ||
| 3. 2-tri-methyl-ammonium ethyl methacrylate chloride (T) (TMAEMC) (cured for 4 min) | *For smooth specimens, no sig. difference b/w all groups | |||||
| 4. sulfobetaine methacrylate (S) (oven at 80°C for 2 h) *Concentrations of coatings at 0%, 25%, 30%, and 35% of the total composition in mmol. |
*For rough surfaces, S30, S35, and HP30 had sig. lower absorbance values than control | |||||
| Additional components in the coating: MMA, TEGDMA, bis-GMA, 4-methyl benzophenone. Also, amino propyl methacrylate for group 4 | ||||||
| *± saliva (non-stimulated) for 30 min at room temp. | ||||||
| Queiroz et al., 2013[22] | Lucitone 550; Dentsply Ind. Com. Ltda, Petropolis, Brazil | *Heat-cured *45 discs (10×5mm) in three groups |
*Polishing of both sides to 1200 grit silicon carbide paper | *Surface roughness (optical, non-contact) | *RBS confirms the presence of carbon in groups 2 and 3 and silver in group 3 | *DLC thin films significantly diminished C. albicans biofilm formation |
|
C. albicans (ATCC 18804) |
*n=15 *Control (no surface treatment) |
*Surface treatment for 15 min: | *Rutherford backscattering spectroscopy (RBS) and atomic force microscopy (AFM) for film characterization | *Surface roughness did not affect the number of Candida adhered | *The films undoped and doped with silver nanoparticles presented similar behavior. | |
| 1. no coating (Gc) | *Anti-microbial activity assessment after 24 h at 37°C b CFU count | *Surface treatment reduced Candida adhesion in groups 2 and 3 compared with control | ||||
| 2. surface coating with DLC fil (Gdlc) | *No additional reducing effect was seen with Ag addition | |||||
| 3. surface coating with DLC doped with Ag-Nps (Gag) | *DLC increased hydrophobicity and lowered surface energy | |||||
| DLC = diamond-like carbon | ||||||
| Al-Bakri et al., 2014[23] | Urban, Shofu Inc., Kyoto, Japan | *Heat-cured *50 discs (10×1.5 mm) in 5 groups |
*Silane-coated glass fibers (1.5 µm, with 15% w/w fluoride) were added to PMMA at concentrations of 0.5%, 1.0%, 2.5%, 5.0%, 10% | *Contact angle | *No sig. difference between all groups regarding contact angle and surface free energy→ fluoride did not have an effect | *Increased loading of the fillers produced increased surface roughness |
| C. albicnas (GDH 2346) | *n=10 *Control |
*Polishing of both sides with 400 grit Al2O3 | *Surface free energy (contact angle cosine value) | *10% filler produced sig. rougher surface than control and 1.0% | *No direct correlation between surface roughness and microbial adhesion | |
| *Surface roughness (non-contact) | *Fluoride addition sig. reduced Candida adhesion to PMMA | *Presence of saliva and fluoride glass fillers significantly reduced Candida adhesion | ||||
| *Adherent Candida count using a light microscope | *Coating PMMA with saliva sig. reduced Candida adhesion | |||||
| Lazarin et al., 2014[24] | Vipi Wave; VIPI | *Microwave-cured *468 discs (13.8×2 mm) in 13 groups |
*Processed against glass (smooth) or against stone (rough) | *Surface roughness | *No sig. differences in surface roughness among groups within each fabrication method | *Experimental photopolymerized coatings did not alter hydrophobicity but changed chemical composition. |
| Industria e Comercio Exportacao e Importacao de Produtos Odontologicos Ltda Pirassununga, SP, Brazil |
*n=36 *Control |
*Photopolymerized coatings: | *contact angle | *Samples prepared against stone were sig. rougher than those prepared against glass | *C. albicans adhesion decreased with coatings sulfobetaine, 2-hydroxypropyl methacrylate, and 2-hydroxyethyl methacrylate | |
|
C. albicans (ATCC 90028) |
1. 2-hydroxyethyl methacrylate (HE) (HEMA) (cured for 4 min) | *Candida adhesion | *Smooth groups HE30, T25, T30, and T35 had sig. higher contact angle | |||
| 2. hydroxypropyl methacrylate (HP) (HPMA) (cured for 4 min) | *Contact angles for rough surface were not sig. different | |||||
| 3. 2-tri-methyl-ammonium ethyl methacrylate chloride (T) (TMAEMC) (cured for 4 min) | *No sig. different in Candida adhesion for saliva-coated specimens | |||||
| 4. sulfobetaine methacrylate (S) (oven at 80°C for 2 h) | *Smooth and non-saliva-coated specimens showed sig. lower Candida with S35, HP35, and HE35 | |||||
| *Concentrations of coatings 0%, 25%, 30%, and 35% of the total composition in mmol. | *Rough specimens ± saliva → no sig. difference between groups regarding Candida adhesion | |||||
| Additional components: MMA, TEGDMA, Bis-GMA, 4-methyl benzophenone. Also, amino propyl methacrylate for group 4 | *Rough S25, S30, HP35, HE30, HE35, T35 with no saliva showed higher Candida adhesion than same smooth groups | |||||
| *± saliva (non-stimulated) for 30 min at room temp. | *XPS showed increase in C, O, Si after HE, HP, and T coating, and S for S coating | |||||
| Yodmongkol et al., 2014[25] | Rodex (Australia) | *Heat-cured | *Silane-SiO2 nanocomposite dip-coating evaporating solvent at 65°C for 20 min and then heating to 110°C for 2 h | *Candida adhesion after 1 h using optical microscope (n=6) | *Sig. higher cell adhesion was seen on uncoated specimens than coated | *Silane-SiO2 nanocomposite films can make acrylic resin more hydrophobic, which decreases C. albicans adhesion. |
|
C. albicans (ATCC 10231) |
*Rectangular specimens (1.5×1.5×1 mm) | *FTIR (n=3) | *FTIR for coated showed a peak for Si-O-Si | *This film improved surface and physical properties of acrylic | ||
| *Control *Roughness (n=5) *Contact angle and SFE (n=3) |
*Surface roughness (contact) (n=5) | *Surface roughness was the same for coated and uncoated | ||||
| *Contact angle of three liquids were used: | *Surface energy was slightly reduced on coated (not sig.) | |||||
| deionized water 18 MΩ/cm, diiodomethane, and glycerol (n=3) → SFE | *Average thickness of coating was 6.8 ±1.0 µm | |||||
| *SEM | ||||||
| Sawada et al., 2014[26] | Natural Resin, Nissin Co., Kyoto, Japan | *Heat-cured | *Addition of 5 wt.%: | *Surface roughness | *Candida binding was sig. decreased in all test groups compared with controls | *Influence of surface characteristics on the adhesion of C. albicans to various denture lining mats |
