Abstract
Genetic testing for prostate cancer is rapidly growing and is increasingly being driven by precision medicine. Rates of germline pathogenic variants have been reported in up to 15% of men with prostate cancer, particularly in metastatic disease, and results of genetic testing could uncover options for precision therapy along with a spectrum of hereditary cancer-predisposition syndromes with unique clinical features that have complex management options. Thus, the pre-test discussion, whether delivered by genetic counsellors or by health-care professionals in hybrid models, involves information on hereditary cancer risk, extent of gene testing, purpose of testing, medical history and family history, potential types of results, additional cancer risks that might be uncovered, genetically based management and effect on families. Understanding precision medicine, personalized cancer risk management and syndrome-related cancer risk management is important in order to develop collaborative strategies with genetic counselling for optimal care of patients and their families.
Subject terms: Prostate, Cancer genetics
In this Review, Russo and Giri describe and discuss germline testing criteria, genetic testing strategies, genetically informed screening, precision management, delivery of genetic counselling or alternative genetic services and special considerations for men with prostate cancer.
Key points
Germline (hereditary) genetic testing is rising in importance for treatment, screening and risk assessment of prostate cancer.
Multiple hereditary cancer syndromes might be associated with prostate cancer, might confer risk of other cancerous and non-cancerous conditions, and can have hereditary cancer implications for family members. The rates of these syndromes can vary based upon the attributed genetic mutations.
Multiple aspects of germline testing should be discussed in the pre-test setting for men to make an informed decision, including the purpose of genetic testing, the benefits and risks of testing, hereditary cancer risk, identification of additional cancer risks, familial implications and the state of genetic discrimination protections.
Genetic evaluation can be conducted by genetic counsellors or a hybrid model can be employed, in which health-care providers deliver pre-test informed consent for testing, order testing and then determine referral to genetic counselling for appropriate patients.
Precision medicine is increasingly driving decisions for germline testing. Poly(ADP-ribose) polymerase (PARP) inhibitors, immune checkpoint inhibitors and various other agents now in clinical trials have clinical activity in patients with certain hereditary cancer gene mutations, such as in DNA repair genes.
Patients’ experiences with germline testing can be variable; taking the patient’s current experience into account, considering referral to genetic counselling when needed and offering germline testing for eligible men at repeated intervals if initially declined are important.
Introduction
Prostate cancer is a common cancer in men, and a subset of men can have a hereditary predisposition to developing this disease1,2. Germline testing (hereditary cancer genetic testing) encompasses testing for genes linked to hereditary syndromes, such as hereditary breast and ovarian cancer (HBOC) syndrome, Lynch syndrome and hereditary prostate cancer1–4. Furthermore, germline testing now involves multigene testing of a host of additional genes, such as DNA repair genes, which might also confer increased risk of additional cancers and be important for therapeutic determination1–7. Germline testing for inherited mutations is important to estimate cancer risks above the general population, with magnitude of risk being gene specific1,8. Population risk for developing prostate cancer is 11%, whereas men with specific genetic mutations (pathogenic or probable pathogenic variants) can have a 2-fold to 10-fold increase in lifetime risk of developing prostate cancer, such as for mutations in BRCA2 or HOXB13 (ref.1). Risk-based screening for prostate cancer is evolving, with current guidelines from the National Comprehensive Cancer Network (NCCN) recommending initiation of prostate cancer screening for BRCA2 carriers at the age of 40 years with consideration of the same for BRCA1 carriers9. Furthermore, prostate cancer germline testing came to the forefront in the precision medicine era1,2,4–6. Multiple targeted agents, such as poly(ADP-ribose) polymerase (PARP) inhibitors and immunotherapeutic drugs, are FDA approved or have FDA designations for use in men with metastatic castration-resistant prostate cancer (mCRPC) owing to demonstrated clinical responses, particularly among men who carry mutations in DNA repair genes such as in BRCA1, BRCA2, ATM, or the DNA mismatch repair genes1–7. Thus, pre-test discussions with patients need to address the potential effects of treatment or screening, hereditary cancer risk and implications for blood relatives1–4,8–10.
Many thousands of men could now be eligible for germline testing and, therefore, strategies for clinical genetic evaluation need to be implemented. Genetics care delivery can occur through genetic counselling or by employing a hybrid model (a health-care provider–genetic counselling collaborative approach). Genetic counselling is a specialized field and genetic counsellors are professionals trained in the principles and practice of genetic testing, hereditary cancer assessment, informed decision-making for genetic testing and the implications of genetic testing for patients and their families10–13 (Box 1). They perform an assessment of a patient’s medical history and family history, discuss cancer heredity and options for genetic testing, implications of test results, and the benefits and risks of testing so that patients can make an informed decision10–13. Upon return of the results, genetic counsellors help patients to understand their results and discuss recommendations based on the results and the patient’s medical history and family history. They also coordinate cascade testing (testing of blood relatives in families with a known genetic mutation) or additional genetic testing in a family9–12.
In the current precision medicine era, in which genetic mutations might guide options for targeted therapies and with the expanded indications for germline testing, a need for alternative delivery of germline testing initiated by health-care providers has arisen given the relative shortage of genetic counsellors and the need to limit additional visits and reduce barriers13–17. Hybrid models have emerged in practice to expand access to germline testing, in which joint care strategies are implemented between health-care providers and genetic counsellors to address pre-test consent strategies, management of men with pathogenic variants, management of subsets of men with variants of uncertain significance (VUS), management of subsets of men with negative results with strong family cancer history, and cascade testing2,14.
Given the growth of indications for germline testing in prostate cancer7,9, this Review addresses major autosomal-dominant hereditary cancer syndromes linked with prostate cancer, germline testing criteria, genetic testing strategies, genetically informed prostate cancer screening and precision management, delivery of genetic counselling or alternative genetic services and special considerations for this population. All of these issues are now of crucial relevance for optimal cancer risk assessment, screening and treatment of patients with prostate cancer or at risk of developing prostate cancer guided by genetic information.
Box 1 Basic elements of genetics care delivery and germline testing.
Aspects of germline genetic testing
Testing DNA from non-cancerous cells to detect variation associated with potential risk of disease or effect on treatment.
Disease risk is predominantly determined by the effect that the variant has on the ability of the gene to translate into a functioning protein.
The goal of germline genetic testing in oncology is to assess an individual’s cancer predisposition and understand why a specific cancer developed based on pathogenic variants in genes implicated in important cellular processes.
Germline testing can also be conducted in oncology to inform targeted therapy options, particularly in the metastatic setting.
Variants detected in germline testing can be passed down or inherited by offspring. Germline testing differs from somatic genetic testing of a tumour assessing for somatic variants, which are usually acquired in tumour formation. Confirmatory germline testing of somatic variants might be indicated to determine hereditary nature.
Aspects of genetic counselling
Evaluating a patient’s medical and family history to assess the likelihood of a genetic predisposition and advise appropriate genetic testing.
Providing education regarding genetic testing, inherited health risk, or effect on treatment.
Supporting patients in making genetic testing decisions.
Interpreting genetic testing results and helping patients to understand and adjust to potential medical, familial and psychosocial implications.
Clinical scope of a genetic counsellor
A medical professional with specialized training in advanced genetics and counselling.
Genetic counsellors often work in a clinic or hospital setting along with physicians and other health-care providers as part of a patient’s care team.
They can specialize in different areas of genetics including, but not limited to, oncological, paediatric, prenatal or preconception, cardiovascular and neurological.
Aspects of a hybrid genetics care delivery model
A collaborative care model between health-care providers and genetic counsellors.
Health-care providers deliver pre-test informed consent.
Health-care providers order germline testing, particularly focusing on the effect on treatment or management.
- Upon return of the results, health-care providers discuss implications for prostate cancer treatment, management or screening. Appropriate patients are referred to genetic counsellors.
- Results of pathogenic or probable pathogenic variants
- Results of variants of uncertain significance with strong family cancer history
- Men with negative results with strong family cancer history
- Complex genetic results
- Some patients might need to be referred to genetic counselling upfront.
