Abstract
Entering the third year into the pandemic, overwhelming evidence demonstrates that Coronavirus disease 2019 (COVID-19) infection is a systemic illness, often with involvement of the central nervous system. Multiple mechanisms may underlie the development of neurologic manifestations of illness, including hypoxia, systemic illness, hypercoagulability, endothelial dysfunction, general critical illness, inflammatory response, and neurotropism of the severe acute respiratory syndrome coronavirus 2 (SARS-Co-V2) virus. COVID-19 infection is associated with neurologic involvement in all stages; acute infection, subacute/post-infection, and growing evidence also suggests during a chronic phase, the post-acute sequalae of COVID-19 (PASC). With over 20,000 published articles on COVID and the brain at the time of writing, it is virtually impossible to present an unbiased comprehensive review of how SARS-Co-V2 impacts the nervous system. In this review, we will present an overview of common neurologic manifestations, in particular focusing on the cerebrovascular complications, and proposed pathophysiology.
Keywords: COVID-19, Neurologic complications, Stroke, SARS-CoV-2, CNS Vasculitis
Neurologic manifestations
Common neurologic signs and symptoms, independent of disease severity
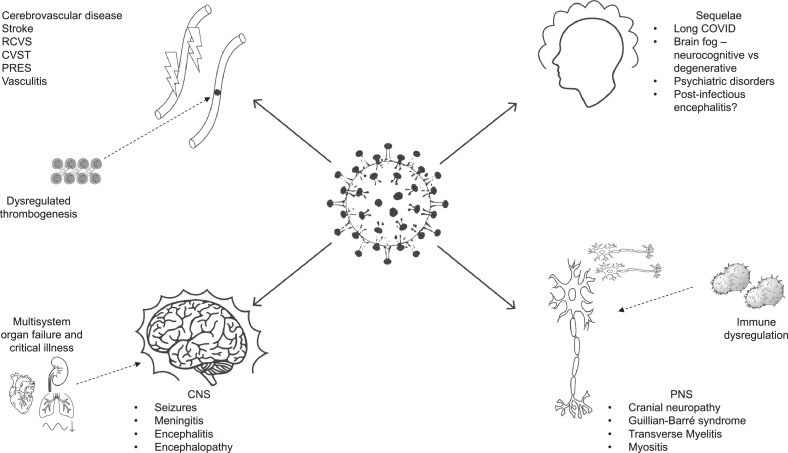
COVID-19 can present with and include a myriad of neurologic signs and symptoms throughout its course, regardless of severity of illness (Fig. 1 ).
Fig. 1.
This schematic demonstrates the spectrum of neurologic complications which have been associated with SARS-CoV-2 infections. The pathologic manifestations are wide ranging from vascular manifestations (hemorrhagic and ischemic strokes, PRES, CVST), generalized CNS disorders (seizures, meningitis, encephalitis), to peripheral neuropathies (GBS, cranial neuropathies) and post-infectious sequelae (long COVID). The proposed mechanisms behind these injuries include immune dysregulation, aberrant thrombogenesis and multi-organ failure in the setting of critical illness. Please refer to table 1 for a more comprehensive list.
Among the most well-discussed symptoms are anosmia and ageusia. While the prevalence varies, anosmia and ageusia has been reported in up to 53% and 44% of patients in one study [1], similarly, the incidence of new onset anosmia is similarly between 34 – 68% with a seemingly female predominance [2]. Anosmia and ageusia are often the initial, and at times the sole, symptoms of the disease course. Unlike in other respiratory diseases, anosmia in COVID-19 occurs in the absence of rhinitis or nasal swelling [3,4,5]. Some studies [6] suggest that the presence of anosmia/dysgeusia is associated with a milder course of illness [7,8] though skepticism exists that perhaps this is due to ascertainment bias in patients with more severe disease unable to endorse these symptoms due to encephalopathy or respiratory failure. Preliminary reports suggest that subsequent SARS-CoV-2 strains (such as omicron) seem to be associated with approximately half the rates of anosmia/dysgeusia [9]. Whether this is explained by the high vaccination rates in the population studied remains to be better understood.
Headache is another common initial or presenting feature and appears to be similar to that which accompanies many viral syndromes. Headaches in patients with COVID-19 are more likely to last for greater than seventy-two hours, be resistant to analgesia, and occur in men [10]. Unfortunately, in rare cases, headache is not a benign symptom and may be caused by COVID-19 associated cerebrovascular disease or meningoencephalitis (see below).
In a study observing hospitalized patients, myalgias were the most commonly reported nonspecific symptom. It appeared significantly more often in non-severe versus severe COVID-19 infection. Furthermore, myalgias, along with other nonspecific symptoms such as headache and dizziness, appeared early in the disease course at 3.8 days from onset on average [11].