| C. albicans (ATCC 1002) | *Rectangular 64×10×33 mm | —FAp-TiO2 (100 nm) —HAp-TiO2 (100 nm) |
*Viable cells determination after incubation for 2 h at 37°C and UVA irradiation | *Candida binding was sig. lower in the FAp-TiO2- and TiO2- containing discs than in the HAp-TiO2-containing discs | ||
| *n=12 *Control (pure PMMA) |
—TiO2 (25 nm) | |||||
| *Polishing up to 2000 grit polishing paper | ||||||
| Compagnoni et al., 2014[27] | Lucitone 550 | *Heat-cured | *Modification with PTBAEMA (0% or 10%) | *Contact angle measurement using 1.0 µL deionized water drop | *Surface roughness increased with PTBAEMA addition | *PTBAEMA slightly increases wettability and roughness of acrylic resin |
| Dentsply International Inc., York, PA, USA | *Control (unmodified) | PTBAEMA= polymer poly (2-tert-butylaminoethyl) | *Atomic force microscopy observations of 100 and 400 µm2 | *Contact angles of PTBAEMA-modified acrylic is lower than controls | *PTBAEMA into acrylic resins did not have an effect against C. albicans at 10% | |
| C. albicans (ATCC 90028) | *Discs (15×3mm) in two groups | Methacrylate | *Adherence assay using CFU counts after 90 min at 37°C | *Contact angle decreased as roughness increased | ||
| *No sig. difference b/w controls and PTBAEMA-modified acrylic regarding Candida count. | ||||||
| Pan et al., 2015[28] | Vertex Rapid Simplified, Vertex-Dental, Zeist, The Netherlands | *Heat-cured | *Polishing to 600 or to 2000 grit silicon carbide | *Contact angle of 2 µL ultrapure water drop (n=18) | *Contact angle sig. reduced after plasma treatment → more hydrophilic | *Ar/O2 plasma treatment improved surface wettability of PMMA without degrading physical properties |
| C. albicans (ATCC 10231) | *36 discs (12×1 mm) in two groups | *± saliva (non-stimulated whole saliva) | *Fungal adherence test after 90 min at 37°C (n=18) using gradient dilution method. | *Sig. reduction in early Candida adhesion for plasma treated | *Ar/O2 plasma treatment sig. reduced early C. albicans adhesion | |
| *n=18 *Rectangular (64×10×3.3 mm) *Control |
*Cold plasma treated or not (98% argon, 2% oxygen, at atmospheric pressure). Discs were treated for 90 s, and rectangular discs were treated for 8.5 min | *Surface roughness (contact) *SEM |
*No sig. difference in surface roughness | |||
| *X-ray photoelectron spectroscopy analysis (XPS) | *XPS revealed fluorine on the surface of plasma treated and reduction of C/O | |||||
| *Optical emission spectroscopy (OES) | *OES revealed abundance of O and OH as active components | |||||
| Qian et al., 2016[29] | Vertex Rapid Simplified, Vertex-Dental | *Heat-cured | *Polished to silicon carbide grit 1000 | *Contact angle (after plasma TX, 48 h, 15 days, 30 days) | *Contact angle decreased after plasma treatment | *Cold plasma treatment resulted in increased hydrophilicity and reduced Candida adhesion |
| BV, Zeist, The Netherlands | *45 discs (12×1 mm) in five groups | *Plasma surface treatment with argon 98%/oxygen 2% for 0, 30, 60, 90, and 120 s | *Surface roughness non-contact (n=9) | *No difference between plasma-treated groups (immediately) | *Prolonged plasma treatment did not improve wettability but affected durability. | |
| C. albicans (ATCC 10231) | *Control *n=9 |
*Candida adhesion by CFU analysis (n=9) | *Contact angle increased after water immersion, 48 h, 15 days | *Reduction in the ratio of C/O, direct relation with treatment time | ||
| *XPS | *No difference b/w all groups after 30 days | *No relation between surface roughness and Candida adhesion | ||||
| *Surface roughness not significantly different b/w all groups | ||||||
| *Lower Candida adhesion for all test groups (90S lowest) | ||||||
| Liu et al., 2017[30] | Lucitone 199; Dentsply Intl Inc. | *Heat-cured | *Smooth and rough surfaces | *Contact angle of sessile drop of distilled water | *Contact angle of coated specimens was higher | *Hydrophobicity increased by TMS coating |
| C. albicans (ATCC 18804) | *60 discs (10×2 mm) in four groups | *Coated with TMS or not coated (trimethylsilane) | *Absorbance of OD (optical density) for Candida | *Absorbance intensity of coated specimens is less than that of controls | *Candida adhesion was decreased by TMS coating | |
| *n=15 *Control |
*MTT assay | *Surface roughness alone did not affect Candida adhesion | ||||
| *SEM and EDS | ||||||
| Türkcan et al., 2018[31] | Meliodent | *Heat-cured | *Polished with silicon carbide paper 600 grit | *Contact angle of 2 µL sessile drop of pure water (n=3) | *Significant decrease in contact angle for 0.25 and 0.75 mol/L MPC → increased wettability | *Surface modification with MPC coating decreased contact angle in 0.25 and 0.75 mol/L MPC groups. |
| Heat Cure, Heraeus Kulzer, Germany | *Disc (6×1.5 mm) in four groups | *Surface coating with MPC (2-methacryloyloxyethyl phosphorylcholine) dissolved in degassed pure water at concentrations of 0.25, 0.5, 0.75 mol/L | *Surface roughness (contact) (n=3) | *MPC coating increased surface roughness, no difference between groups | *Graft polymerization of MPC does not cause a significant change in surface roughness. | |
| C. albicans (ATCC 90028) | *Contact angle and roughness (n=3) | *FTIR spectroscopy with attenuated total reflection (ATR) equipment (n=2) | *MCP increased hydrophilicity (increased water absorption) | *Graft polymerization of MPC decreased C. albicans adhesion onto PMMA surface. | ||
| *Candida adhesion (n=10) | *Candida adhesion assay using CFU (n=10) | *Reduction in Candida adhesion as concentration increased, no difference between 0.5 and 0.75 mol/mL | ||||
| *Control | *SEM (n=2) | |||||
| Hirasawa et al., 2018[32] | Natural resin, Nissin Co., Kyoto, Japan | *Heat-cured | *Polished on both sides to 8000 grit | *XTT reduction assay (n=10) | *Significant difference among all groups for XTT and CFU | *Coating with cross-linkable co-polymers containing SBMAm significantly reduced the initial adhesion of C. albicans |
| C. albicans (JCM2085) | *250 discs (12×2 mm) | *In laboratory-made co-polymer coating plasma cleaning → primer → drying → immersion (10 s) in prepared polymer at concentrations SM0%, SM15%, SM30%, and SM50% → UV (27s) | *CFU (n=10) | *Significant reduction in biofilm for all test groups compared with controls | ||