- Men who prefer to see a genetic counsellor
- Men with a high level of anxiety or psychosocial issues
- Men with known mutations in the family
- Men with rare or complex syndromes
Inherited cancer syndromes contributing to prostate cancer
Multiple hereditary cancer syndromes can be associated with prostate cancer, such as HBOC syndrome and Lynch syndrome, which have specific clinical manifestations and management1,2 (Table 1). These syndromes require unique attention when performing genetic counselling and/or pre-test informed consent and germline testing (Table 1). HBOC is associated with mutations in BRCA1 and BRCA2 (ref.9). BRCA1 and BRCA2, which are located on chromosomes 17 and 13, respectively, are tumour suppressor genes involved in DNA homologous recombination repair18–20. HBOC is inherited in an autosomal-dominant manner (in which a single copy of a mutation on a non-sex chromosome is sufficient to predispose to disease)19,20 and is associated with breast cancer (male and female), ovarian cancer, pancreatic cancer, prostate cancer and melanoma9. Up to 15% of men with metastatic prostate cancer have been reported to carry germline mutations in DNA repair genes such as BRCA1 and BRCA2, whereas ~5–7% of men with early-stage prostate cancer are carriers1,2,21–23. BRCA2 mutations are more strongly associated with prostate cancer than BRCA1 mutations, with an approximately eightfold versus approximately threefold increase in risk, respectively1,24. Furthermore, BRCA2 mutations have been associated with aggressive prostate cancer with decreased prostate cancer-specific survival1,25–27. Individuals with Ashkenazi Jewish ancestry are at an increased risk of carrying a mutation in BRCA1 or BRCA2 owing to three known founder mutations in this population: 185delAG (BRCA1), 5382insC (BRCA1) and 6174delT (BRCA2)28,29. The combined population risk of carrying one of these variants is 1 in 40 for Ashkenazi Jewish individuals compared with 1 in 400 for the general population28,30.
Table 1.
Major hereditary cancer syndromes linked with prostate cancer
| Gene | Syndrome | Clinical manifestations affecting cancer management (patient and familial implications)a | Prostate cancer management considerations by selected guidelines for patients with mutationsb |
|---|---|---|---|
|
BRCA1 BRCA2 |
HBOC |
Female and male breast cancer Ovarian cancer Prostate cancer Pancreatic cancer Melanoma |
NCCN Guidelines Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 1.2022 (ref.9) Prostate cancer: PSA screening starting at the age of 40 years with annual intervals Recommend screening for BRCA2 carriers and consider screening for BRCA1 carriers NCCN Prostate Cancer (Version 2.2021)7 For mCRPC, DNA genes such as BRCA1, BRCA2, ATM, etc. might inform early platinum therapy or PARP inhibitor therapy NCCN Prostate Cancer Early Detection (Version 2.2020)49 Consider shared decision-making for men with BRCA1 or BRCA2 mutations regarding prostate cancer screening starting at the age of 40 years and to consider annual versus biannual screening Philadelphia Prostate Cancer Consensus Conference 2019 (ref.2) Recommend precision medicine clinical trials mCRPC: BRCA2 and BRCA1 inform PARP inhibitor therapy; also inform use of platinum chemotherapy. Consider immunotherapy for DNA MMR gene mutation carriers Consider BRCA2 in active surveillance discussions Prostate cancer screening at the age of 40 years or 10 years before the youngest person in the family was diagnosed with prostate cancer and continue screening with annual intervals (recommended for BRCA2 carriers with consideration for BRCA1 carriers) AUA/ASTRO/SUO Advanced Prostate Cancer 2020 (ref.40) Offer PARP inhibitors to patients with deleterious or suspected deleterious germline or somatic homologous recombination repair gene-mutated mCRPC following previous treatment with enzalutamide or abiraterone acetate, and/or a taxane-based chemotherapy. Consider platinum-based chemotherapy as an alternative for patients who cannot use or obtain a PARP inhibitor EAU Prostate Cancer 2020 (ref.41) Genetic testing for DNA repair mutations for PARP inhibitor therapy in mCRPC |
|
MLH1 MSH2 MSH6 PMS2 EPCAM |
Lynch syndrome |
Colorectal Endometrial Ovarian Urothelial cancer (renal pelvis, ureteral cancers) Gastric and/or small bowel Pancreatic Prostate Brain |
NCCN Prostate Cancer (Version 2.2021)7 For mCRPC, consider dMMR or MSI-H status for pembrolizumab Philadelphia Prostate Cancer Consensus Conference 2019 (ref.2) Consider DNA MMR gene mutations for pembrolizumab Consider prostate cancer screening at age 40 years or 10 years before the youngest person in the family was diagnosed with prostate cancer and continue screening with annual intervals AUA/ASTRO/SUO Advanced Prostate Cancer 2020 (ref.40) In patients with dMMR or MSI-H mCRPC, offer pembrolizumab |
| HOXB13 | Hereditary prostate cancer | Prostate cancer |
Philadelphia Prostate Cancer Consensus Conference 2019 (ref.2) Consider prostate cancer screening at age 40 years or 10 years before the youngest person in the family was diagnosed with prostate cancer and continue screening with annual intervals |
ASTRO, American Society for Radiation Oncology; AUA, American Urological Association; dMMR, mismatch repair deficiency; EAU, European Association of Urology; HBOC, hereditary breast and ovarian cancer syndrome; mCRPC, metastatic castration-resistant prostate cancer; MMR, mismatch repair; MSI-H, microsatellite instability high; NCCN, National Comprehensive Cancer Network; PARP, poly(ADP-ribose) polymerase; SUO, Society of Urologic Oncology. aClinical trials are important for patients with mutations concerning treatment and management of prostate cancer. Many of the hereditary syndromes in this table have cancerous and non-cancerous disease features beyond prostate cancer that also require management. bMultiple NCCN guidelines address management of cancer risks, risk reduction and treatment affecting men and their families.
Hereditary prostate cancer, with generational and/or young onset of disease, is another hereditary syndrome associated with prostate cancer development1,31. HOXB13, the first classic hereditary prostate cancer gene identified in 2012 is associated with an approximately eightfold increased risk of prostate cancer and approximately tenfold increased risk of early-onset disease1,24,32. HOXB13 is currently not known to confer increased risk of developing other cancers. The G84E pathogenic variant of HOXB13 has an established association with prostate cancer; its overall frequency was reported to be 1.34% among men with prostate cancer in a pooled analysis across multiple studies33, whereas the highest carrier rate of 6.25% has been reported in men with prostate cancer in Finland34. HOXB13 codes for a homeobox transcription factor and is located on chromosome 17 (ref.34); this gene is part of a large group of transcription factors called the homeobox protein family, which has a role in prostate development35. HOXB13 mutations are inherited in an autosomal-dominant manner1,32.
Prostate cancer is also within the spectrum of Lynch syndrome cancers1,24,36. Lynch syndrome, classically known as hereditary non-polyposis colorectal cancer syndrome, predisposes to development of cancers of the colon and/or rectum, pancreas, ovary, uterus, upper bowel and urinary tract, and sebaceous carcinoma36,37. Lynch syndrome is associated with mutations in the following genes involved in DNA mismatch repair (MMR): MLH1 (chromosome 3), MSH2 (chromosome 2), MSH6 (chromosome 2) and PMS2 (chromosome 7) and is inherited in an autosomal-dominant manner36,37. Certain deletions in the EPCAM gene have also been shown to cause Lynch syndrome by causing an MSH2 epimutation37. However, associated cancer risks vary based on the study and DNA MMR genes studied1,36–39. Overall, mutations in the DNA MMR genes, particularly MSH2 and MSH6, are associated with an approximately threefold increased risk of developing prostate cancer1,38.
Other genes, such as CHEK2 or NBN, have varying degrees of association with prostate cancer, but are included in prostate cancer testing panels owing to implications for therapy1,2. Multiple other genes involved in DNA repair, such as ATM, PALB2, RAD51C and RAD51D, could also be important for testing to inform response to precision therapies such as PARP inhibitors1,2,4,5.
These data show that multiple genes linked with a spectrum of hereditary cancer syndromes are associated with varying degrees of prostate cancer risk, disease characteristics and manifestations, and additional cancers1,7,9,24; thus, multigene testing for prostate cancer has become a standard of genetic testing practice1,2,7. Several of these syndromes have established guidelines to inform hereditary cancer assessment in families and guide screening for at-risk individuals7,9,39 (Table 1).