Neurologic manifestations in hospitalized patients
Neurologic involvement in patients with illness requiring hospitalization is nearly ubiquitous. In the GCS-NeuroCOVID study, a large multicenter international study of three separate cohorts enrolling over 3500 patients, 80% reported any neurologic manifestation, with the most common being encephalopathy [12]. Other serious neurologic manifestations of interest appear to be rarer including stroke, seizure, meningoencephalitis, and Guillain-Barre syndrome (GBS). Most studies demonstrate that neurologic involvement in COVID-19 occurs in older patients, often with a prior history of a neurologic disorder, and often in patients with vascular risk factors [3,12]. Neurologic involvement appears to be uniformly associated with worse outcomes including ICU admission, mortality, and increased disability in survivors. Though some studies have shown that neurologic syndromes are not limited to the critically ill [6,13].
Encephalopathy
Encephalopathy is a broad term referring to alterations in generalized brain function, encompassing the spectrum of agitation to confusion and somnolence, synonymous with hyper and hypoactive delirium. Encephalopathy is the most common severe manifestation of COVID-19 infection, occurring with increasing frequency with severity of illness. An emergency department study reported an incidence of 28% [14], present in up to 55% of critically ill [15], and 49% in GCS-NeuroCOVID study [12].
Encephalopathy in COVID-19 infection is likely multifactorial as a consequence of metabolic derangement, hypoxia, medications, ICU delirium but evidence suggests an important role of vascular disease especially in severe cases [16]. Several imaging studies suggest that cerebrovascular abnormalities contribute heavily to encephalopathy with infarction often seen on magnetic resonance imaging (MRI) even when not clinically apparent as a focal deficit [17,18,19]. Encephalopathy has been associated with poorer hospital outcomes and may increase risk for morbidity following discharge in survivors. Encephalopathy during hospitalization was associated with increased risk of stroke in the 6 months following discharge in a large electronic health records based study [20].
Cerebrovascular disease
The spectrum of cerebrovascular disease has been reported in the context of COVID-19 acute infection [21]. The reported incidence ranges from 0.5 – 5% of hospitalized cases [22,23,24,25,26,27,28] depending upon severity of illness in the cohort studied and ischemic strokes predominate. The majority of studies [24,25], but not all [29,30], report strokes predominantly in patients with critical illness. A meta-analysis of the existing literature concludes that stroke in patients with COVID-19 is associated with older age, co-morbidities and critical illness [31]. Strokes which occur in the context of COVID-19 tend to be more severe which corresponds to the predominant etiology being large vessel occlusions and cardioembolism [32,33]. Whether strokes in COVID-19 tend to be more severe or whether strokes tend to occur more frequently in patients who are more severely ill or both, is not clear. Regardless, stroke in the context of COVID-19 has been uniformly associated with poorer outcomes. In one meta-analysis with over 100,000 patients, the odds of in-hospital mortality was 5 times that of uninfected controls [32].
Ischemic stroke
Acute ischemic strokes (AIS) that occur in the context of COVID-19 tend to be more severe with the predominant etiology being large vessel occlusions and cardioembolism [32,33]. In a large multinational registry sponsored by the Society of Vascular and Interventional Neurology, 42.6% of stroke etiologies were determined to be cryptogenic [34]. In this study, patients with cryptogenic strokes had higher levels of leukocytosis and a higher national institutes of health stroke scale (NIHSS) at baseline, and using a multivariable regression model, the authors concluded that acute stroke from a cryptogenic source was significantly associated with in-hospital mortality compared to stroke from any other mechanism [34].
Most patients with cerebrovascular complications have traditional underlying risk factors [22,23,24,26,32], but a sizeable minority do not [32, ]suggesting a potentially important role of virally mediated hypercoagulable state, cytokine storm, cardiac effects, or cerebrovascular arteriopathy [35,36,37,38,39,40].
The duration of increased risk of AIS following SARS-CoV-2 infection is uncertain. In one Swedish controlled case series, incident rate ratio was 6.18 (4.06 – 9.42) in the week following infection and dropped to 2.14 (1.36 – 3.38) by the 3-4th week after infection [41]. However, a large electronic medical records based study showed that increased risk of stroke was seen in the subsequent 6 months following infection, with those suffering encephalopathy during hospitalization at highest risk [20].
With regards to treatment, studies do not support changing the approach to acute stroke management in COVID-19 infected patients. In observational studies of people with an active COVID-19 infection and acute ischemic stroke, administration of tissue plasminogen activator (TPA) showed efficacy and did not have any association with worse morbidity or mortality compared to patients with acute ischemic stroke without COVID-19 infection [29,42]. Thrombectomy and intravenous thrombolysis have both shown benefit and no substantial increased risk [43,44,45]. No robust studies have shown the benefit of anticoagulation over antiplatelet agents specifically for primary or secondary prevention of stroke in COVID-19.
A thorough investigation into traditional mechanisms of stroke is warranted. Additional investigations into hypercoagulable state including lupus anticoagulant and antiphospholipid antibodies is controversial [26,46,47]. In atypical cases where vasculopathy is suspected, investigation using vessel wall imaging may be helpful. Endotheliitis, identified both radiographically and in neuropathology, may be a cause for cerebrovascular disease in some cases. There may be a role for steroids in this circumstance [40,48].