| *n=10 | *SEM (n=10) | *Surface roughness was less than 0.005 µm for all groups (no difference) | ||||
| *Control | *Surface roughness (non-contact) | *Film thickness was less than 5 µm for all groups—thicker for SM30% than SM0% and SM50% | ||||
| *Film thickness (spectroscopic ellipsometer) | *All coated groups had lower contact angle than control, SM15% had lowest contact angle and highest hydrophilicity | |||||
| *Contact angle of 1 mL purified water drop | ||||||
| Darwish et al., 2019[33] | Lucitone 199 (Dentsply Intl, York, PA, USA) | *Heat-cured | *Polished to 4000 grit silicon carbide paper | *Surface roughness (non-contact) (n=10) | *Surface roughness of coated specimens was less than that of non-coated | *Titanium oxide coating improved wettability, surface smoothness, and increased resistance to microbial adherence. |
| *Rectangular specimens (20×20×1 mm) | *TiO2 coating at 65°C for 3 h to form 30 nm film | *Contact angle using sessile drop of 5 µL deionized water (n=10) | *Contact angle of coated was lower than that of non-coated | |||
| *Roughness and contact angle (n=10) | *Candida adhesion (n=5) after 12 h at 37°C | *Sig. reduction in viable attached Candida cells to coated surfaces | ||||
| *Candida adhesion (n=5) *Control |
*Biofilm formation (n=5) | *Sig. reduction in viable Candida biofilm on coated surfaces | ||||
| Acosta et al., 2019[34] | Lucitone 199 (Dentsply Sirona) and ProBase Hot (Ivoclar Vivadent AG) | *Heat-cured | Acrylic acid or itaconic acid coatings | Surface roughness (non-contact) (n=30) | Affected surface roughness | PMMA acrylic resin base material was superficially modified through the incorporation of carboxylic acid groups by using PAA and PIA coatings that reduced the adherence of C. albicans biofilm by 90%. |
| (ATCC 90028) | *Discs (13–14×4–5 mm) | *Contact angle using sessile drop of 5 µL deionized water (n=30) Candida biofilm adhesion | Increased surface wettability | |||
| *n=30 | PMMA disks modified with PIA or PAA showed a 90% reduction of C. albicans | |||||
| *Control | ||||||
| Fouda et al., 2019 [35] | Major.Base.20 Resin |
*Heat-cured | Nano-diamond at 0.5%, 1.0%, and 1.5% | *Surface roughness (non-contact) (n=30) | Decreased surface roughness at 1% NDs and 0.5% NDs | PMMA/ND composites could be valuable in the prevention of denture stomatitis, which is considered one of the most common clinical problems among removable denture wearers. |
| C. albicans | *Square (10×10×3 mm) | *Contact angle using sessile drop of 5 µL deionized water (n=30) | Decreased C. albicans adhesion | |||
| (ATCC 10231) | *n=30 | C. albicans adhesion | No significant effect was observed on the contact angle. | |||
| *Control | ||||||
| AlBin-Ameer et al., 2020[36] | Major.Base.20, Major Prodotti Dentari SPA, Moncalieri, Italy | *Heat-cured | Nanocoat | *Surface roughness (non-contact) | Nano-coat, Optiglaze, Nano-silica decrease Ra whereas cyanoacrylate increased | Coating of removable prosthesis with nano-coat, Optiglaze, or nanosilica is an effective method to reduce C. albicans adhesion |
| C. albicans | *Rectangular specimens (12×10×2.5 mm) | Optiglaze | *Contact angle using sessile drop of 5 µL deionized water | Nano-coat, Optiglaze, Nano-silica decrease contact angle, whereas cyanoacrylate increased | ||
| (ATCC 10231) | *n=14 | Nano-silica | *C. albicans adhesion | Nano-coat, Optiglaze, Nano-silica decrease C. albicans adhesion, whereas cyanoacrylate increased | ||
| *Control | Cyanoacrylate |
CA = contact angle, SFE = surface free energy, SR = surface roughness, H = hydrophobicity, GS = Google scholar, S = Scopus
Table 3.
Type of tests applied in the included studies
| No. | Year | Author | Tests undertaken in the study |
||||
|---|---|---|---|---|---|---|---|
| Contact angle | Surface free energy | Candida albicans | Candida adherence | Surface roughness | |||
| 1 | 2005 | Yildirim et al. | × | ATCC 10321 | × | ||
| 2 | 2007 | Tokita et al. | × | JCM 1542 | × | ||
| 3 | 2010 | Zamperini et al. | × | ATCC 90028 | × | × | |
| 4 | 2010 | Zamperini et al. | × | ATCC 90028 | × | × | |
| 5 | 2012 | Wady et al. | × | ATCC 90028 | × | × | |
| 6 | 2013 | Lazarin et al. | × | × | ATCC 90028 | × | × |
| 7 | 2013 | Queiroz et al. | ATCC 18804 | × | × | ||
| 8 | 2014 | Al-Bakri et al. | × | × | GDH 2346 | × | × |
| 9 | 2014 | Lazarin et al. | × | ATCC 90028 | × | × | |
| 10 | 2014 | Yodmongkol et al. | × | × | ATCC 10231 | × | × |
| 11 | 2014 | Sawada et al. | ATCC 1002 | × | × | ||
| 12 | 2014 | Compagnoni et al. | × | ATCC 90028 | × | ||
| 13 | 2015 | Pan et al. | × | ATCC 10231 | × | × | |
| 14 | 2016 | Qian et al. | × | ATCC 10231 | × | × | |
| 15 | 2017 | Liu et al. | × | ATCC 18804 | × | ||
| 16 | 2018 | Turkan et al. | × | ATCC 90028 | × | × | |
| 17 | 2018 | Hirasawa et al. | × | JMC 2085 | × | × | |
| 18 | 2019 | Darwish et al. | × | ATCC 90028 | × | × | |
| 19 | 2019 | Acosta et al. | × | ATCC 90028 | × | × | |
| 20 | 2019 | Fouda et al. | × | ATCC 10231 | × | × | |
| 21 | 2020 | AlBin-Ameer et al. | × | ATCC 10231 | × | × | |
ASSESSMENT OF RISK OF BIAS
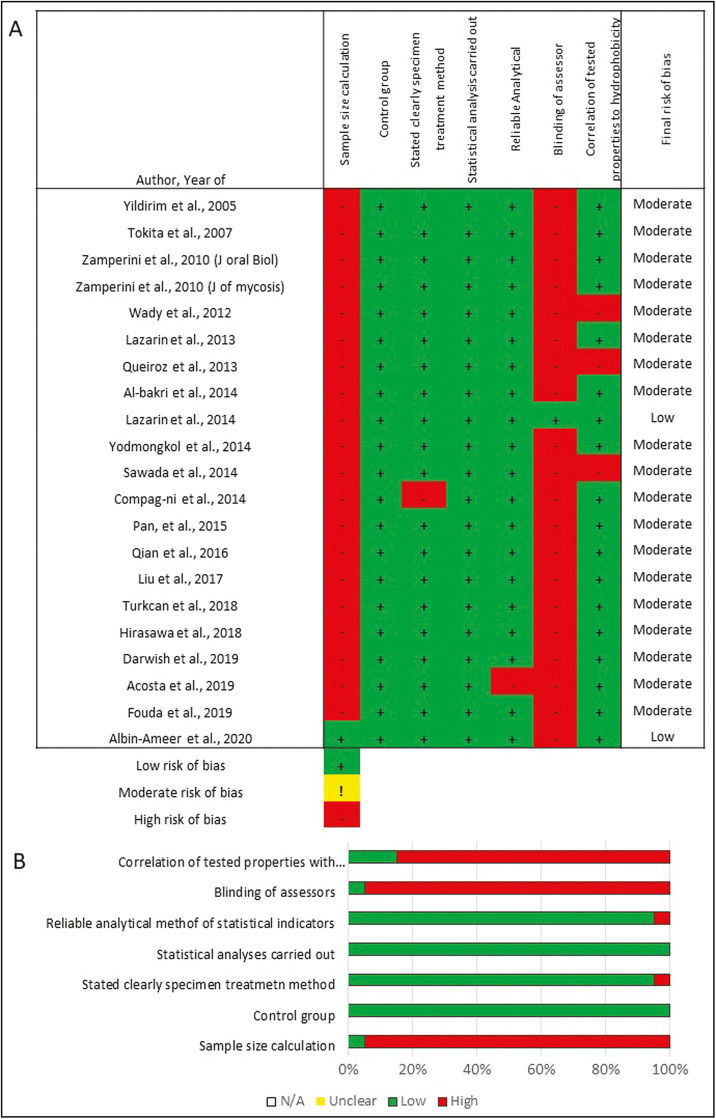
A modification of the method used in previous systematic reviews was used by two authors (MMAG and RA) to independently assess the quality and risk of bias of each study.[12,13,14] The characteristics were tabulated (n=21) and the parameters were reported as “+ve” if the parameter was described in the text or “−ve” if the information was missing or unclear. The parameters assessed were: sample size calculation, the use of a control group, stating the treatment method, statistical analysis performed, reliable analytical methods, blinding of the evaluators, and correlation of the reported properties with hydrophobicity. The risk of bias was classified according to the sum of “+ve” marks obtained as follows: 1 to 3= high-, 4 to 5= medium-, 6 to 7= low-risk of bias.[15]
Meta-analysis was performed for each treatment modality separately. Moreover, due to the variability of outcomes and methodology per treatment method, quantitative meta-analysis was done for 16 studies, whereas the rest of the studies were descriptively analyzed.
