Abstract
Background
Autistic spectrum disorder encompasses a wide variety of behavioural and communicative problems. Both the core features and non‐core features of autism have been targeted in a variety of therapies. Atypical antipsychotic medications, including risperidone, have been used for symptom and behaviour improvement and have shown beneficial outcomes, particularly in certain aspects of the disorder. However, given the nature of the condition presenting in young patients, the risks of these potentially long term therapies must be weighed against the benefits.
Objectives
To determine the efficacy and safety of risperidone for people with autism spectrum disorder.
Search methods
Electronic databases: CENTRAL (Cochrane Central Register of Controlled Trials) 2006 (Issue 3); MEDLINE (1966 to April 2006); EMBASE (1980 to April 2006); PsycINFO (1887 to April 2006); CINAHL (1982 to April 2006); LILACS (1982 to April 2006 ); Clinicaltrials.gov (USA) (accessed April 2006); ZETOC (1993 to April 2006); National Research Register (NRR) (UK) 2006 (Issue 1) were searched. In addition further data were retrieved through contact with pharmaceutical companies and authors of published trials.
Selection criteria
All randomised controlled trials of risperidone versus placebo for patients with a diagnosis of autism spectrum disorder. All trials had to have at least one standardised outcome measure used for both intervention and control group.
Data collection and analysis
Data were independently evaluated and analysed by the reviewers. Data were evaluated at the end of each randomised controlled trial. Unpublished data were also considered and analysed.
Main results
Only three randomised controlled trials were identified. Meta‐analysis was possible for three outcomes. Some evidence of the benefits of risperidone in irritability, repetition and social withdrawal were apparent. These must however be considered against the adverse effects, the most prominent being weight gain.
Authors' conclusions
Risperidone can be beneficial in some features of autism. However there are limited data available from studies with small sample sizes. In addition, there lacks a single standardised outcome measure allowing adequate comparison of studies, and long‐term followup is also lacking. Further research is necessary to determine the efficacy pf risperidone in clinical practice.
Plain language summary
Risperidone for autism spectrum disorder
Risperidone is an antipsychotic medication that has been used for symptom relief and behavioural improvement in autism. This review encompasses three randomised controlled trials and concludes that risperidone may be beneficial for various aspects of autism including irritability, repetition and hyperactivity. However, all studies were relatively small and used different ways to assess effectiveness, making comparisons difficult. In addition, side effects were identified, notably weight gain. Further studies are therefore necessary to determine the long term benefits, if any, compared with the potential risks.
Background
Description of the condition
Autism spectrum disorder
Described for the first time in 1943 by Leo Kanner infantile autism was characterised by inability to relate to people, speech development failure and abnormal responses to environmental objects and events (Kanner 1943). Today autism is defined by three core features; abnormal interaction, communication impairment and stereotyped behaviours with limited activities and interests.
There is no universal classification for autism spectrum disorder (ASD) with diagnoses made using a number of instruments (eg, ADOS (Lord 1989), ADI‐R (Lord 1994) , DISCO (Wing 1999), CARS (Schopler 1988), Developmental, Dimensional and Diagnostic Interview (3di) (Skuse 2004)), or by using established diagnostic criteria (ICD‐10 (WHO 1993), DSM‐IV (APA 1994)). Conditions included in the autism spectrum are Childhood autism or autistic disorder, Pervasive Developmental Disorder ‐ Not Otherwise Specified (PDD‐NOS), Asperger Syndrome and Atypical autism. In addition ICD‐10 categories of other childhood disorders of social functioning, specified (F94.8) and unspecified (F94.9), and other codes for behaviour and language disturbances may also be relevant.
The largest study to date reported a prevalence of 1.1 per 1000 children with full syndrome autism (Croen 2002). Autism is approximately four times more common in boys (Fombonne 1999). The aetiology of autism is unknown, possibly genetic, with most cases having no identifiable underlying condition (Lissauer 2002). Problems usually present in early childhood and continue throughout life. Autistic children have a triad of difficulties (social relationships, social language and communication skills) evident before the age of 3 years and about two‐thirds have a general learning disability (Lissauer 2002). Non‐core features of the condition may present with serious behavioural disturbances such as self‐injurious behaviour, aggression and tantrums.