Germline testing criteria and approach to testing for prostate cancer
Germline testing criteria for prostate cancer encompasses personal cancer history, cancer features and pathology, family history and precision therapy indications7,9. The NCCN publishes guidelines for consideration of germline testing across multiple cancer types, including prostate cancer7,9. Germline testing criteria for prostate cancer have been provided by multiple NCCN panels7,9, an international expert consensus statement from the Philadelphia Prostate Cancer Consensus Conference 2019 (ref.2), American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology (AUA/ASTRO/SUO) 2020 guideline for advanced prostate cancer40, and the European Association of Urology (EAU) 2020 guideline2,7,9,41 (Box 2). Prostate cancer germline testing criteria from the NCCN are uniform among guidelines regarding cancer features (stage, grade) and ancestry; however, they differ by family history criteria7,9. NCCN guidelines agree regarding recommending germline testing for men with any one of the following: metastatic prostate cancer, regional or node-positive disease, very high-risk or high-risk prostate cancer (defined by grade group, T stage, and serum PSA levels at diagnosis), intraductal or cribriform histology, Ashkenazi Jewish ancestry, or family history of a relative or relatives with a germline mutation7,9. Eliciting family history of prostate cancer (age at diagnosis, known metastatic disease, death from prostate cancer) is important to inform for suspicion of hereditary prostate cancer and to help to inform regarding the full scope of genes to test2,7,9. Furthermore, knowing the family history of cancers of the breast, ovary, pancreas, colon and/or rectum, uterus, small bowel, urinary tract and skin is important7. Clinical practice might not be straightforward, but clinicians can refer to all guidelines to determine which family history criteria might match their patient’s family history to determine eligibility for germline testing7,9. Thus, health-care providers need to become familiar with the nuances of these guidelines and remain current, as the guidelines are regularly updated.
Multiple professional organizations address germline testing for prostate cancer in various ways (Table 1; Box 2). The EAU 2020 guideline41 recommends conducting germline testing for men with metastatic prostate cancer. This guideline also recommends performing genetic testing for men with mCRPC for DNA repair mutations to determine eligibility for PARP inhibitors. The AUA/ASTRO/SUO 2020 advanced prostate cancer guideline40 states that patients with metastatic prostate cancer should be offered genetic counselling and genetic testing regardless of age and family history30. It also recommends offering PARP inhibitors to patients with deleterious or suspected deleterious germline or somatic homologous recombination repair gene-mutated mCRPC following treatment with enzalutamide or abiraterone acetate, and/or a taxane-based chemotherapy. It also recommends considering platinum-based chemotherapy as an alternative for patients who cannot use or obtain a PARP inhibitor and offering pembrolizumab to patients with MMR-deficient or microsatellite instability-high mCRPC.
Clinical germline testing has now progressed from single gene testing to multigene testing options for patients2,3,42. Multiple genetic testing laboratories offer germline testing options for patients with prostate cancer or those concerned about risk of prostate cancer. In general, testing options include guideline-focused panels, tumour-specific panels and large comprehensive cancer panels2,3 (Table 2). Furthermore, some genetic testing laboratories have the option of initially conducting testing with a small set of genes, and then reflex testing a larger set of genes if initial testing does not reveal pathogenic and/or probable pathogenic variants in the genes tested2. These reflex options typically need to be conducted within the laboratory-specified time frame of test ordering for the test to be cost free to patients in the USA.
Table 2.
Prostate cancer panels available through experienced USA-based clinical laboratories
| Testing laboratory | Multigene panels and genes | Considerations |
|---|---|---|
| Invitae |
Prostate cancer panel: ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PMS2, TP53 Prostate cancer HRR panel: ATM, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, FANCL, PALB2, RAD51C, RAD51D |
Ability to customize gene panelsa Reflex testing is available within a specific time frame Offers paired RNA testingb Accepts skin punch biopsy specimens for testing |
| Ambry Genetics | ProstateNext panel: ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PALB2, PMS2, RAD51D, TP53 |
Ability to customize gene panelsa Reflex testing is available within a specific time frame Offers paired RNA testing (RNAinsight)b Accepts skin punch biopsy specimens for testing |
| GeneDx | Hereditary prostate cancer panel: ATM, BRCA1, BRCA2, BRIP1, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PALB2, PMS2, RAD51C, RAD51D, TP53 |
Ability to customize gene panelsa Reflex testing is available Accepts skin punch biopsy specimens for testing |
| Myriad Genetics | myRisk panel: APC, ATM, AXIN2, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, GREM1, HOXB13 GALNT12, MLH1, MSH2, MSH3, MSH6, MUTYH, NBN, NTHL1, PALB2, PMS2, POLE, POLD1, PTEN, RAD51C, RAD51D, RNF43, RPS20, SMAD4, STK11, TP53 |
HOXB13 sequencing only Reflex testing from small panels to largest panel (Myriad myRisk) is available, but might require an additional specimen Accepts skin punch biopsy specimens for testing |
| Color | Hereditary cancer panel: APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, GREM1, MITF, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLE, POLD1, PTEN, RAD51C, RAD51D, SMAD4, STK11, TP53 |
PMS2: only covers exons 1–11. Variant interpretation outside of this area of the gene is difficult owing to a pseudogene PMS2CL that shares a similar coding sequence HOXB13 not included |
As of August 2021; furthermore, multiple additional cancer panels are available at these laboratories. HRR, homologous recombination repair. aCustomization involves being able to choose the genes to test rather than a premade gene panel. bRNA testing involves assessing the function of DNA variants usually reported as variants of uncertain significance (VUS). These VUS are usually in genes associated with hereditary cancer and disrupt RNA splicing88.
Multigene panel testing has become the standard of care for many genetic testing indications7,9; thus, dedicated discussion of the considerations of panel tests is needed before a patient pursues genetic testing2,42. The size and availability of multiple gene panels varies by laboratory and the process of helping the patient choose the best panel for them can be complex2,42,43. A thorough discussion of benefits and limitations of panel testing is necessary before pursuing multigene panel testing, with recognition that these discussions need to be tailored to the purpose of testing2,7,9,42,43. For example, multigene testing in a patient with metastatic prostate cancer might include large panel testing to optimize targeted therapy or clinical trial options2,4; however, in early-stage prostate cancer, genetic testing might be more tailored and encompass genes that account for the patient’s cancer and family history2. Patients need to understand the benefits and limitations of various multigene panel testing options2,42,43. Benefits of large-panel testing include potentially increased chance of uncovering a mutation, cost efficiency, and reduced testing fatigue, which can occur with repeat testing42,43. Limitations of large-panel testing include an increased rate of detecting VUS, unexpected secondary findings, mutations in genes in which the cancer risk is unknown or not well established, and finding mutations in genes without current management guidelines2,3,7,9,42.
Acceptable specimens for germline genetic testing at most laboratories include peripheral blood, saliva, and cheek and/or buccal swab. For some patients, such as those with certain haematological malignancies or a history of transplant, a skin punch biopsy sample might be needed to avoid testing tumour cells for genetic mutations. Germline genetic testing through most commercial laboratories generally has a 3–4-week turnaround time depending on testing techniques, sample used and billing practices. Each laboratory has individual practice that providers should become familiar with.
Importantly, the difference between ‘medical genetic testing’ or ‘clinical genetic testing’ and ‘recreational genetic testing’ or ‘direct-to-consumer’ testing needs to be distinguished44–47. Medical or clinical genetic testing is a comprehensive process that involves extensive interrogation of multiple genes or genetic regions to detect thousands of pathogenic variants in disease-related genes of interest. The technology can detect multiple types of mutations and deletions and/or duplications and/or rearrangements. Genetic evaluation is guided by a genetics professional or health-care professional and is tailored to the patient’s medical history, family history and personal preferences2,7,9,42. Recreational genetic tests or direct-to-consumer tests are primarily designed for population-level testing, with some advantages and important disadvantages to consider45–47. The main advantage is increased access to genetic testing without practice barriers or delays that can be involved in scheduling appointments with genetic counselling15,45,46. However, multiple important considerations need to be noted: the testing might not be suited to a particular patient’s medical condition or address family cancer history; the testing might be incomplete compared with clinical genetic testing (testing only a few mutations versus complete sequencing and alteration assessment; limited genes tested)44–47; if test results return with no pathogenic variants, individuals might have false reassurance of no hereditary cancer condition in themselves or their families, with subsequent missed hereditary cancer management and lack of appropriate follow-up care4,44–47; misunderstanding of various types of genetic results (such as VUS) can occur, with propagation of misinformation in families, potential adverse effects on life insurance or long-term care insurance for mutation carriers, and adverse psychological consequences without preparatory pre-test genetic counselling44–47. Furthermore, patients might present to their physicians or genetic counsellors with results of at-home genetic tests, which could then require repeat or confirmatory germline testing, often at a cost to the patient.