Intracerebral hemorrhage
Intracerebral hemorrhage has been less frequently reported in COVID-19 [49,50,51]. Therapeutic anticoagulation appears to be a significant risk factor, in one study increasing the risk for hemorrhage 5-fold [51]. The use of extra corporeal membrane oxygenation (ECMO) for treatment of refractory acute respiratory distress syndrome (ARDS) has also been associated with intracerebral hemorrhage. In the ELSO registry, ischemic stroke occurred in 1% of patients and hemorrhagic stroke in 7% of all patients on ECMO [52]. Hemorrhagic stroke is thought to be induced by a few mechanisms as well. First, as ACE2 receptor expression is downregulated and angiotensin 2 expression is increased, endothelial dysfunction and blood pressure dysregulation ensue. Further, the cytokine storm induced by SARS-CoV-2 infection results in breakdown of the BBB; taken together with elevated blood pressure, hemorrhagic stroke risk is increased [53]. It should be noted that while both hemorrhagic and ischemic strokes occur in COVID-19, ischemic strokes are far more common. A notable exception are critically ill patients on therapeutic anticoagulation and patients on ECMO [54,55].
Cerebral venous sinus thrombosis
Systemic venous thromboembolic complications, including deep vein thromboses (DVT), are frequently observed in hospitalized COVID-19 patients. Cerebral venous sinus thrombosis (CVST) appear to be less common than DVT but have also been reported in those infected with SARS-CoV-2 [56,57]. Notably, CVST have been noted often times in the weeks that follow acute infection and often present with isolated headache and without focal neurologic deficit. Unlike most cerebrovascular complications, CVST occur more often in women [58]. CVST can often be associated with seizure and can lead to both ischemic and hemorrhagic strokes. Given the frequency of primary headache with COVID-19 infection and the potentially devastating complications of untreated CVST, this is an important diagnosis to keep in mind.
Cerebrovascular vasculopathies
Given vascular endothelial expression of ACE-2 receptors, it is not surprising that uncommon cerebrovascular disorders have been reported with COVID-19 infection. The posterior reversible encephalopathy syndrome (PRES), which is most commonly associated with malignant hypertension or chemotherapy agents, has been reported in several cases of COVID, in some, associated with much less dramatic alterations in blood pressure and with immunologic treatment of COVID-19 [3,21]. The reversible cerebral vasoconstriction syndrome (RCVS) is also speculated to be a disorder of endothelial function and has been associated with SARS-CoV-2 infections [59,60]. Cerebrovascular and systemic endotheliitis has been observed, in some cases successfully treated with steroids [48], as well as vasculitis [61].
SARS-CoV-2 vaccine related Stroke
Notably, cerebrovascular complications have been noted in patients receiving the modified adenovirus based SARS-CoV-2 vaccine. Initially this was reported as cases of CVST, occurring solely in young woman. The pathophysiology is similar to heparin-inducted thrombocytopenia with high titer antibodies to platelet factor 4 which activates platelets leading to thrombosis. As such, the syndrome has been name vaccine-inducated immune thrombocytopenia and thrombosis (VITT) [62]. Since the initial reports, VITT has also been associated with arterial and venous thrombosis [63]. Though the incidence in those less than 50 years of age is low (estimated at 1:50,000), given the wide availability of mRNA based vaccines, adenovirus based immunizations are no longer preferred. Interim surveillance data of 6.2 million persons receiving 11.8 million doses of mRNA vaccines have not shown an increase risk of AIS or CVT compared to controls [64].
Seizure
Seizures appear to be a rare complication of COVID-19 infection, reported in less than 2% of hospitalized cohorts [12,65,66]. A similar number is reported by an Iranian study which showed that of the approximately 6000 patients who tested positive for COVID-19 in the emergency department, about 1% had solely seizure as their presenting symptom [67]. Most seizures are described in hospitalized patients in the context of metabolic derangement, notably uremia, and/or critical illness, and many patients have a history of epilepsy or prior neurologic condition which may predispose to triggering seizures. However, new onset seizures have been reported in the context of COVID-19 infection [68]. In a case series of such cases with mild COVID-19 infection, one patient had electroencephalography (EEG) showing abnormalities including bilateral dysfunction without epileptiform discharges three weeks after initial presentation [69]. Electrographic seizures have been seen in patients with COVID-19 who are in ICUs with altered consciousness and put on long-term EEG monitoring [3].
Meningitis/encephalitis
Meningitis and encephalitis have been infrequently reported in conjunction with COVID-19 disease, only 0.5% in the GCS-Neuro COVID study [12]. The first reported case of meningitis/encephalitis occurred in Japan, in which a 24-year-old man had MRI hyperintensities of the right lateral ventricle wall and right mesial temporal lobe. His cerebrospinal fluid (CSF) study was positive for SARS-CoV-2 RNA, suggesting potential direct infection versus a para-infectious immune-mediated process [70]. Meningitis can also be the initial presenting symptom of COVID-19 as seen in emerging case reports [71,72]. The ENCOVID study is the largest cohort of patients with encephalitis in the context of COVID, representing 25 patients within Northern Italy [73]. The most commonly seen presentation was that of delirium followed by stroke like symptoms of aphasia/dysarthria and seizures. Intriguingly, most patients in their study only had moderately severe respiratory illness and mortality was 16% [73].
In some cases of meningitis/encephalitis with COVID-19, CSF has shown a lymphocytic pleocytosis typical for viral meningoencephalitis but SARS-CoV-2 RNA was not found [3,74].