Description of the intervention
Management Options for Autism
Despite the regular claims for curative interventions, there is no specific treatment for autism and therapies target the symptoms of autism. Given the devastating nature of the condition, parents are often keen to try any intervention available. The heterogeneity of the symptoms means that therapies should be tailored to the individual. The varying age at which ASD is diagnosed, and subsequent timing of delivery of intervention, are important. Early detection, and interventions (which may include education for parents, behavioural therapy and /or pharmacological management) may all play a role in improving outcomes (Bryson 2003).
Most behavioural treatment programs for children with autism include clear instructions to the child, prompting him or her to perform specific behaviours, within a framework of gradually increasing reinforcement as target tasks are made more complex. Pharmacological interventions have been used as adjuncts to behavioural treatments in both children and adults, and may reduce specific autistic symptoms and behaviours (such as self‐injury or aggression) which may interfere with the success of behavioural treatments (Posey 2001). Pharmacological treatments available include antipsychotics, selective serotonin reuptake inhibitors, stimulants, antihistamines and anxiolytics. Potential benefits include improvements in sleep disturbance, mood disorder, concentration/attention and self‐harm or aggression (Gringas 2000).
Atypical Antipsychotics
Antipsychotic drugs generally tranquillise without impairing consciousness and relieve psychotic symptoms (BNF 2003). Atypical antipsychotics are termed 'atypical' because of their tendency to cause fewer unwanted motor side effects than the typical antipsychotics. Examples of this class of drug include amisulpride, clozapine, olanzapine, quetiapine and risperidone. They are a mainstay treatment for schizophrenia and other psychoses. They act by blocking dopamine receptors (D2) in the brain, and may also affect cholinergic, alpha‐adrenergic, histaminergic and serotonergic receptors.
Autism and risperidone
Antipsychotic drugs are the most frequently prescribed psychoactive agent used in autism (Campbell 1999). Typical antipsychotics, specifically haloperidol, have been found to be useful in reducing motor stereotypies, hyperactivity, temper tantrums and improving social relatedness (Anderson 1984). Extrapyramidal side effects have limited the use of these drugs (Campbell 1997), resulting in the introduction of the atypical antipsychotics.
Why it is important to do this review
Risperidone has been the most widely used atypical antipsychotic in autism. In one open trial of risperidone in children with autism, the authors concluded that risperidone may be effective for ameliorating dysfunctional behaviours in children with autistic spectrum (Findling 1997). Another study which looked at olanzapine suggested that the drug improved language use and response after eight and 12 weeks of treatment (Potenza 1999).
Atypical antipsychotics can have quite different pharmacodynamic properties and different adverse effects. This review set out to evaluate the efficacy and potential harms of risperidone in people with autism spectrum disorder.
Objectives
To determine the efficacy and safety of risperidone in the treatment of autism spectrum disorder.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with at least one standardised measure (eg a behaviour checklist) used for the intervention and control group.
Types of participants
Participants of any age with a diagnosis of disorder on the autism spectrum using either a standardised diagnostic instrument or established diagnostic criteria..
Types of interventions
Risperidone, any dose and duration, by any means of delivery. The control group had to be a placebo.
Types of outcome measures
1. Core features of autism such as social interaction, communication and behavioural problems including stereotypy or obsessional behaviour; 2. Non‐core behaviours such as sleep disturbance, self‐mutilation, aggression, attention and concentration problems, and gastrointestinal function 3. Global impression of health 4. Adverse events 5. Quality of life for the individual or their carers 6. Economic outcomes
Outcomes were divided into short‐term (less than three months) medium term (three‐12 months) and long term (over one year).