DATA ANALYSIS
Comprehensive meta-analysis (version 3, NJ, USA) was used for analysis. Visual inspection of forest plots and χ2 tests were used to evaluate the presence of heterogeneity. Random-effects model was used when the data were found to be heterogenic, whereas the fixed-effects model was used otherwise. Egger’s and Begg’s tests were used to check for the possibility of publication bias. P-values less than 0.05 were considered statistically significant.
RESULTS
DATA SELECTION
Twenty-one studies met the inclusion criteria [Figure 1] and submitted for data extraction and result analysis. Tables 2 and 3 summarize the studies’ details, methods, results, and outcomes.
RISK OF BIAS
Figure 2 presents the risk of bias for the included studies. Out of the 21 studies, 19 showed medium risk of bias and two showed low risk of bias. The risk of bias was mainly linked to the absence of sample size calculation and non-blinding of investigators.
Figure 2.
Risk of bias for the included studies
Applying the inclusion criteria, out of the 21 included articles,[16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] 16 used surface coating, 4 added antimicrobial fillers, and 1 modified the chemical composition of PMMA (refer to Tables 2 and 3 for details). In addition to that, several included studies compared between smooth and rough surfaces of the modified specimens.[17,30] Results revealed that hydrophobicity of DBRs was affected by surface coating, antimicrobial additives, or chemical composition modifications. Therefore, the results of this study were categorized based on the effects of these modifications on the hydrophobicity of DBR and its correlations with CA, Ra, and C. albicans adhesion.
META-ANALYSIS
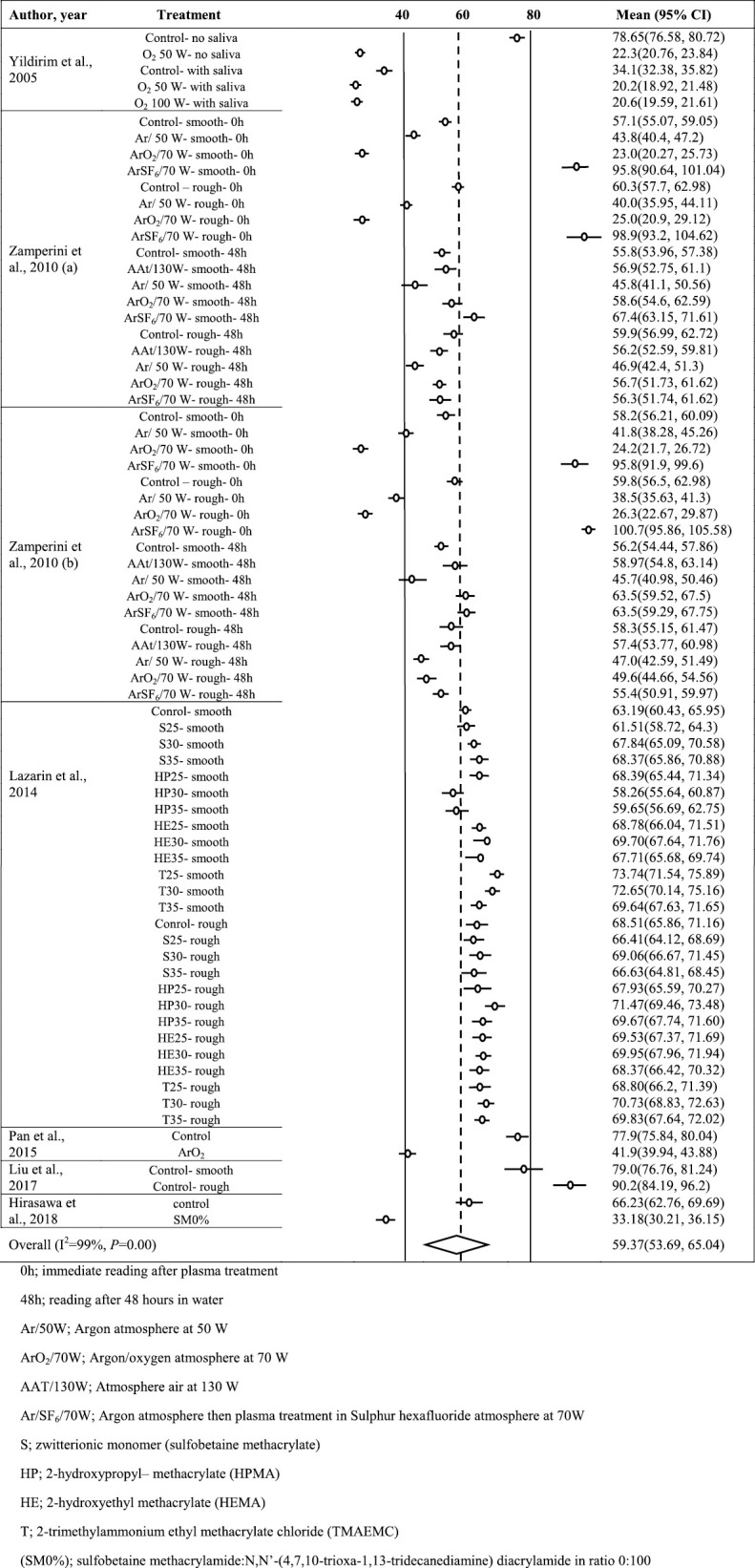
In coating vs. CA (Supplementary Appendix 1), after exclusion of outliers, 74 groups underwent meta-analysis. Due to the considerable heterogeneity found (I2 >75%, P<0.001), random-effects model was used and the average CA after coating was found to be 59.37° [95% confidence interval (CI): 53.7–65.0]. The trim and fill method suggested inclusion of 33 more groups to remove publication bias after getting significant results of Begg’s and Eggers’ tests (P=0.002 and P=0.001, respectively).