Movement disorders
Movement disorders have been occasionally reported in hospitalized patients. These have mostly involved myoclonus [75]. In one case series, myoclonus was often heralded by hypoxia and was seen in patients in the peri-intubation setting and was short lived after recovery [76]. The significance of myoclonus with regards to treatment and prognostication remains unknown given its rare nature. Taken together with worsening myoclonus with movement or tactile stimuli and exaggerated startle response, proposed mechanisms of disease include post- or para-infection immune mediated mechanisms or SARS-CoV-2 virus invasion of olfactory bulb and brainstem [75].
Other movement disorders have been rarely described. Parkinsonism showed no significant association with COVID-19 infections in cohort studies where patients were matched to influenza infections or other respiratory tract infections [20] however, late parkinsonism as seen with the encephalitic lethargica after the Spanish influenza epidemic remains a possibility. Rare case reports of ataxias and tremors have also been associated with COVID-19 infections.
Post/para-infectious complications
GBS in the setting of COVID-19 has been seen as a post-infectious, likely immune mediated, phenomenon, present mostly as its classic form with sensorimotor dysfunction but also as rare variants such as Miller Fisher syndrome, in which oculomotor insults and ataxia are more common. CSF studies in these patients have been negative for SARS-CoV-2 RNA [77]. Within the spectrum of inflammatory neuropathies, COVID-19 infections have also been hypothesized to provoke cranial nerve problems, most prominently affecting the olfactory nerve causing anosmia. Other prominent associations of COVID-19 infections include facial nerve palsy [78]. In one large case series, cranial nerve deficits were found in over 40% of the 300 or so patients examined, with many patients demonstrating multi-cranial nerve impairments along the spectrum of polycranialis neuritis [79]. COVID-19 has also shown rare associations with acute disseminated encephalomyelitis (ADEM). In a case series compiled by Wang and colleagues, ADEM associated with COVID-19 infections seemed to have a longer incubation period after viral infection and affected more adult populations compared to typical ADEM which affects children [80]. Furthermore, these patients had poorer prognoses and higher mortality compared to patients with typical ADEM. Transverse myelitis, a disease process causing sensory, motor and autonomic dysfunction due to spinal cord lesions has also been seen, albeit rarely with COVID-19 infections. Transverse myelitis has been reported in a case series of 43 patients with a post-infectious latency period of up to 6 weeks in some cases [81] with a mechanism that is proposed to be an immune cross-reaction to viral antigens.
PASC and long-term neurologic complications
The trajectory of recovery, including neurologic recovery, is a hot area for research. A particular concern in PASC is “long covid” or long-haul COVID, characterized by severe fatigue, headache, subjective cognitive impairment (“brain fog”), mood disorders, sleep disorders that persist greater than 4 weeks after acute illness [82,83]. This syndrome appears to be independent of acute illness severity and may occur in patients with and without laboratory confirmed SARS-CoV-2 infection. Many consider long haul COVID as a similar entity to the myalgic encephalomyelitis or chronic fatigue syndrome frequently associated with Epstein Barr virus (EBV), and others have found evidence of autonomic dysfunction reminiscent of postural orthostatic tachycardia syndrome (POTS) [84,85,86]. A prospective study by Carfi et al. found that of the patients discharged from the hospital after recovery from COVID-19, about 87% reported the persistence of at least one symptom, and while the most common persistent symptom was dyspnea, neurologic symptoms including anosmia, headaches and dysgeusia were also prominently noted [87]. Though many long COVID patients have undergone extensive diagnostic evaluation, the findings are often negative or nonspecific. In one study by Graham et al., in a post COVID clinic at Northwestern, long COVID appears to be more common in women with a ratio of 2.3 :1, which is similar to other autoimmune illnesses such as multiple sclerosis [88]. In this cohort there was a high prevalence of other autoimmune diseases and high ANA titers all suggesting that there may be an autoimmune etiology, but also some overlay with pre-morbid psychiatric conditions. In this same cohort, 42% of patients had pre-morbid depression or anxiety, compared with 21.4% in the US with a mood disorder.