Types of measures 1. Standardised diagnostic assessment instruments (eg Aberrant Behaviour Checklist (Aman 1986), Autism Behaviour Checklist (Krug 1980), Childhood Autism Rating Scale (Schopler 1988), Autism Diagnostic Interview‐Revised (Lord 1994), Autism Diagnostic Observation Schedule (Lord 1989), Diagnostic Interview for Social and Communication Disorders (Wing 1999)) 2. Standardised communication assessments 3. Quality of life questionnaires 4. Behaviour scales 5. Global Impression Rating Scales 6. Other Health Outcome Rating Scales
Search methods for identification of studies
Relevant trials were identified through searching the following databases:
CENTRAL (Cochrane Central Register of Controlled Trials) 2006 (Issue 3) MEDLINE, accessed through OVID (1966 to April 2006) EMBASE, accessed through OVID (1980 to April 2006) PsycINFO, accessed through Silverplatter (1887 to April 2006) CINAHL, accessed through OVID (1982 to April 2006) LILACS (1982 to April 2006) Clinicaltrials.gov (USA) (July 2005) ZETOC (1993 to April 2006) National Research Register (NRR) (UK) 2006 (Issue 1) ERIC, accessed through DataStar (1966 to April 2006)
The search strategy was devised to capture trials both for this review and for future reviews on other antipsychotics and ASD. Search terms for MEDLINE were as follows:
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trials.sh. 4. random allocation.sh. 5. double blind method.sh. 6. single‐blind method.sh. 7. or/1‐6 8. (animal not human).sh. 9. 7 not 8 10. clinical trial.pt. 11. exp clinical trials/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. Placebos.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. research design.sh. 18. or/10‐17 19. 18 not 8 20. 19 not 9 21. comparative study.sh. 22. exp evaluation studies/ 23. follow up studies.sh. 24. prospective studies.sh. 25. (control$ or prospectiv$ or volunteer$).ti,ab. 26. or/21‐25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. (substituted adj benzamide$).mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 31. amisulpiride.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 32. antipsycho$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 33. anti‐psycho$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 34. exp Antipsychotic Agents/ 35. beclamide.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 36. benperidol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 37. benzamide.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 38. butyrophenone$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 39. chlorpromazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 40. clozapine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 41. dibenzoxazepine$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 42. diphenylbutylpiperidine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 43. dipiperone.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 44. dixyrazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 45. droperidol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 46. eltoprazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 47. flupenthixol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 48. flupentixol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 49. fluphenazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 50. haloperidol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 51. loxapine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 52. methotrimeprazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 53. milenperone.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 54. neuroleptic$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 55. olanzapine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 56. oxypertine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 57. penfluridol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 58. pericyazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 59. perphenazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 60. phenothiazine$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 61. pimozide.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 62. pipamperone.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 63. pipothiazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 64. pipotiazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 65. prochlorperazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 66. promazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 67. prothipendyl.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 68. quetiapine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 69. risperidone.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 70. sertindole.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 71. sulpiride.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 72. thioridazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 73. thioxanthene$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 74. tranquil$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 75. trifluoperazine.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 76. zuclopenthixol.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 77. autistic disorder.mp. or exp Autistic Disorder/ 78. autis$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 79. kanner$.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 80. (childhood adj3 schizophren$).mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 81. (speech adj3 disorder$).mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 82. (language adj3 delay$).mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 83. pdd.mp. (mp=title, original title, abstract, name of substance, mesh subject heading) 84. exp Child Development Disorders, Pervasive/ 85. 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 86. zotepine.mp. 87. aripiprazole.mp. 88. ziprasidone.mp. 89. atypical antipsychotic$.mp. 90. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 86 or 87 or 88 or 89 91. 29 and 85 and 90
Search terms were altered where necessary, to meet the requirements of individual databases. The optimally sensitive search strategy for randomised controlled trials, developed for the Cochrane Collaboration (Higgins 2005), was combined with medical subject headings and text words specific for antipsychotics and autism and pervasive development disorders. Search terms were modified to meet the requirements of individual databases regarding differences in fields and syntax. The aim of the search strategy was for high precision and recall. There were no language restrictions. Authors of retrieved papers were contacted for ongoing or unpublished work (Shea 2004b, Shea 2005, Scahill 2005a, Scahill 2005b, Zarcone 2005), as were relevant pharmaceutical companies (S‐Synthelabo 2004, Novartis 2004, Eli Lilley 2004, Astra Zeneca 2004, Orion Pharma 2004, Lundbreck 2004, Janssen‐Cilag 2004). We searched data bases designed to identify grey literature and ongoing studies including ZETOC, ClinicalTrials.gov (USA) and the National Research Register (NRR UK).
Data collection and analysis
Selection of studies
Titles and abstracts identified in the search were considered independently by two authors (OJ, MA). Full text articles were retrieved for all that appeared to meet the inclusion criteria. These were then independently assessed by the two authors (OJ, MA). Disagreement was resolved by discussion with the third author (EC) and articles that did not fulfil inclusion criteria were excluded.
Data extraction and management
Data were organised using Review Manager 4.2. Data extraction forms were developed a priori and included information regarding methods, participant details, dose and frequency of administration, other concurrent interventions and/or health problems, and outcomes. Data were independently extracted by two reviewers (OJ,MA).