Overall, criteria for genetic testing for prostate cancer are tailored to the clinical features, pathological features and family history7,9. Genetic testing options have expanded and providers should have an understanding of the benefits and limitations of genetic test options2,7,9,42. Close collaboration between health-care providers and genetic counsellors is important for optimal testing and comprehensive recommendations to patients2,7,9,14,42.
Box 2 Examples of germline testing criteria for prostate cancer.
NCCN Guidelines Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic (Version 1.2022) 9
- Personal history of prostate cancer at any age with
- Metastatic, intraductal or cribriform histology, or high-risk or very-high-risk group (see NCCN Prostate Cancer guideline7 criteria below) regardless of age
- Any NCCN risk group (see NCCN Prostate Cancer guideline7 below) with the following
- ≥1 close blood relative diagnosed with breast cancer at ≤50 years old, pancreatic, ovarian, or metastatic, intraductal or cribriform prostate cancer at any age
- ≥2 close blood relatives with either breast or prostate cancer at any age
- Ashkenazi Jewish ancestry
NCCN Guidelines Prostate Cancer (Version 2.2021) 7
Regardless of age
Metastatic prostate cancer
Very high risk: T3b–T4 or primary Gleason pattern 5 or >4 cores with grade group 4 or 5
High risk: T3a or grade group 4 or 5 or PSA >20 ng/ml
Any other risk group with any of the following
Ashkenazi Jewish ancestry
Prostate cancer family history: brother, father, or multiple family members diagnosed with prostate cancer at <60 years old or died from prostate cancer (grade groups 2–5)
≥3 cancers on the same side of the family (especially if diagnosed at ≤50 years old): bile duct, breast, colorectal, endometrial, gastric, kidney, melanoma, ovarian, pancreatic, prostate (grade groups 2–5), small bowel, or urothelial
Philadelphia Prostate Cancer Consensus Conference 2019 (ref.2)
Recommend to
Men with metastatic prostate cancer
- Men with a family history of prostate cancer: men with one first-degree relative or ≥2 male relatives with one of the following:
- Diagnosed with prostate cancer at the age of <60 years
- Any of whom died from prostate cancer or had metastatic prostate cancer
Consider for
- Men with non-metastatic prostate cancer with one of the following
- Diagnosed with prostate cancer at <60 years old
- Advanced disease (T3a or higher)
- Intraductal or ductal pathology
- Grade group 4 (Gleason sum 8) or above
Men with Ashkenazi Jewish ancestry
Men with two or more cancers within the hereditary breast and ovarian cancer or Lynch syndrome spectrum in any relatives on the same side of the family (especially if diagnosed at <50 years old)
AUA/ASTRO/SUO Clinically Localized Prostate Cancer 2017 (ref.89)
Referral to genetic counselling should be considered for patients (and their families) with high-risk localized prostate cancer and a strong family history of specific cancers (such as breast, ovarian, pancreatic, other gastrointestinal tumours)
AUA/ASTRO/SUO Advanced Prostate Cancer 2020 (ref.40)
Patients with metastatic hormone-sensitive prostate cancer should be offered genetic counselling and genetic testing regardless of age and family history
EAU guidelines: Prostate Cancer 41
Conduct germline testing for men with metastatic prostate cancer
ASTRO, American Society for Radiation Oncology; AUA, American Urological Association; EAU, European Association of Urology; NCCN, National Comprehensive Cancer Network; SUO, Society of Urologic Oncology.
Management of prostate cancer based on germline mutations
Historically, germline testing for cancer predisposition syndromes was performed to inform cancer risk, screening and cancer risk-reduction measures44. However, advances in precision medicine have heralded a new era of expanded therapeutic utility of germline testing, which is now highly relevant to prostate cancer1,2,4–7,9 (Table 1). Hereditary cancer management for syndrome-associated cancers including and beyond prostate cancer is also crucial7,9.
The influence of genetic testing in prostate cancer screening has been of long-standing interest, with emerging data influencing screening approaches2,7,48. Updated results from the IMPACT prostate cancer screening trial were reported in 2019 (ref.48). This study included men aged 40–69 years, 919 of whom were BRCA1 carriers, 709 of whom were BRCA1 non-carriers, 902 of whom were BRCA2 carriers, and of whom 497 were BRCA2 non-carriers, who underwent 3 years of screening48. Higher prostate cancer incidence was observed among BRCA2 carriers than non-carriers (19.4 versus 12.0; P = 0.03). BRCA2 carriers were also diagnosed with prostate cancer at a younger age (61 versus 64 years; P = 0.04) and were more likely to have clinically significant disease than BRCA2 non-carriers (77% versus 40%; P = 0.01)48. Cancer incidences per 1,000 person-years were 19 in BRCA2 carriers, 12 in BRCA2 non-carriers, 14 in BRCA1 carriers and 11 in BRCA1 non-carriers48. Thus, the NCCN Genetic/Familial High Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022) guideline recommends that men with BRCA2 mutations start PSA screening at the age of 40 and that men with BRCA1 mutations consider the same9. The NCCN Prostate Cancer Early Detection Guideline (Version 2.2020) recommends considering shared decision-making for men carrying BRCA1 or BRCA2 mutations regarding prostate cancer screening starting at the age of 40 years and to consider annual versus every other year screening49 (Table 1). Initial results from prostate cancer screening in men with pathogenic variants in DNA MMR genes were also reported in the IMPACT study in 2021 (ref.50). Among 962 men enrolled, including those carrying and not carrying DNA MMR mutations, prostate cancer incidence (using a PSA threshold of >3.0 ng/ml) was higher in MSH2 carriers than in MSH2 non-carriers (4.3% versus 0.5%; P = 0.011) and in MSH6 carriers than in MSH6 non-carriers (3.0% versus 0%; P = 0.034)50. The overall positive predictive value of biopsy using a PSA threshold of 3.0 ng/ml was 51.4% (95% CI 34.0–68.6)50. To inform further strategies for prostate cancer screening and to encompass other genes now available on prostate cancer multigene panels, recommendations from the Philadelphia Prostate Cancer Consensus Conference 2019 were published for prostate cancer screening based on mutation status and age at diagnosis of prostate cancer in the family2 (Table 1). In these recommendations, agreement to start PSA screening at the age of 40 years or 10 years before the youngest age at prostate cancer diagnosis among men carrying BRCA2 mutations, and to consider the same in men with BRCA1, ATM, HOXB13 and DNA MMR mutations is strong2. Currently, prophylactic prostatectomy is not indicated for mutation carriers.
Genetically based management of men with early-stage prostate cancer is also evolving regarding active surveillance discussions51. In one study, 6 of 11 men with BRCA2 mutations on active surveillance had significant upgrading of biopsy samples, either scheduled or prompted by serum PSA levels, compared with 283 of 1,200 non-BRCA2 carriers (adjusted HR 2.74; 95% CI 1.26–5.96, P = 0.01)51. Surveillance biopsies were performed at 1–2 years after prostate cancer diagnosis based on the cohorts included in the analysis51. These early results point to the potential need to include germline test results, particularly for BRCA2 mutations, in active surveillance discussions; further data are needed for definitive recommendations and current practice is evolving based on expert consensus2,52.
Prostate cancer is an important urological malignancy with increasing options for precision medicine for men with mCRPC2,4–7,53–64. Genetic results are increasingly influencing choice of therapy, such as PARP inhibitors for men with mCRPC with mutations in DNA repair genes, particularly BRCA1 and/or BRCA2 (refs4–7,53–56,62). Genetic mutations that influence treatment decisions can be from germline testing or somatic testing and, therefore, might be based on hereditary or acquired genetic mutations, respectively7.