Cognitive dysfunction post-COVID may be multifactorial. Not surprisingly, patients who were critically ill with COVID-19 often had prolonged hospitalizations and residual cognitive impairment following recovery is common [89]. The brain fog, described in long-COVID, seen at a rate of 81% in a specialized post-COVID clinic. Objectively, patients were found to have significantly worse attention and working memory function than expected based on demographic profile, which in turn negatively impacted patients’ quality of life [88]. For those admitted in intensive care units, patients who were under intensive care for significant periods of time with COVID-19 have been known to have residual cognitive impairment following recovery [89]. This long COVID “brain-fog” has been noted on fluorodeoxyglucose-positron emission tomography scans as a hypometabolic state in the cingulate cortex [90]. With regards to non-hospitalized patients, a prospective study found cognitive impairment in patients as assessed by the NIH toolbox when compared to non-infected age matched peers, 5 months after initial symptom onset [88]. Mechanistically, persistent and widespread neurologic viral infection does not seem to be the case when trying to understand these symptoms. Transcriptome analysis of parenchymal and barrier cells such as the glia and choroid in autopsy samples suggests a substantial upregulation of inflammatory genes particularly the interferon and complement system in the brains of patients infected with COVID-19, which has been previously linked to cognitive dysfunction seen with aging [91,92]. The same study also found peripheral T cell infiltration of the brain parenchyma and microglial subpopulations that share features with pathologic cell states in neurodegenerative diseases [92]. Further evidence of immune system dysregulation during acute COVID-19 infections comes from B cell profiling of critically ill patients. Studies show extrafollicular B cell activation and proliferation as seen in diseases like lupus and the generation of antiphospholipid antibodies that potentiate thromboses [93,94]. The persistence or memory of these antibodies in long COVID-19 remains to be explored; however, pathologic changes that may precede recovery are hypothetically attributable to these autoimmune type processes
Mechanisms of neurologic injury
Given the various neurological manifestations of COVID-19, it is not surprising that the SARS-CoV-2 virus has multiple processes by which it can cause a neurologic insult (Fig. 1 and Table 1 ).
Table 1.
Mechanisms of Cerebrovascular Disease in COVID-19
| Exacerbation of underlying risk factors |
| Viral mediated hematologic derangement/hypercoagulable state (+/- PFO) |
| Effects on the Renin-Angiotensin-Aldosterone System |
| Hyperinflammatory condition (Cytokine storm) |
| Myocarditis, Stress cardiomyopathy |
| Atrial Fibrillation |
| Cerebrovascular endotheliitis |
| General critical illness, hypoxia, hypotension |
| Complication of COVID treatments (medications, ECMO) |
| Cervical artery dissections |
| * Vaccine induced thrombotic thrombocytopenia |
Viral pathogenesis
The SARS-CoV-2 virus enters cells by binding its viral spike protein on the angiotensin converting enzyme (ACE)-2 receptor on mammalian host cells and undergoes spike protein priming via serine protease transmembrane protease serine 2 (TMPRSS2) [95]. Both ACE2 and TMPRSS2 have been found on ciliated epithelial cells and oligodendrocytes, the latter of which could provide a means to central nervous system (CNS) penetration [96]. While the ACE2 receptor is found on epithelial cells of oral, nasal, and respiratory tract mucosa, it also exists at various regions of the brain, including motor cortex, ventricles, the olfactory system, hippocampi, substantia nigra, and various areas of the brainstem involved in autonomic function [4,5]. As SARS-CoV-2 enters cells, it downregulates ACE2 and thus disrupts a balance between ACE1 and ACE2; this increased ACE signal overall results in excessive vasoconstriction and disrupted cerebral autoregulation [96] which may contribute to cerebrovascular complications.
Viral neurotropism
Neuroinvasion of the SARS-CoV-2 virus can occur through various potential mechanisms, including transsynaptic transfer, olfactory nerve entry, vascular endothelial infection, and leukocyte migration across the blood brain barrier (BBB). Using transsynaptic transfer, the virus has been proposed to move in a retrograde manner exocytosis and endocytosis once across the synaptic cleft, and then via fast axonal transport along microtubules to neuronal cell bodies. The olfactory epithelium, another area of invasion, putatively allows spread through the cribriform place and into the olfactory nerve then olfactory bulb within the CNS. Endothelial cells, which express ACE2 throughout the body, including within the brain, can become infected with SARS-CoV-2, which can travel across capillaries and into glial cells within the CNS within vesicles. Furthermore, using a Trojan horse mechanism, the virus can infect T lymphocytes which then can cross the BBB and infect the CNS [5,96]. CSF studies have been particularly difficult to rely on, given that the full mechanism of how COVID-19 crosses the blood brain barrier remains to be elucidated. That coupled to the lack of robust sensitivity of CSF fluid testing for SARS-CoV-2, particularly in the initial stages of the pandemic has made it difficult to rely on the reliability of the results of the lumbar puncture, especially when negative.
Mechanism of cerebrovascular injury
Though the majority of patients with stroke during COVID-19 likely have exacerbation or provocation of underlying cardiovascular and cerebrovascular disease, there are many proposed mechanisms by which cerebrovascular insult can occur (Fig. 1,Table 1).
Etiologies behind ischemic and hemorrhagic stroke in the setting of COVID-19 infection have been under investigation. In a European case series of six consecutive patients with acute ischemic stroke in the form of large vessel occlusion, five of six patients had a positive lupus anticoagulant; of note, one of the patients in this group had medium titer IgM anticardiolipin and low titer IgG and IgM anti-β2-glycoprotein-1 antibodies [46]. In another European study, Lupus anticoagulant assays were positive in 91% of patients with SARS-CoV-2 infection; all positive blood specimens had accompanying prolonged activated partial thromboplastin time (aPTT). The percentage of lupus anticoagulant positive studies in this group was significantly higher than a control group [97].
As the SARS-CoV-2 virus invades, it enforces a pro-inflammatory effect by reducing ACE2 expression, which increases angiotensin 2, activating macrophages that release pro-inflammatory cytokines, which in turn activates tissue factor. Tissue factor gets released from endothelial cells and macrophages, and it works by activating the extrinsic coagulation pathway, which leads to fibrin deposition and blood clotting [53]. This leads to the distinctive feature of COVID-19 infection with diffuse small vessel platelet-fibrin thrombosis in venules, arterioles, and capillaries, as well as intravascular megakaryocytes [35].