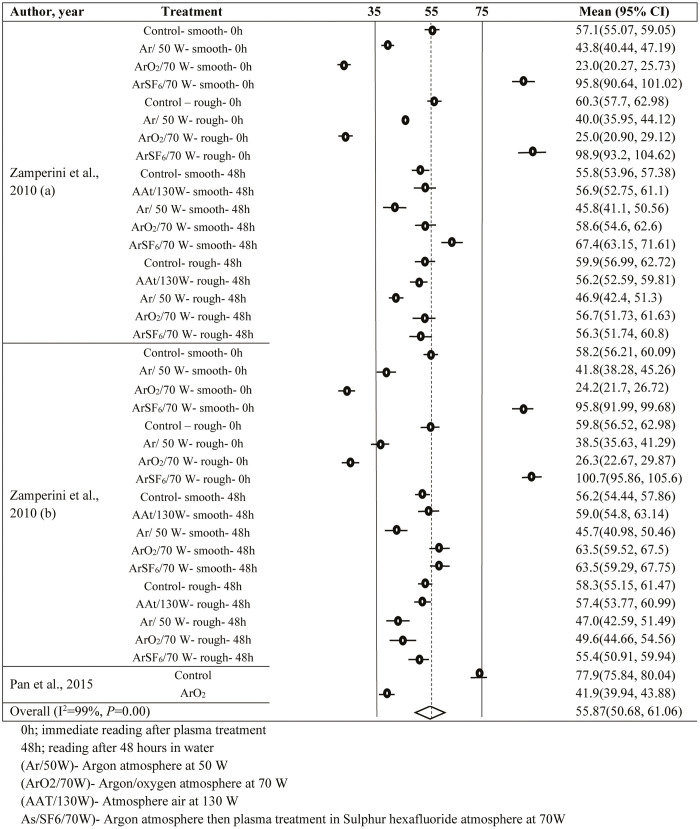
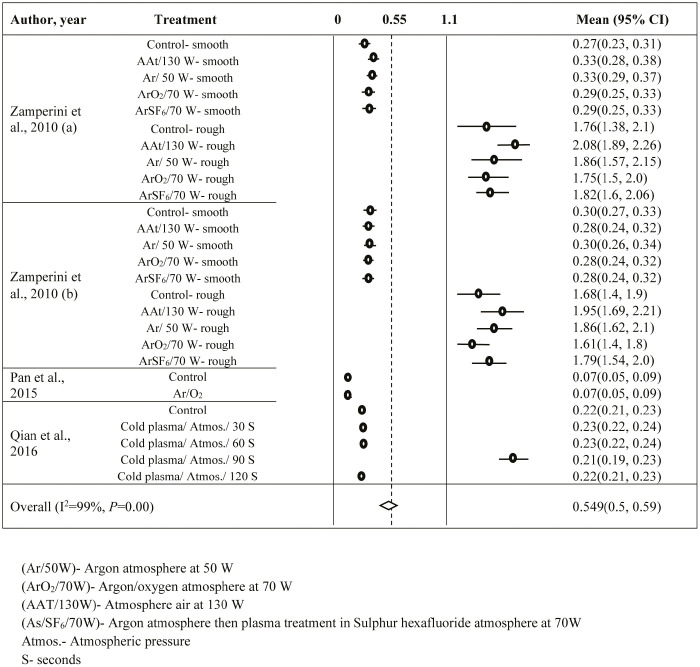
In the plasma coating vs. CA (Supplementary Appendix 2) and coating vs. Ra (Supplementary Appendix 3), a total of 38 and 91 observations were, respectively, included in the analysis. Due to significant heterogeneity (I2 >70%, P<0.001) in both the groups, random-effects model was used. The average CA and Ra were found to be 55.87° (95% CI: 50.68–61.06) and 0.552 µm (95% CI: 0.524–0.58), respectively. In plasma coating vs. CA, Begg’s and Eggers’ tests provided insignificant results; hence, the trim and fill method was not used. However, in coating vs. Ra, the trim and fill method provided insertions of 32 more observations to avoid publication bias.
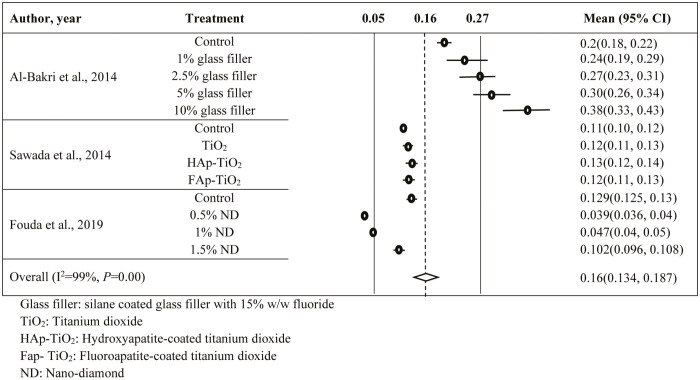
In plasma coating vs. Ra (Supplementary Appendix 4) and filler vs. Ra (Supplementary Appendix 5), 27 and 13 observations were included in the analysis. Both data sets reflected the presence of heterogeneity (I2 >70%, P<0.001) and hence the random-effects model was used for both. The estimated average Ra for plasma coating and filler addition were 0.549 µm (95% CI: 0.504–0.593) and 0.161 µm (95% CI: 0.134–0.187), respectively. Significant P-values for Eggers’ and Begg’s tests proved the presence of publication bias for both data sets. Hence, the trim and fill method suggested to insert 12 and 7 observations, respectively, to remove the publication bias.
Concerning these factors and their direct and indirect relations to C. albicans adhesion, almost all treatment modalities decreased C. albicans adhesion to modified DB in comparison to unmodified DB.
DISCUSSION
The results of this review revealed that the different treatment modalities (filler incorporation, surface coating, and chemical composition modification) affected the Ra, CA, and hydrophobicity of the DBR resulting in Candida adhesion modification, and therefore, the null hypothesis was rejected.
WHY COATING? AND WHAT IS THE OUTCOME?