Initially, data from phase II trials led to FDA designations for PARP inhibitors including olaparib, rucaparib and niraparib for men with mCRPC with germline or somatic alterations in DNA repair genes involved in homologous recombination repair (such as BRCA1 and BRCA2) and DNA damage signalling and checkpoint regulation (for example, ATM)53–55,61,62. In 2020, the FDA approved two PARP inhibitors, olaparib and rucaparib, for men with mCRPC with specific DNA repair defects on progression after standard lines of therapy5,6. PARP inhibitors work by using the concept of ‘synthetic lethality’, in which the co-ordinated effort of repairing single-strand DNA breaks by PARP1 and double-strand breaks by homologous recombination repair is compromised62. In individuals who carry BRCA1 or BRCA2 mutations, homologous recombination repair is defective and, therefore, PARP inhibition has clinical activity62. Rucaparib was granted accelerated approval for BRCA1-mutated or BRCA2-mutated mCRPC with previous treatment with androgen receptor-directed therapy and taxane-based chemotherapy based on demonstrated clinical responses in the TRITON2 study6. In TRITON2, overall response rates were reported to be 43.5% in men with BRCA1 or BRCA2 mutations with measurable disease (95% CI 31.0–56.7%) and PSA response rate was 54.8% (95% CI 45.2–64.1%)6. Olaparib was FDA approved for the treatment of mCRPC in men with deleterious or suspected deleterious germline or somatic homologous recombination repair gene mutations who had progressed following previous treatment with enzalutamide or abiraterone based on the PROfound study5. Men with BRCA1 or BRCA2 or ATM (cohort A) or multiple other DNA repair mutations (BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PP2R2A, RAD51B, RAD51C, RAD51D, RAD54L) (cohort B) were randomized to receive olaparib 300 mg twice a day or enzalutamide or abiraterone. Progression-free survival was significantly longer for men in cohort A treated with olaparib than treatment with abiraterone or enzalutamide (median 7.4 versus 3.6 months; P < 0.001)5. Furthermore, overall survival was also superior for men in cohort A treated with olaparib to those who received abiraterone or enzalutamide (median 19.1 versus 14.7 months, P = 0.02)64. Multiple PARP inhibitors are being studied for the treatment of prostate cancer56. These trials include PARP inhibitors alone or in combination with androgen receptor signalling inhibitors, immune checkpoint inhibitors, chemotherapy or other agents56.
Immunotherapy with pembrolizumab for prostate cancer is another area of advance in treatment for which assessment of DNA MMR deficiency is performed on tumour tissue, potentially leading to suspicion of Lynch syndrome7,57,58. The mechanism of action is complex; agents such as pembrolizumab promote tumour cell death by binding to T cell PD1 receptors and disrupting interaction with PDL1 molecules on tumour cells, enabling immune attack on a tumour57,63. In 2017, the FDA approved pembrolizumab for the treatment of all solid tumours, including prostate cancer, that exhibit MMR deficiency and/or exhibit microsatellite instability57,58. Approximately 5–10% of prostate cancers exhibit MMR deficiency and, therefore, a subset of these patients with prostate cancer might be candidates for pembrolizumab59. Clinical trials are ongoing and further data are emerging regarding additional biomarkers of response to immune checkpoint inhibitors in men with prostate cancer58,60. Pembrolizumab eligibility is based on MMR deficiency or microsatellite instability status determined in tumours, but the somatic genomic analysis results might point to the need to evaluate the germline for Lynch syndrome39.
NCCN guidelines address precision management of prostate cancer and are regularly updated7. The NCCN Prostate Cancer (Version 2.2021) guideline states that for men with mCRPC, alterations in DNA repair genes such as BRCA1, BRCA2 and ATM might indicate PARP inhibitor therapy7. This guideline further suggests consideration of MMR deficiency or microsatellite status for pembrolizumab candidacy7.
Overall, genetic information has become central to management approaches for prostate cancer, with a rising role in determining which precision medicine to choose, early-stage disease management and screening7,9. Olaparib and rucaparib are FDA approved for the treatment of men with mCRPC after progression on various standard therapies, and have unique approvals based on a spectrum of germline mutations5,6. Thus, genetic testing and genetic counselling need to be considered by urologists and oncologists for appropriate patients during the course of management or treatment.
Genetics care delivery
Traditionally, genetics care delivery has been conducted through genetic counselling by specialist genetics counsellors; however, novel approaches are also being used to improve accessibility and speed.
Genetic counselling
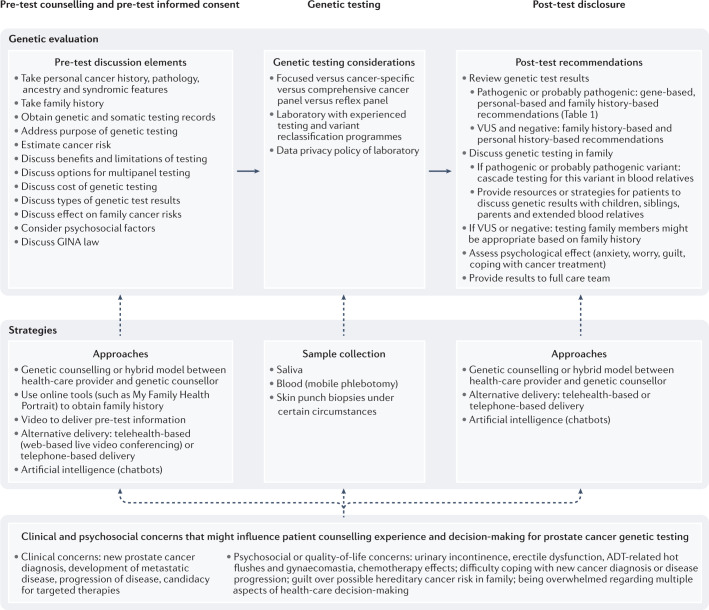
Many patients with prostate cancer or at risk of developing prostate cancer are now increasingly in need of germline testing2,7,9, necessitating pre-test informed decision-making for germline testing that addresses precision management and screening and encompasses hereditary cancer management. Genetics care delivery can occur through the traditional approach of referral to genetic counselling or, increasingly, by using hybrid approaches to handle the rising volume of patients in need of germline testing and reduce barriers to care14–17. Health-care providers and genetic counsellors need to have a working knowledge of hereditary cancer assessment, the nuances of genetic evaluation, and prostate cancer management across the stage and risk spectrum (Fig. 1; Box 1).
Fig. 1. Genetic evaluation process for patients with or at risk of developing prostate cancer.
The process and elements involved in pre-test counselling and informed consent, genetic testing and post-test disclosure. Special considerations in each step in the process and unique clinical and psychosocial concerns that can influence decision-making are shown. ADT, androgen-deprivation therapy; GINA, Genetic Information Nondiscrimination Act; VUS, variants of uncertain significance.
The generally accepted model of genetic counselling can be divided into pre-test counselling (before genetic testing) and post-test counselling (after test results return)8,10–12 (Fig. 1). The pre-test counselling visit entails gaining knowledge of a patient’s personal medical history, family history and previous genetic testing in the family8,10–12. A three-generation pedigree, including the patient’s medical history, cancers in male and female relatives, and ethnicity, is gathered at the pre-test session. This information is used to assess the patient’s risk of carrying a mutation and informs the strategy for germline testing (genes to include, panel to use, etc.) to fully assess for an associated cancer predisposition syndrome8,10–12. Some ‘red flags’ indicating a suspected hereditary cancer syndrome include personal and/or family history of cancers and other clinical features known to be associated with specific genetic syndromes, cancers diagnosed at unusually young ages, multiple blood relatives across generations diagnosed with similar or genetically linked cancers, a personal history of bilateral and/or multiple primary cancers, and an ethnic background known to increase the risk of hereditary cancer (such as Ashkenazi Jewish ancestry)8–12,28,29.
Genetic counsellors typically have an education-oriented approach to the counselling session that enables the patient to make an informed decision while addressing psychosocial concerns10. The pre-test discussion comprises multiple components, including the purpose of testing (precision therapy, surgical decision-making, risk assessment, effect on screening, cascade testing), estimates of cancer risk, risks and benefits of testing, types of test results that could be returned (pathogenic or probable pathogenic variant, VUS, negative), options for multigene panel testing, cost of genetic testing, and psychological counselling and support8,10–13. Communication of genetic risk for at-risk individuals also involves a discussion of statistical probabilities for mutation presence and implications for personal and family cancer risk. In the advanced-cancer setting, genetic counsellors need to adapt counselling models towards how genetic testing might inform options for targeted therapy and guide panel testing2,4,7,14.