Cardiovascular complications secondary to COVID-19 occur due to direct effects of the infection but can also arise from systemic illness as well as drug effects including proarrhythmic reactions. Myocardial ischemia seen in COVID-19 predisposes patients to arrhythmias, which can be fatal in and of themselves but also can cause worse morbidity due to increased stroke risk [98]. Atrial Fibrillation (AF) is a commonly seen arrhythmia in patients hospitalized due to COVID-19, even in patients without a prior history of arrhythmias [99]. Proposed mechanisms for onset of AF include virus-induced perimyocarditis, hypoxemia, systemic infection, and sympathetic nervous system hyperactivity and whether this has long-term implications for lifelong anticoagulation therapy remains an outstanding question [98].
Pathophysiology of the post-acute COVID-19 sequelae
With long-term consideration of neurologic deficits, known cytokine release and inflammatory mediators are thought to take part in a systemic inflammatory response that creates an environment susceptible to cognitive decline and neurodegenerative disease. Specifically, NOD-Like Receptor Proteins (NLRP3) inflammasome-mediated inflammation in patients with ventilation-induced hypercapnia, suffered by many with COVID-related ventilation, has experimentally been shown to cause pathological accumulation of amyloid-β. Combined with interleukin-mediated modulation of phosphokinases and phosphatases, patients with COVID-19 are also likely to have accumulation of neurofibrillary tangles. Taken together, there is likely induction or aggravation of processes seen pathologically in diseases such as Alzheimer's disease [100]. Other neurologic diseases potentially implicated as sequelae COVID-19 survivors include Parkinson's disease, multiple sclerosis and narcolepsy. Patients with COVID-19 present with anosmia and ageusia, two prodromal features of Parkinson's disease. Dysregulated cytokine activation is hypothesized to initiate α-synuclein seeding by altering CNS homeostasis, the pathologic underpinning in Parkinson's disease [101]. Multiple sclerosis (MS) and its associated demyelination can be seen years after infection by EBV. Co-infection with EBV has been reported in patients with COVID-19, especially in those with a lower CD4 to CD8 ratio; both T cell counts are notably lower in affected patients with lymphopenia. The inflammatory response to the virus could also initiate a pro-inflammatory status of T cell subsets implicated in MS, causing an autoimmune response. Lastly, the inflammatory response seen in COVID-19 can also cause a response in which T cells damage orexin neurons in the hypothalamus; this could lead to greater incidence of narcolepsy as orexin neuron degeneration is strongly associated with narcolepsy [101].
Structurally, patients who have recovered from COVID-19 have been found to have significantly enlarged volumes along with micro-structural abnormalities, such as grey matter volume changes, in the central olfactory system which includes bilateral olfactory cortices, hippocampi, insulae, among other structures three months after recovery [102].
Conclusion
In this review, we have attempted to highlight the neurologic complications associated with COVID-19 infections. Commonly seen phenomena include headaches and anosmia and now are often considered part of the typical prodrome of COVID-19 infections. Cerebrovascular complications are important conditions to consider in these patients but fortunately occur in only a minority of patients. COVID-19 infection appears to lower seizure threshold and severe infections are often associated with encephalopathy. Meningoencephalitis in COVID-19 is extremely rare. Emerging reports are shedding light on post-acute COVID-19 sequelae such as cranial neuropathies, GBS and transverse myelitis most of which are thought to originate via an autoimmune mechanism. Finally, there remains the unsolved mystery of long COVID-19. Several questions remain in the wake of recovery from COVID-19 and the widespread dissemination of vaccines. Assuming less severe infections, will neurologic complications be limited to cranial neuropathies and headaches? Will patients who have recovered from COVID-19 infections be at increased risks for future cerebrovascular disease due to irreparable vascular damage? Will there be post-infectious sequelae such as the encephalitis lethargica cases which were seen after the Spanish influenza epidemic? Does infection with COVID-19 variants target the nervous system in selective ways? Are the evolving SARS-CoV-2 variants less neurotropic? We await the answers to these and many more as landscape of COVID-19 infections changes in the coming years.
Ethical statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that all authors are responsible for the content and have read and approved the manuscript; and that the manuscript conforms to the Uniform Requirements for Manuscripts Submitted to Biomedical Journals published in Annals in Internal Medicine
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Signed by all authors as follows:
Karan S. Hingorani MD, PhD
Shivkumar Bhadola MD
Anna M. Cervantes-Arslanian MD
Footnotes
Search terms: COVID-19, SARS-CoV-2, Neurologic complications, stroke
Financial disclosures: None.