In recent years, the DB surface has been modified with various coatings in an attempt to increase its hydrophilicity and to reduce C. albicans adhesion.[3,21] These coatings can be in the form of plasma-based treatment, photopolymerized coatings, and hydrophilic polymer coatings, among others. In plasma-based treatment, partial ionization of the gas is brought up by electrical discharge which creates an environment that contains reactive species such as electrons, ions, and free radicals. Plasma treatment helps clean debris, generates reactive groups on the surface, and makes the surface more attractive to specific cells depending on the treatment atmosphere.[16] The newly formed surface has higher SFE, improved wettability, and diminished CA, which reduces the adherence of C. albicans.[18,19,28,29]
Plasma treatment of PMMA in the presence of O2 gas improved the wettability of the surface even in the presence of salivary pellicle.[16] Similarly, plasma coating in argon, argon-oxygen, and atmospheric air resulted in lower CAs.[18] Conversely, TMS coating increased the hydrophobicity, lowered the wettability of the DB surface, and significantly reduced C. albicans adhesion.[30] Silane-SiO2 nanocomposite films were found to improve the surface, augment the physical properties of PMMA, and increase surface hydrophobicity which decreases C. albicans adhesion.[25]
Coating with TiO2 created smoother surfaces that are more resistant to wear and less porous, which prevent microorganisms from diffusing into the acrylic resin and colonizing on the surface.[33] UV irradiation of TiO2 activates oxidative species that produce irreversible damage to the cells.[33] Additionally, TiO2 coating creates a super-hydrophilic surface with “water sheathing” effect. The ability of TiO2 to improve surface wettability is essential to reduce or inhibit Candida attachment on DBR.[33]
Surface modification with photopolymerized coating of poly(acrylic acid) (PAA) or poly(itaconic acid) (PIA) followed by UV irradiation has been achieved. The coatings decreased the CA and increased the SFE, which may have resulted from changes in the surface polar groups after coating[21] and the acidic environment in the presence of (-OH) groups.[34] In a similar manner, surface modification by polymerization of 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer provided a statistical decrease in CA and C. albicans adhesion.[31]
Other hydrophilic coatings like 3-hydroxypropyl methacrylate (HPMA) and polymers containing sulfobetaine methacrylate (SBMA) were found to enhance the wettability of the DB surface and to reduce C. albicans adhesion as a result of limited hydrophobic interactions.[2,21,22] The CA and C. albicans adhesion were significantly reduced after coating the DBR with nanocoat or Optiglaze. This effect was brought up by changes in the carbon and oxygen content and different types of interactions.[36] Conversely, cyanoacrylate coating increased the C. albicans adhesion with no effect on CA.[36]
The main advantage of coating is that it allows surface alteration at a relatively low cost and preserves the properties of the original material.[18,19] The low viscosity can produce thin films (~50 nm[22] and <5 µm[32]) on the surface that do not interfere with the fit of the denture.[22,36] Coating PMMA with ceramic materials improves its resistance to abrasion[33] and protects the surface from attacks of different solutions.[22] Cold plasma treatment is performed at room temperature avoiding possible damage or warpage of acrylic resin with thermal treatments.[30] As most of the aforementioned coatings produce hydrophilic surfaces and improve the wettability of the PMMA, their application on the fitting surface of a denture could enhance the retention of the DBs by increasing affinity to saliva/liquid molecules that would create a denture seal.[28] In contrast, some of the coating materials require a certain preservation temperature and consumption within a short duration after preparation.[25] Also, the durability of different coatings needs further investigation.
ROUGHNESS (Ra), HYDROPHOBICITY, RESIN SURFACE CHEMISTRY, AND candida ADHESION
High Ra may enhance microbial retention because a rougher surface provides more area for microbial adhesion and promotes fungal adhesion and colonization.[7,8,37,38,39] Hahnel et al.[40] did not find a linear relationship between Ra and C. albicans adhesion. However, many other studies reported that greater C. albicans adhesion is associated with higher Ra.[7,38,39] Studies indicated that Ra was not altered following plasma treatment or film deposition process.[29] Thus, these opposing results suggest that the reduced C. albicans biofilm was due to the chemical modification of the PMMA surface represented by increased hydrophilicity and SFE that was promoted by film coating.[22] Hirasawa et al.[32] reported that roughness of different coated specimens was not the main determining factor in Candida reduction, rather it was surface hydrophilicity that played the major role.
REINFORCEMENT/Ra/CA
Incorporation of antifungal agents within DBR affected C. albicans adhesion and the development of DS.[41] The antimicrobial efficiency of the added AgNPs is associated with ingress of water molecules into the material and the outward movement of the silver ions to the aqueous solution.[20] Others suggested that the inhibitory effect was due to the greater antimicrobial effect of the smaller particles which provides more surface area in direct contact with the nanoparticles.[20] PMMA containing FAp-TiO2 exhibited strong photocatalytic activity following irradiation through the production of reactive oxygen species such as (-OH) and (H2O2) which inhibit C. albicans attachment.[26] This filler has clinical advantages especially for elderly patients through maintenance of proper denture hygiene.[26]
The addition of nano-diamonds showed an improvement of the specimen surface, which may contribute to the significant reduction in C. albicans adhesion. Regardless of the increase in Ra at a high concentration, a reduction in Candida adhesion was detected. Moreover; the inclusion of nano-diamonds within PMMA did not alter the CAs of the modified specimens in comparison to the unmodified specimen.[35] However, the mechanism of antifungal activity of ND was not described clearly and requires further investigations.
CHEMICAL COMPOSITION MODIFICATION
The addition of phosphate into DBR by monomer substitution was reported to improve the surface hydrophilicity.[28,42] The quantity of adherent C. albicans was associated with the wettability properties of the DB, emphasizing the role of acrylic resin chemistry on the initial attachment of C. albicans.[16]
CLINICAL SIGNIFICANCE
The literature reported that hydrophobicity and Ra of DBs influence the attachment and colonization of C. albicans. Therefore, to reduce Candida adhesion, the surface of the DB must be smooth, hydrophilic, and has no porosities.[5] Improving the hydrophilicity of the DB allows contact with more liquid molecules which helps in forming the seal that keeps the denture tight to air leakage.[28] Additionally, it has been reported that hydrophilic surfaces have fewer adherent C. albicans.[2] Therefore, increasing the surface hydrophilicity would hinder Candida attachment.[36]
Additionally, the intaglio surface provides the best environment for C. albicans adhesion, as it cannot be finished or polished to preserve its accuracy and fit. Therefore, surface coatings can be of great use in such situations in which the coating films are extremely thin and less likely to induce any misfit between the DB and oral tissues, affect the occlusion, or affect the texture of the resin.[24,25,43] The different coating modalities mentioned earlier can reduce C. albicans adhesion and biofilm formation.[25]
The limitations of this review could be attributed to a wide range of different treatments in each section, such as different coating materials, fillers, and minimal studies on chemical modification, which made the comparison more difficult as a result of the wide range of properties of each material and its effect on the studied properties.
CONCLUSION
Based on this review, it could be concluded that the hydrophobicity of DBRs and C. albicans adhesion were affected by the interrelated following factors: wettability (CA), SFE, and surface structure of DBR. Incorporation of antifungal agents or surface coating of DBR affected its hydrophobicity. Future studies evaluating the long-term biocompatibility and antifungal efficacy of different modifications are required to correlate between factors affecting the hydrophobicity and C. albicans adhesion.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Not applicable.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable.
PATIENT DECLARATION OF CONSENT
Not applicable.
DATA AVAILABILITY STATEMENT
Not applicable.
ACKNOWLEDGEMENTS
Not applicable.
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY APPENDIX 1: FOREST PLOT FOR COATING VS CONTACT ANGLE (°).