Another component of pre-test counselling is discussion of the benefits and limitations of genetic testing2,8–13. Benefits of genetic testing include information for medical management, precision therapy options, identification of additional cancer risks to inform screening, and identification of at-risk family members to address cancer risks. Limitations of genetic testing include technical issues (such as the inability to detect certain genetic variants with standard testing technology), limited knowledge of cancer risk or management for some genes, identification of VUS and complex genetic findings8–13. The chances of having a VUS reported are increased when an increased number of genes are tested2,65. Furthermore, VUS rates are increased in patient populations with diverse race or ethnic backgrounds65. Additional considerations include genetic discrimination protections and gaps in protection under the 2008 Genetic Information Nondiscrimination Act (GINA)66. The GINA law provides protection related to health insurance and employment discrimination for mutation carriers in most scenarios. The GINA law does not provide protections for military health insurance and employment, life insurance, disability and long-term care insurance66. Currently, this law only applies to adults who have been tested; how the law will apply to blood relatives regarding effect on insurance might evolve over time.
In a post-test disclosure session, genetic test results are discussed with the patient along with recommendations. Genetic results are reviewed to enhance understanding of associated medical implications for patients and their family members while also addressing emotional and psychosocial needs8–13. Medical management discussion is based on genetic testing results and the patient’s specific family history. Pathogenic or probable pathogenic variants can influence medical management for urological malignancies, cancer risk recommendations and cascade testing of blood relatives2,7,9. Coordination of cancer screening for mutations can be complex, as multiple cancer risks might need to be addressed. For example, men with BRCA2 mutations without prostate cancer are usually recommended to start prostate cancer screening at the age of 40 years by their urologist or their primary-care doctor9; they are also recommended to have clinical breast examinations by their physician starting at the age of 35 years, discuss pancreatic cancer screening (if a family history of pancreatic cancer exists) with a gastroenterologist and be evaluated by a dermatologist for risk of melanoma9. These visits can be coordinated by genetic counsellors, the patient’s primary-care doctor or be the responsibility of the patient. Improved support for patients who carry mutations to have multidisciplinary screening is needed.
VUS are also reported in genetic test results; patients need to understand how to interpret these findings. VUS do not affect management at the time of reporting2,10. VUS are followed over time by genetic testing laboratories for evidence in support of or against pathogenicity67,68. A 2018 retrospective cohort study including over 1 million genetic tests showed that 7.7% of VUS results were reclassified, of which 91.2% were downgraded to benign or probably benign68. Thus, counselling sessions need to provide this information to patients and include plans to recontact patients if VUS reclassification updates occur, to provide revised recommendations as needed. For patients who receive VUS results or totally negative genetic results, referral to genetic counselling might be needed, and, if family cancer history is strong, they might need to receive comprehensive family history-based recommendations or to determine whether additional relatives need germline testing1,2,4.
Cascade testing in a family of a patient with a pathogenic variant or probable pathogenic variant (mutation) takes co-ordinated effort between a genetic counsellor, the patient, and their family. Once a patient is identified as having a pathogenic variant or probable pathogenic variant, the genetic counsellor typically needs to obtain a release of information from the patient to use this information to guide genetic testing of blood relatives. The patient needs to communicate information of their genetic test results to blood relatives, who then need to contact their local genetics services to make an appointment for genetic counselling and genetic testing. These genetic counselling sessions for relatives will include the genetic results of the proband (original patient), the relative’s medical history and family history, discussion of pre-test elements, and optimal testing approach10. The relative will next undergo genetic testing and then return for disclosure of results and recommendations based on their test results10.
Communication of genetic results and recommendations in families can be challenging. Providing resources or strategies for patients to discuss genetic results with children, siblings, parents and extended blood relatives is an important part of genetic counselling69,70. Some controversies that genetic counsellors can encounter centre around difficult family dynamics, when family members do not wish to discuss their genetic results with relatives or have little contact with family members69,70. In such cases, balancing a patient’s autonomy and confidentiality with a duty to warn relatives of the potential genetic risk becomes difficult69–71. The American Society of Human Genetics permits a provider to disclose information to a third party only under specific circumstances, such as when encouragement to disclose to family members has failed and a serious risk that is identifiable and can be prevented, treated or reduced by early monitoring exists71.
In summary, the genetic counselling process encompasses pre-test and post-test sessions, focuses on educating and aiding the patient in choosing appropriate genetic testing, and considers psychosocial concerns that might influence decision-making for genetic testing8–13. Genetic test results are not always straightforward as genetic testing technology is limited and guidelines can vary7,9,10,39. Results and implications for patients and family members are important to address, along with sharing of results with family members to enable cascade testing10.
Novel genetics care delivery approaches
In the era of expanded germline testing and precision medicine, the traditional model of referral of all patients to genetic counselling has needed to be adapted for increased access to testing, rapid return of results, and to mitigate the relative shortage of genetic counselling14–16. Adaptations to the traditional genetic counselling model have, therefore, been emerging, in which oncologists and urologists are becoming more involved in both pre-test and post-test contexts (Fig. 1; Box 1). In this hybrid, point-of-care model, the physician performs the pre-test consent and orders genetic testing. When results are returned, they review the results with the patient regarding effect on treatment and then refer a subset of patients to a genetic counsellor to discuss genetic results, provide full recommendations based on personal and family history, and discuss cascade testing or further testing in the family as indicated14,17. This approach facilitates rapid return of genetic results, but some complexities in practice need to be considered and addressed proactively (Box 1). One consideration is the time needed to perform appropriate pre-test informed consent for genetic testing. Multiple elements need to be discussed and understood by patients to make an informed decision for testing2,7,9,10. To facilitate pre-test information delivery, videos are being studied in which patients view a genetics education video to understand the genetic testing process and considerations before pursuing genetic testing72. Patient-reported outcomes in a male population undergoing prostate cancer germline testing from one study (n = 127) revealed that most men (71%) chose a pre-test video compared with genetic counselling (29%) (P < 0.001), with no negative effect on patient satisfaction, decisional conflict for genetic testing, cancer genetics knowledge or uptake of genetic testing72.
In a hybrid model, intake of a three-generation pedigree to accurately determine extent of testing might be challenging in busy clinics. One approach to increase the yield of full family history intake in the pre-test setting includes use of online tools (such as My Family Health Portrait from the Centers for Disease Control and Prevention)73 into which patients can input family history at home before their appointments. Furthermore, consideration of optimal panels for testing by ordering physicians, such as focused, guideline-based, comprehensive or reflex panels, which cover the patient’s medical history, family history and take patient preferences into account, becomes important2. In particular, reflex testing might be preferable in hybrid models to enable flexibility for upfront ordering and expanded testing based on full family history information obtained along the care pathway2. In the USA, insurance coverage can also be a limiting factor as some policies require the patient to undergo genetic counselling with a certified genetics professional before undergoing genetic testing in order for insurance to cover the cost of testing. Various countries have national or private policies that also need to be followed. Urologists or oncologists seeking to employ hybrid genetics care delivery models in their practices are encouraged to have increased working knowledge of the principles and practice of genetic counselling and genetic testing2,74. Finally, proactive development of genetics care delivery and referral criteria between health-care providers and genetic counsellors is encouraged when instituting hybrid models in practice to streamline pre-test and post-test care and optimize genetic testing14 (Box 1; Fig. 1).
To enhance access to genetic counselling, novel technology-based approaches are in use or under development. Telephone-based and telehealth-based (also called telegenetics when in the context of providing web-based video-conferencing genetic consultation) are alternative ways of disseminating genetic counselling75,76. More research is needed concerning the use of these approaches in patients with prostate cancer, but previous research in women primarily engaged in genetic counselling for HBOC reported that telephone-based genetic counselling was not inferior to standard genetic counselling for patient-reported outcomes77,78. Results of one randomized trial that included 669 women undergoing genetic counselling for HBOC showed the non-inferiority of telephone-based genetic counselling versus in-person genetic counselling for knowledge, satisfaction, decision conflict, distress and quality of life77. Results of another study showed that anxiety, cancer-specific distress, perceived personal control, and decisional conflict for genetic testing were not inferior at the 1-year follow-up point between telephone-based counselling and in-person counselling among women undergoing evaluation for HBOC78. Web-based telegenetics has also been reported to have high satisfaction rates among patients undergoing genetic counselling and genetic testing76. A randomized trial of 130 patients (90% of whom were women) undergoing cancer genetic counselling primarily for HBOC or Lynch syndrome reported no difference in patient satisfaction between telegenetics and in-person genetic counselling (P = 0.03)76. Key barriers to address include comfort with and access to technology, reimbursement, co-ordination of sample collection for genetic testing, and privacy and ethical issues. In the virtual era of the COVID-19 pandemic, the importance of remote genetics care delivery, such as telegenetic visits, and remote sample collection, such as through mobile phlebotomy, have increased79,80. Finally, chatbots are also being studied in genetic education, consent and counselling delivery81. A chatbot is a computer programme that simulates human conversations and enables communication of information by messages or voice command81. Chatbots have been developed to aid delivery of genetic testing consent, follow-up monitoring and cascade testing of family members, with patients supporting this technology from focus group data81. In one study of focus group participants enrolled in a genomics research programme in which chatbots were used, participants were supported using chatbots to consent to genomics research and to interact with health-care providers for recommendations and co-ordination of care, as well as to share genetic information with relatives81. Further research is needed among men with prostate cancer regarding utility and satisfaction with genetics care delivery models and tools for male-specific patient-reported outcomes.