References
- 1.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 2.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divani AA, Andalib S, Biller J, Di Napoli M, Moghimi N, Rubinos CA, et al. Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20:60. doi: 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltani Zangbar H, Gorji A, Ghadiri T. A review on the neurological manifestations of COVID-19 infection: a mechanistic view. Mol Neurobiol. 2020 doi: 10.1007/s12035-020-02149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand P, Zhou L, Bhadelia N, Hamer DH, Greer DM, Cervantes-Arslanian AM. Neurologic findings among inpatients with COVID-19 at a safety-net US hospital. Neurol Clin Pract. 2020 doi: 10.1212/CPJ.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, et al. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. 2020;419 doi: 10.1016/j.jns.2020.117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U, et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway. Eurosurveillance 2021. December 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hashel JY, Abokalawa F, Alenzi M, Alroughani R, Ahmed SF. Coronavirus disease-19 and headache; impact on pre-existing and characteristics of de novo: a cross-sectional study. J Headac Pain. 2021;22:97. doi: 10.1186/s10194-021-01314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou SH-Y, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo MS, Malsy J, Pöttgen J, Seddiq Zai S, Ufer F, Hadjilaou A, et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia JM, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pun BT, Badenes R, Heras La, Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respirat Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uginet M, Breville G, Hofmeister J, Machi P, Lalive PH, Rosi A, et al. Cerebrovascular Complications and Vessel Wall Imaging in COVID-19 Encephalopathy—A Pilot Study. Clin Neuroradiol. 2021:1–7. doi: 10.1007/s00062-021-01008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer S, Lersy F, Anheim M, Merdji H, Schenck M, Oesterlé H, et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 19.Lin E, Lantos JE, Strauss SB, Phillips CD, Campion TR, Navi BB, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York city. AJNR Am J Neuroradiol. 2020;41:2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand P, Lau KHV, Chung DY, Virmani D, Cervantes-Arslanian AM, Mian AZ, et al. Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. J Stroke Cerebrovascul Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jillella DV, Janocko NJ, Nahab F, Benameur K, Greene JG, Wright WL, et al. Ischemic stroke in COVID-19: an urgent need for early identification and management. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0239443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kihira S, Schefflein J, Mahmoudi K, Rigney B, Delman-B N, Mocco J, et al. Association of coronavirus disease (COVID-19) with large vessel occlusion strokes: a case-control study. Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23847. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51 doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegler JE, Cardona P, Arenillas JF, Talavera B, Guillen AN, Chavarría-Miranda A, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int J Stroke. 2020 doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaghi S, Ishida K, Torres J, Mac-Grory B, Raz E, Humbert K, et al. SARS-CoV-2 and stroke in a New York Healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carneiro T, Dashkoff J, Leung LY, Nobleza COS, Marulanda-Londono E, Hathidara M, et al. Intravenous tPA for acute ischemic stroke in patients with COVID-19. J Stroke Cerebrovascul Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the Young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A, Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J Stroke Cerebrovascul Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2020.105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Y-K, Goh C, Leow AST, Tambyah PA, Ang A, Yap E-S, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-Araque ME, Siegler JE, Ribo M, Requena M, López C, de Lera M, et al. Stroke etiologies in patients with COVID-19: the SVIN COVID-19 multinational registry. BMC Neurology. 2021;21:43. doi: 10.1186/s12883-021-02075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, Biller J, et al. Coronavirus Disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovascul Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel Wall Enhancement and Focal Cerebral Arteriopathy in a Pediatric Patient with Acute Infarct and COVID-19 Infection. AJNR Am J Neuroradiol. 2020;41:2348–2350. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendren NS, Drazner MH, Bozkurt B, Cooper LT. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirschenbaum D, Imbach LL, Rushing EJ, Frauenknecht KBM, Gascho D, Ineichen BV, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2020 doi: 10.1111/nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Connolly A-MF. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet. 2021;398:599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abootalebi S, Aertker BM, Andalibi MS, Asdaghi N, Aykac O, Azarpazhooh MR, et al. Call to action: SARS-CoV-2 and cerebrovascular disorders (CASCADE) J Stroke Cerebrovascul Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Havenon A, Yaghi S, Mistry EA, Delic A, Hohmann S, Shippey E, et al. Endovascular thrombectomy in acute ischemic stroke patients with COVID-19: prevalence, demographics, and outcomes. J NeuroIntervent Surg. 2020;12:1045–1048. doi: 10.1136/neurintsurg-2020-016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. 2020;51:2540–2543. doi: 10.1161/STROKEAHA.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. Ischemic Stroke n.d.:6. [DOI] [PubMed]
- 46.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugin D, Vargas M-I, Thieffry C, Schibler M, Grosgurin O, Pugin J, et al. COVID-19–related encephalopathy responsive to high-dose glucocorticoids. Neurology. 2020;95:543–546. doi: 10.1212/WNL.0000000000010354. [DOI] [PubMed] [Google Scholar]
- 49.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, et al. Hemorrhagic stroke and anticoagulation in COVID-19 n.d.:7. [DOI] [PMC free article] [PubMed]