SUPPLEMENTARY APPENDIX 2: FOREST PLOT FOR PLASMA SURFACE TREATMENT VS CONTACT ANGLE (°)
SUPPLEMENTARY APPENDIX 3: FOREST PLOT FOR SURFACE COATING VS. SURFACE ROUGHNESS (MM)
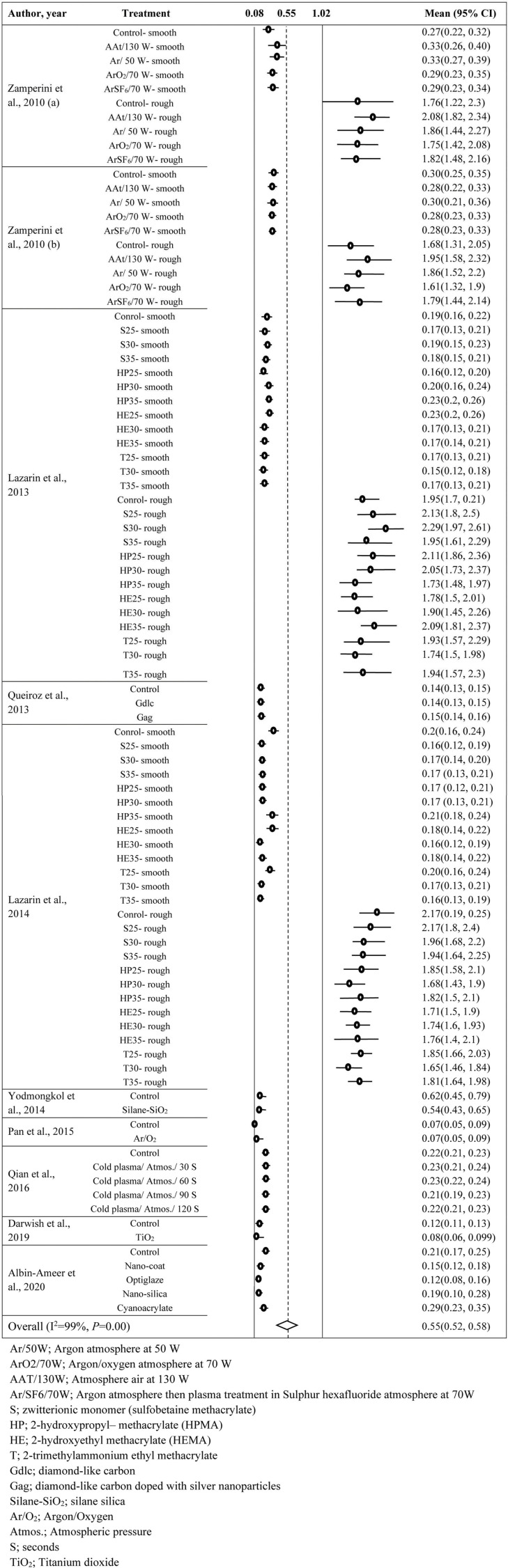
SUPPLEMENTARY APPENDIX 4: FOREST PLOT FOR PLASMA TREATMENT VS ROUGHNESS (MM)
SUPPLEMENTARY APPENDIX 5: FOREST PLOT FOR FILLERS VS ROUGHNESS (MM)
REFERENCES
- 1.Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 2.Yoshijima Y, Murakami K, Kayama S, Liu D, Hirota K, Ichikawa T, et al. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida. Mycoses. 2010;53:221–6. doi: 10.1111/j.1439-0507.2009.01694.x. [DOI] [PubMed] [Google Scholar]
- 3.Arai T, Ueda T, Sugiyama T, Sakurai K. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. J Oral Rehabil. 2009;36:902–8. doi: 10.1111/j.1365-2842.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshizaki T, Akiba N, Inokoshi M, Shimada M, Minakuchi S. Hydrophilic nano-silica coating agents with platinum and diamond nanoparticles for denture base materials. Dent Mater J. 2017;36:333–9. doi: 10.4012/dmj.2016-243. [DOI] [PubMed] [Google Scholar]
- 5.Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–60. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 6.von Fraunhofer JA, Loewy ZG. Factors involved in microbial colonization of oral prostheses. Gen Dent. 2009;57:136–43. [PubMed] [Google Scholar]
- 7.Pereira-Cenci T, Del Bel Cury AA, Crielaard W, Ten Cate JM. Development of Candida-associated denture stomatitis: New insights. J Appl Oral Sci. 2008;16:86–94. doi: 10.1590/S1678-77572008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radford DR, Sweet SP, Challacombe SJ, Walter JD. Adherence of Candida albicans to denture-base materials with different surface finishes. J Dent. 1998;26:577–83. doi: 10.1016/s0300-5712(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 9.Choi SY, Habimana O, Flood P, Reynaud EG, Rodriguez BJ, Zhang N, et al. Material- and feature-dependent effects on cell adhesion to micro injection moulded medical polymers. Colloids Surf B Biointerf. 2016;145:46–54. doi: 10.1016/j.colsurfb.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Gad MM, Fouda SM, Al-Harbi FA, Näpänkangas R, Raustia A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int J Nanomed. 2017;12:3801–12. doi: 10.2147/IJN.S130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangal U, Kim JY, Seo JY, Kwon JS, Choi SH. Novel poly(methyl methacrylate) containing nanodiamond to improve the mechanical properties and fungal resistance. Materials (Basel) 2019;21:12. doi: 10.3390/ma12203438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astudillo-Rubio D, Delgado-Gaete A, Bellot-Arcís C, Montiel-Company JM, Pascual-Moscardó A, Almerich-Silla JM. Mechanical properties of provisional dental materials: A systematic review and meta-analysis. PLoS One. 2018;13:0196264. doi: 10.1371/journal.pone.0193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goujat A, Abouelleil H, Colon P, Jeannin C, Pradelle N, Seux D, et al. Marginal and internal fit of CAD-CAM inlay/onlay restorations: A systematic review of in vitro studies. J Prosthet Dent. 2019;121:590–597.e3. doi: 10.1016/j.prosdent.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Faggion CM., Jr Guidelines for reporting pre-clinical in vitro studies on dental materials. J Evid Based Dent Pract. 2012;12:182–9. doi: 10.1016/j.jebdp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Yildirim MS, Hasanreisoǧlu U, Hasirci N, Sultan N. Adherence of Candida albicans to glow-discharge modified acrylic denture base polymers. J Oral Rehab. 2005;32:518–525. doi: 10.1111/j.1365-2842.2005.01454.x. [DOI] [PubMed] [Google Scholar]
- 17.Nevzatoğlu EU, Ozcan M, Kulak-Ozkan Y, Kadir T. Adherence of Candida albicans to denture base acrylics and silicone-based resilient liner materials with different surface finishes. Clin Oral Investig. 2007;11:231–6. doi: 10.1007/s00784-007-0106-3. [DOI] [PubMed] [Google Scholar]
- 18.Zamperini CA, Machado AL, Vergani CE, Pavarina AC, Giampaolo ET, da Cruz NC. Adherence in vitro of Candida albicans to plasma treated acrylic resin. Effect of plasma parameters, surface roughness and salivary pellicle. Arch Oral Biol. 2010;55:763–70. doi: 10.1016/j.archoralbio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Zamperini CA, Machado AL, Vergani CE, Pavarina AC, Rangel EC, Cruz NC. Evaluation of fungal adherence to plasma-modified polymethylmethacrylate. Mycoses. 2011;54:e344–51. doi: 10.1111/j.1439-0507.2010.01921.x. [DOI] [PubMed] [Google Scholar]
- 20.Wady AF, Machado AL, Zucolotto V, Zamperini CA, Berni E, Vergani CE. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J Appl Microbiol. 2012;112:1163–72. doi: 10.1111/j.1365-2672.2012.05293.x. [DOI] [PubMed] [Google Scholar]
- 21.Lazarin AA, Machado AL, Zamperini CA, Wady AF, Spolidorio DM, Vergani CE. Effect of experimental photopolymerized coatings on the hydrophobicity of a denture base acrylic resin and on Candida albicans adhesion. Arch Oral Biol. 2013;58:1–9. doi: 10.1016/j.archoralbio.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Queiroz JR, Fissmer SF, Koga-Ito CY, Salvia AC, Massi M, Sobrinho AS, et al. Effect of diamond-like carbon thin film coated acrylic resin on Candida albicans biofilm formation. J Prosthodont. 2013;22:451–5. doi: 10.1111/jopr.12029. [DOI] [PubMed] [Google Scholar]
- 23.Al-Bakri IA, Harty D, Al-Omari WM, Swain MV, Chrzanowski W, Ellakwa A. Surface characteristics and microbial adherence ability of modified polymethylmethacrylate by fluoridated glass fillers. Aust Dent J. 2014;59:482–9. doi: 10.1111/adj.12218. [DOI] [PubMed] [Google Scholar]