Overall, with the rising volume of patients in need of genetic counselling and germline testing, current and future models of genetic counselling need to be adapted to provide responsible delivery of genetic counselling to patients on an individualized basis.
Unique aspects of genetic counselling for men with prostate cancer
With the expansion of genetic testing criteria for prostate cancer7,9, improved insight is needed in to how men process the pre-test counselling discussion, understand their genetic results and handle the anxiety or guilt of having an inherited genetic mutation. Many men might have high satisfaction with their genetic evaluation experience, but some men may have difficulty with the process. Patients might need additional resources or support to understand their genetic results. For example, results are emerging that report that some men who undergo prostate cancer genetic testing might have limited understanding of VUS82. Given that ~30% of men undergoing prostate cancer germline testing will have VUS reported1,83, this proportion represents a substantial number of men in need of reinforcement of information to enhance understanding of results and to limit propagation of misinformation in families82.
Furthermore, understanding quality-of-life issues for men dealing with prostate cancer treatment across the stage spectrum is needed when discussing germline testing. Men might be dealing with treatment-related effects, such as urinary incontinence, erectile dysfunction, fatigue, hot flushes, weight gain, gynaecomastia, or loss of libido and/or sexual dysfunction caused by treatments including prostatectomy, radiotherapy, androgen deprivation therapy or chemotherapy84–86. Men on active surveillance might fear disease progression and men with biochemical recurrence might fear disease recurrence (Fig. 1). Clinical experience shows that most of the time these issues are not barriers for men to undergo germline testing, but occasionally current quality-of-life issues might make processing genetic information difficult or overwhelming87. Difficulty coping with progressive metastatic disease, new development of metastatic disease, or a new diagnosis of prostate cancer with the multitude of treatment or management options could make informed decision-making for germline testing challenging for some men. If men initially decline germline testing, repeated offering of testing to eligible men would be encouraged. Dedicated patient-reported outcomes data in male populations undergoing genetic testing for prostate cancer are needed. A patient-driven registry (PROGRESS Registry) is currently actively recruiting men in the USA who have had prostate cancer-related germline testing to garner patient experiences to support development of resources for men and their families.
Overall, identifying and addressing the clinical, genetic and psychosocial issues for men is important for facilitating decision-making on genetic testing and for supporting men in their cancer treatment and genetic evaluation experience.
Conclusions
Genetic evaluation for men with prostate cancer or at risk of prostate cancer development is a specialized area requiring knowledge of pre-test genetic information, genetic testing options and approaches, complex hereditary cancer syndromes, and unique considerations important for patients and providers to understand. Genetic counselling, hybrid models, and technology-based approaches are essential to provide access to genetic testing for men and their families. This population of patients deserves dedicated studies of patient-reported outcomes and increased knowledge of genetics contributing to prostate cancer across diverse populations to enhance the genetic evaluation impact and experience for men and their families.
Author contributions
Both authors made substantial contributions to discussion of content, researched data for the article, wrote the manuscript and reviewed and edited the article before submission.
Peer review
Peer review information
Nature Reviews Urology thanks Olivier Cussenot, George Netto, Celestia Higano, Alexandra Sokolova, Rana McKay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
My Family Health Portrait: https://www.cdc.gov/genomics/famhistory/knowing_not_enough.htm
PROGRESS Registry: www.progressregistry.com
References
- 1.National Cancer Institute. Genetics of Prostate Cancer (PDQ®) – Health Professional Version. NIHhttps://www.cancer.gov/types/prostate/hp/prostate-genetics-pdq (2022). [PubMed]
- 2.Giri VN, et al. Implementation of germline testing for prostate cancer: Philadelphia Prostate Cancer Consensus Conference 2019. J. Clin. Oncol. 2020;38:2798–2811. doi: 10.1200/JCO.20.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaDuca H, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet. Med. 2020;22:407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng HH, et al. Germline and somatic mutations in prostate cancer for the clinician. J. Natl Compr. Cancer Netw. 2019;17:515–521. doi: 10.6004/jnccn.2019.7307. [DOI] [PubMed] [Google Scholar]
- 5.de Bono J, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 6.Abida W, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 2020 doi: 10.1200/JCO.20.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Clinical Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer (Version 2.2021). NCCNhttps://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459 (2021).
- 8.National Cancer Institute. Cancer Genetics Overview (PDQ®) — Health Professional Version. NIHhttps://www.cancer.gov/about-cancer/causes-prevention/genetics/overview-pdq (2022). [PubMed]
- 9.National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2022). NCCNhttps://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (2022).
- 10.Riley BD, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J. Genet. Couns. 2012;21:151–161. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- 11.Resta R, et al. A new definition of genetic counseling: national society of genetic counselors’ task force report. J. Genet. Couns. 2006;15:77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- 12.Uhlmann, W. R., Schuette, J. L., & Yashar, B. A Guide to Genetic Counseling 2nd edn (Wiley-Blackwell, 2009).
- 13.Schneider, K. A. Counseling about Cancer: Strategies for Genetic Counseling 3rd edn (Wiley-Blackwell, 2009).
- 14.Giri VN, Hyatt C, Gomella LG. Germline testing for men with PCA: navigating an expanding new world of genetic evaluation for precision therapy and precision management. J. Clin. Oncol. 2019;37:1455–1459. doi: 10.1200/JCO.18.02181. [DOI] [PubMed] [Google Scholar]
- 15.Hughes KS. Genetic testing: what problem are we trying to solve? J. Clin. Oncol. 2017;35:3789–3791. doi: 10.1200/JCO.2017.74.7899. [DOI] [PubMed] [Google Scholar]
- 16.Abacan M, et al. The global state of the genetic counseling profession. Eur. J. Hum. Genet. 2019;27:183–197. doi: 10.1038/s41431-018-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MA, et al. Coordinating an oncology precision medicine clinic within an integrated health system: lessons learned in year one. J. Patient Cent. Res. Rev. 2019;6:36–45. doi: 10.17294/2330-0698.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sztupinszki Z, et al. Detection of molecular signatures of homologous recombination deficiency in prostate cancer with or without BRCA1/2 mutations. Clin. Cancer Res. 2020;26:2673–2680. doi: 10.1158/1078-0432.CCR-19-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Human Genome Research Institute. Talking glossary of genetic terms. NIHhttps://www.genome.gov/genetics-glossary (2022).
- 20.Welcsh PL, King M-C. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001;10:705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard CC, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolosi P, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5:523–528. doi: 10.1001/jamaoncol.2018.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giri VN, et al. Inherited mutations in males undergoing multigene panel testing for prostate cancer — emerging implications for personalized prostate cancer genetic evaluation. J. Clin. Oncol. Precis. Oncol. 2017;1:1–17. doi: 10.1200/PO.16.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giri VN, Beebe-Dimmer JL. Familial prostate cancer. Semin. Oncol. 2016;43:560–565. doi: 10.1053/j.seminoncol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro E, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbari MR, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br. J. Cancer. 2014;111:1238–1240. doi: 10.1038/bjc.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro E, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur. Urol. 2015;68:186–193. doi: 10.1016/j.eururo.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 28.King MC, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;02:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff T, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin. Cancer Res. 2004;10:2918–2921. doi: 10.1158/1078-0432.CCR-03-0604. [DOI] [PubMed] [Google Scholar]
- 30.Abul-Husn NS, et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder variants in a diverse population-based biobank. Genome Med. 2020;12:2. doi: 10.1186/s13073-019-0691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter BS, et al. Hereditary prostate cancer: epidemiologic and clinical features. J. Urol. 1993;150:797–802. doi: 10.1016/S0022-5347(17)35617-3. [DOI] [PubMed] [Google Scholar]
- 32.Ewing CM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witte JS, et al. HOXB13 mutation and prostate cancer: studies of siblings and aggressive disease. Cancer Epidemiol. Biomark. Prev. 2013;22:675–680. doi: 10.1158/1055-9965.EPI-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laitinen VH, et al. HOXB13 G84E mutation in Finland: population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2013;22:452–460. doi: 10.1158/1055-9965.EPI-12-1000-T. [DOI] [PubMed] [Google Scholar]
- 35.Brechka H, Bhanvadia RR, VanOpstall C, Vander Griend DJ. HOXB13 mutations and binding partners in prostate development and cancer: function, clinical significance, and future directions. Genes Dis. 2017;4:75–87. doi: 10.1016/j.gendis.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond VM, et al. Elevated risk of prostate cancer among men with Lynch syndrome. J. Clin. Oncol. 2013;31:1713–1718. doi: 10.1200/JCO.2012.44.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch H, et al. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez-Valentin M, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020;22:15–25. doi: 10.1038/s41436-019-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Comprehensive Cancer Network. Clinical Guidelines in Oncology (NCCN Guidelines®): Lynch syndrome (Version 2.2021) (NCCN, 2021).