- 50.Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, et al. Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York Healthcare system. Neurocrit Care. 2020 doi: 10.1007/s12028-020-01077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melmed KR, Cao M, Dogra S, Zhang R, Yaghi S, Lewis A, et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, et al. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pantel T, Roedl K, Jarczak D, Yu Y, Frings DP, Sensen B, et al. Association of COVID-19 with intracranial hemorrhage during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a 10-year retrospective observational study. J Clin Med. 2021;11:28. doi: 10.3390/jcm11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usman AA, Han J, Acker A, Olia SE, Bermudez C, Cucchiara B, et al. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothor Vascu Anesth. 2020;34:3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J Stroke Cerebrovasc Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Cantú C, Bousser M-G, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40:2356–2361. doi: 10.1161/STROKEAHA.108.543884. [DOI] [PubMed] [Google Scholar]
- 59.Arandela K, Samudrala S, Abdalkader M, Anand P, Daneshmand A, Dasenbrock H, et al. Reversible cerebral vasoconstriction syndrome in patients with coronavirus disease: a multicenter case series. J Stroke Cerebrovascul Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mansoor T, Alsarah AA, Mousavi H, Eliyas JK, Girotra T, Hussein O. COVID-19 associated reversible cerebral vasoconstriction syndrome successfully treated with nimodipine and aspirin. J Stroke Cerebrovascul Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanafi R, Outteryck O. COVID-19 Neurologic Complication with CNS Vasculitis-Like Pattern n.d.:4. [DOI] [PMC free article] [PubMed]
- 62.Lacy J, Pavord S, Brown KE. VITT and second doses of Covid-19 vaccine. N Engl J Med. 2022;386:95. doi: 10.1056/NEJMc2118507. –95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charidimou A, Samudrala S, Cervantes-Arslanian AM, Sloan JM, Dasenbrock HH, Daneshmand A. Vaccine-induced immune thrombotic thrombocytopenia with concurrent arterial and venous thrombi following Ad26.COV2.S vaccination. J Stroke Cerebrovascul Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.106113. [DOI] [PubMed] [Google Scholar]
- 64.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danoun OA, Zillgitt A, Hill C, Zutshi D, Harris D, Osman G, et al. Outcomes of seizures, status epilepticus, and EEG findings in critically ill patient with COVID-19. Epilep Behav. 2021;118 doi: 10.1016/j.yebeh.2021.107923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emami A, Fadakar N, Akbari A, Lotfi M, Farazdaghi M, Javanmardi F, et al. Seizure in patients with COVID-19. Neurol Sci. 2020;41:3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keshavarzi A, Janbabaei G, Kheyrati L, Ghavamabad LH, Asadi-Pooya AA. Seizure is a rare presenting manifestation of COVID-19. Seizure. 2021;86:16–18. doi: 10.1016/j.seizure.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anand P, Al-Faraj A, Sader E, Dashkoff J, Abdennadher M, Murugesan R, et al. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilep Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaughan M, Connolly S, Direkze S, Kinsella JA. Acute new-onset symptomatic seizures in the context of mild COVID-19 infection. J Neurol. 2020 doi: 10.1007/s00415-020-10214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mardani M, Nadji SA, Sarhangipor KA, Sharifi-Razavi A, Baziboroun M. COVID-19 infection recurrence presenting with meningoencephalitis. New Microb New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naz S, Hanif M, Haider MA, Ali MJ, Ahmed MU, Saleem S. Meningitis as an initial presentation of COVID-19: a case report. Front Public Health. 2020;8:474. doi: 10.3389/fpubh.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilotto A, Masciocchi S, Volonghi I, Crabbio M, Magni E, De Giuli V, et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID multicenter study. J Infect Dis. 2021;223:28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24:421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D, Sánchez-Tejerina D, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anand P, Zakaria A, Benameur K, Ong C, Putman M, O'Shea S, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med. 2020;48:1664–1669. doi: 10.1097/CCM.0000000000004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;268:1133–1170. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta S, Jawanda MK, Taneja N, Taneja T. A systematic review of Bell's Palsy as the only major neurological manifestation in COVID-19 patients. J Clin Neurosci. 2021;90:284–292. doi: 10.1016/j.jocn.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Doblan A, Kaplama ME, Ak S, Basmacı N, Tarini EZ, Göktaş ŞE, et al. Cranial nerve involvement in COVID-19. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2021.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Wang Y, Huo L, Li Q, Chen J, Wang H. SARS-CoV-2-associated acute disseminated encephalomyelitis: a systematic review of the literature. J Neurol. 2021 doi: 10.1007/s00415-021-10771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12:879. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 83.Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20:1144. doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 85.Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Á-Steig B, Gaini S, et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salmon-Ceron D, Slama D, De Broucker T, Karmochkine M, Pavie J, Sorbets E, et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect. 2021;82:e1–e4. doi: 10.1016/j.jinf.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carfì A, Bernabei R, Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann Clin Transl Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol. 2020 doi: 10.1007/s12035-020-02152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hugon J, Msika E-F, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2021 doi: 10.1007/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12:eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care. 2020 doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Musikantow DR, Turagam MK, Sartori S, Chu E, Kawamura I, Shivamurthy P, et al. Atrial fibrillation in patients hospitalized with COVID-19. JACC. 2021;7:1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alz Res Therapy. 2020;12:69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schirinzi T, Landi D, Liguori C. COVID-19: dealing with a potential risk factor for chronic neurological disorders. J Neurol. 2020 doi: 10.1007/s00415-020-10131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Li X, Geng D, Mei N, Wu P-Y, Huang C-C, et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]