- 24.Lazarin AA, Zamperini CA, Vergani CE, Wady AF, Giampaolo ET, Machado AL. Candida albicans adherence to an acrylic resin modified by experimental photopolymerised coatings: An in vitro study. Gerodontology. 2014;31:25–33. doi: 10.1111/j.1741-2358.2012.00688.x. [DOI] [PubMed] [Google Scholar]
- 25.Yodmongkol S, Chantarachindawong R, Thaweboon S, Thaweboon B, Amornsakchai T, Srikhirin T. The effects of silane-SiO2 nanocomposite films on Candida albicans adhesion and the surface and physical properties of acrylic resin denture base material. J Prosthet Dent. 2014;112:1530–8. doi: 10.1016/j.prosdent.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Sawada T, Sawada T, Kumasaka T, Hamada N, Shibata T, Nonami T, et al. Self-cleaning effects of acrylic resin containing fluoridated apatite-coated titanium dioxide. Gerodontology. 2014;31:68–75. doi: 10.1111/ger.12052. [DOI] [PubMed] [Google Scholar]
- 27.Compagnoni MA, Pero AC, Ramos SM, Marra J, Paleari AG, Rodriguez LS. Antimicrobial activity and surface properties of an acrylic resin containing a biocide polymer. Gerodontology. 2014;31:220–6. doi: 10.1111/ger.12031. [DOI] [PubMed] [Google Scholar]
- 28.Pan H, Wang G, Pan J, Ye G, Sun K, Zhang J, et al. Cold plasma-induced surface modification of heat-polymerized acrylic resin and prevention of early adherence of Candida albicans. Dent Mater J. 2015;34:529–36. doi: 10.4012/dmj.2015-035. [DOI] [PubMed] [Google Scholar]
- 29.Qian K, Pan H, Li Y, Wang G, Zhang J, Pan J. Time-related surface modification of denture base acrylic resin treated by atmospheric pressure cold plasma. Dent Mater J. 2016;35:97–103. doi: 10.4012/dmj.2015-162. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Xu C, Hong L, Garcia-Godoy F, Hottel T, Babu J, et al. Effects of trimethylsilane plasma coating on the hydrophobicity of denture base resin and adhesion of Candida albicans on resin surfaces. J Prosthet Dent. 2017;118:765–70. doi: 10.1016/j.prosdent.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Türkcan İ, Nalbant AD, Bat E, Akca G. Examination of 2-methacryloyloxyethyl phosphorylcholine polymer coated acrylic resin denture base material: Surface characteristics and Candida albicans adhesion. J Mater Sci Mater Med. 2018; 29:107. doi: 10.1007/s10856-018-6116-7. [DOI] [PubMed] [Google Scholar]
- 32.Hirasawa M, Tsutsumi-Arai C, Takakusaki K, Oya T, Fueki K, Wakabayashi N. Superhydrophilic co-polymer coatings on denture surfaces reduce Candida albicans adhesion—An in vitro study. Arch Oral Biol. 2018;87:143–50. doi: 10.1016/j.archoralbio.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Darwish G, Huang S, Knoernschild K, Sukotjo C, Campbell S, Bishal AK, et al. Improving polymethyl methacrylate resin using a novel titanium dioxide coating. J Prosthodont. 2019;28:1011–7. doi: 10.1111/jopr.13032. [DOI] [PubMed] [Google Scholar]
- 34.Acosta LD, Pérez-Camacho O, Acosta R, Escobar DM, Gallardo CA, Sánchez-Vargas LO. Reduction of Candida albicans biofilm formation by coating polymethyl methacrylate denture bases with a photopolymerized film. J Prosthet Dent. [DOI] [PubMed]
- 35.Fouda SM, Gad MM, Ellakany P, Al-Thobity AM, Al-Harbi FA, Virtanen JI, et al. The effect of nanodiamonds on Candida albicans adhesion and surface characteristics of PMMA denture base material—An in vitro study. J Appl Oral Sci. 2019;27:e20180779. doi: 10.1590/1678-7757-2018-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AlBin-Ameer MA, Alsrheed MY, Aldukhi IA, Matin A, Khan SQ, Abualsaud R, et al. Effect of protective coating on surface properties and Candida albicans adhesion to denture base materials. J Prosthodont. 2020;29:80–6. doi: 10.1111/jopr.13118. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi M, Yamamoto K, Wakabayashi M, Kawano J. In vitro adherence of microorganisms to denture base resin with different surface texture. Dent Mater J. 1990;9:19–24. doi: 10.4012/dmj.9.19. [DOI] [PubMed] [Google Scholar]
- 38.Pereira-Cenci T, Pereira T, Cury AA, Cenci MS, Rodrigues-Garcia RC. In vitro candida colonization on acrylic resins and denture liners: Influence of surface free energy, roughness, saliva, and adhering bacteria. Int J Prosthodont. 2007;20:308–10. [PubMed] [Google Scholar]
- 39.Verran J, Maryan CJ. Retention of Candida albicans on acrylic resin and silicone of different surface topography. J Prosthet Dent. 1997;77:535–9. doi: 10.1016/s0022-3913(97)70148-3. [DOI] [PubMed] [Google Scholar]
- 40.Hahnel S, Rosentritt M, Handel G, Bürgers R. In vitro evaluation of artificial ageing on surface properties and early Candida albicans adhesion to prosthetic resins. J Mater Sci Mater Med. 2009;20:249–55. doi: 10.1007/s10856-008-3570-7. [DOI] [PubMed] [Google Scholar]
- 41.Alzayyat ST, Almutiri GA, Aljandan JK, Algarzai RM, Khan SQ, Akhtar S, et al. Antifungal efficacy and physical properties of poly(methylmethacrylate) denture base material reinforced with SiO2 nanoparticles. J Prosthodont. 2021;30:500–8. doi: 10.1111/jopr.13271. [DOI] [PubMed] [Google Scholar]
- 42.Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, et al. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–8. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali AA, Alharbi FA, Suresh CS. Effectiveness of coating acrylic resin dentures on preventing Candida adhesion. J Prosthodont. 2013;22:445–50. doi: 10.1111/jopr.12046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.