- 40.Lowrance, W et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline. AUAwww.auanet.org/guidelines/advanced-prostate-cancer (2020).
- 41.Mottet, N et al. EAU guidelines: prostate cancer. EAUhttps://uroweb.org/guideline/prostatecancer (2020).
- 42.Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J. Clin. Oncol. 2013;31:1267–1270. doi: 10.1200/JCO.2012.46.9403. [DOI] [PubMed] [Google Scholar]
- 43.Lynce F, Isaacs C. How far do we go with genetic evaluation. Am. Soc. Clin. Oncol. Educ. Book. 2016;35:e72–e78. doi: 10.1200/EDBK_160391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cancer Genetics Risk Assessment and Counseling. National Cancer Institute PDQ®. NIHhttps://www.cancer.gov/about-cancer/causes-prevention/genetics/risk-assessment-pdq (2022). [PubMed]
- 45.Oh B. Direct-to-consumer genetic testing: advantages and pitfalls. Genomics Inform. 2019;17:e33. doi: 10.5808/GI.2019.17.3.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horton R, et al. Direct-to-consumer genetic testing. BMJ. 2019;367:l5688. doi: 10.1136/bmj.l5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts JS, Ostergren J. Direct-to-consumer genetic testing and personal genomics services: a review of recent empirical studies. Curr. Genet. Med. Rep. 2013;1:182–200. doi: 10.1007/s40142-013-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page EC, et al. Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur. Urol. 2019;76:831–842. doi: 10.1016/j.eururo.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Comprehensive Cancer Network Clinical Guidelines in Oncology (NCCN Guidelines®): prostate cancer early detection (Version 2.2020) (NCCN, 2020).
- 50.Bancroft EK, et al. A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study. Lancet Oncol. 2021;22:1618–1631. doi: 10.1016/S1470-2045(21)00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter HB, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur. Urol. 2019;75:743–749. doi: 10.1016/j.eururo.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loeb S, Giri VN. Clinical implications of germline testing in newly diagnosed prostate cancer. Eur. Urol. Oncol. 2021;4:1–9. doi: 10.1016/j.euo.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Mateo J, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abida W, et al. Preliminary results from TRITON2: a phase 2 study of rucaparib in patients with mCRPC associated with homologous recombination repair gene alterations. Ann. Oncol. 2018;29(Suppl. 8):viii271–viii302. [Google Scholar]
- 55.Smith MR, et al. Pre-specified interim analysis of GALAHAD: a phase II study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD) Ann. Oncol. 2019;30(Suppl. 5):V884–V885. doi: 10.1093/annonc/mdz394.043. [DOI] [Google Scholar]
- 56.Virtanen V, et al. PARP inhibitors in prostate cancer — the preclinical rationale and current clinical development. Genes. 2019;10:565. doi: 10.3390/genes10080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graff JN, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–52817. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng HH. The resounding effect of DNA repair deficiency in prostate cancer. Urol. Oncol. 2018;36:385–388. doi: 10.1016/j.urolonc.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antonarakis ES, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013;5:a012716. doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mateo J, et al. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019;30:437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyiadzis MM, et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer. 2018;6:35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain M, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;383:2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 65.Caswell-Jin JL, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet. Med. 2018;20:234–239. doi: 10.1038/gim.2017.96. [DOI] [PubMed] [Google Scholar]
- 66.U.S. Government Publishing Office Genetic Information Nondiscrimination Act of 2008 Public Law 110–233, 122 Stat. 881 (2008).
- 67.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mersch J, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA. 2018;320:1266–1274. doi: 10.1001/jama.2018.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallo AM, Angst DB, Knafl KA. Disclosure of genetic information within families. Am. J. Nurs. 2009;109:65–69. doi: 10.1097/01.NAJ.0000348607.31983.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly MB. A family-centered model for sharing genetic risk. J. Law Med. Ethics. 2015;43:545–551. doi: 10.1111/jlme.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perry TJ, et al. The duty to warn at-risk relatives — the experience of genetic counselors and medical geneticists. Am. J. Med. Genet. 2019;182:314–321. doi: 10.1002/ajmg.a.61425. [DOI] [PubMed] [Google Scholar]
- 72.Russo J, et al. Pretest genetic education video vs. genetic counseling for men considering prostate cancer germline testing: a patient-choice study to address urgent practice needs. J. Clin. Oncol. Precis. Oncol. 2021;5:PO.21.00238. doi: 10.1200/PO.21.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention. My Family Health Portrait: a tool from Surgeon General. CDChttps://phgkb.cdc.gov/FHH/html/index.html (2022).
- 74.Mark JR, McDougall C, Giri VN. Genetic testing guidelines and education of health care providers involved in prostate cancer care. Urol. Clin. North. Am. 2021;48:311–322. doi: 10.1016/j.ucl.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. Am. J. Med. Genet. 2018;178C:24–37. doi: 10.1002/ajmg.c.31602. [DOI] [PubMed] [Google Scholar]
- 76.Buchanan AH, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: cost, patient satisfaction and attendance. J. Genet. Couns. 2015;24:961–970. doi: 10.1007/s10897-015-9836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwartz MD, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J. Clin. Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinney AY, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow-up. J. Clin. Oncol. 2016;34:2914–2924. doi: 10.1200/JCO.2015.65.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gadzinski AJ, Ellimoottil C. Telehealth in urology after the COVID-19 pandemic. Nat. Rev. Urol. 2020;17:363–364. doi: 10.1038/s41585-020-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mauer C, et al. Adapting genetic counseling operations amidst the COVID-19 pandemic. J. Genet. Couns. 2021;30:949–955. doi: 10.1002/jgc4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidlen T, Schwartz M, DiLoreto K, Kirchner HL, Sturm AC. Patient assessment of chatbots for the scalable delivery of genetic counseling. J. Genet. Couns. 2019;28:1166–1177. doi: 10.1002/jgc4.1169. [DOI] [PubMed] [Google Scholar]
- 82.Giri VN, et al. Understanding of multigene test results among males undergoing germline testing for inherited prostate cancer: implications for genetic counseling. Prostate. 2018;78:879–888. doi: 10.1002/pros.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giri VN, et al. Germline genetic testing for inherited prostate cancer in practice: implications for genetic testing, precision therapy, and cascade testing. Prostate. 2019;79:333–339. doi: 10.1002/pros.23739. [DOI] [PubMed] [Google Scholar]
- 84.Zeliadt SB, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106:1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 85.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 86.Sharpley CF, Birsika V, Denham JW. Factors associated with feelings of loss of masculinity in men with prostate cancer in the RADAR trial. Psychooncology. 2014;23:524–530. doi: 10.1002/pon.3448. [DOI] [PubMed] [Google Scholar]
- 87.Bester J, Cole C, Kodish E. The limits of informed consent for an overwhelmed patient: clinicians’ role in protecting patients and preventing overwhelm. AMA J. Ethics. 2016;18:869–886. doi: 10.1001/journalofethics.2016.18.9.peer2-1609. [DOI] [PubMed] [Google Scholar]
- 88.Karam R, et al. Assessment of diagnostic outcomes of RNA genetic testing for hereditary cancer. JAMA Netw. Open. 2019;2:e1913900. doi: 10.1001/jamanetworkopen.2019.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanda, M. G. et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline (2017). AUAhttps://www.auanet.org/guidelines/guidelines/prostate-cancer-clinically-localized-guideline (2017).