Assessment of risk of bias in included studies
Quality Assessment
Two independent reviewers (OJ, MA) evaluated included studies for methodological quality and appropriateness. Two reviewers (OJ, MA) independently assigned each selected study to quality categories described in the Cochrane Collaboration Reviewer's Handbook (Higgins 2005). The following relates to allocation concealment alone:
(A) indicates adequate concealment of the allocation (for example, by telephone randomisation, or use of consecutively numbered, sealed, opaque envelopes); (B) indicates uncertainty about whether the allocation was adequately concealed (for example, where the method of concealment was not known); (C) indicates that the allocation was definitely not adequately concealed (for example, open random number lists or quasi‐randomisation such as alternate days, odd/even date of birth, or hospital number).
The reviewers (OJ, MA) also assessed the extent to which both participants and outcome assessors were blind to the allocation status of participants. The blinding was recorded as 'met', 'unmet' or 'unclear'. The use of intention‐to‐treat was also documented (see below).
Measures of treatment effect
Binary data
Meta‐analysis of binary data was conducted and results expressed as relative risk with a 95% confidence interval. A random effects model was used.
Continuous data
Differences in means were calculated using a 95% confidence interval. A random effects model was used.
Dealing with missing data
Loss to follow up and dropouts were assessed for each included study and reported in the review. In addition missing outcome data were noted.
Assessment of heterogeneity
Heterogeneity was assessed in the using the I2 measure (Higgins 2002) and the Chi squared test of heterogeneity as well as visual inspection of the graph. Due to heterogeneity in the results, random effects analysis was undertaken. Clinical heterogeneity was discussed in the text of the review.
Assessment of reporting biases
A funnel plot was not considered relevant due to an insufficient number of studies. Should more studies be identified at the next update, we plan to reconsider issues around any relationship between effect size and study precision, as such a relationship could have been due to publication or related biases or due to systematic differences between small and large studies. If a relationship is identified in future, clinical diversity of the studies will be further examined as a possible explanation. (Egger 1997). Every attempt has been made, and will in future be made, to obtain unpublished data.
Data synthesis
Meta‐analyses were performed for three outcomes: ‐ Clinical Global Impression Scale (CGI) ‐ where data were available, represented as 'numbers of participants improved/ significantly improved', in all three included studies (McDougle 1998, RUPP 2002, Shea 2004); ‐ 'Aberrant Behavior Checklist' (ABC) between the two trials which utilised the scale (RUPP 2002 and Shea 2004). ‐ weight gain
Subgroup analysis and investigation of heterogeneity
A sufficient number of studies was not retrieved and thus subgroup analyses were not performed.
Sensitivity analysis
Sensitivity analyses to assess the impact of study quality on results of meta‐analysis were inappropriate, as insufficient data were identified.
Results
Description of studies
Results of the search
580 citations were found using the search strategy used in June 2004, of which 24 qualified for further inspection. One investigator responded to the authors' request for unpublished trials with information concerning further data (Zarcone 2005). One study (RUPP 2002) produced two additional papers (McDougle 2005, Arnold 2003). Searches were rerun in April 2006 at which time 180 citations were found of which 8 required further inspection. Of these seven were randomised controlled trials, of which three were eligible for inclusion within the review.
Included studies
The three studies included within this review were all described as both "randomised" and "double blind" (McDougle 1998, RUPP 2002, Shea 2004b) . The participant groups were adults (McDougle 1998), 5‐17 year olds (RUPP 2002), 5‐12 year olds (Shea 2004b) with a diagnosis of autism spectrum disorder or other pervasive developmental disorders based on DSM IV criteria (APA 1994). The numbers of participants in the studies were small (ranging from 31 to 101) and included people of both sexes.
Interventions
The intervention was risperidone and permitted comparison groups comprised placebo only. Risperidone can be found in the British National Formulary 2005 and is a licensed indication for acute and chronic psychoses and mania (BNF 2005).
Outcomes
In the three included studies, the primary outcome was improvement in common symptoms of autism spectrum disorder, as per inclusion criteria for this review. A variety of validated and unvalidated rating scales were used, but each study included at least one validated measure. Outcome measures used are summarised below and are also listed in Table 1:
1. Scales used in the review.
| Scale / measure | McDougle 1998e | RUPP 2002 | Shea 2004 |
| Clinical Global Impression Scale (CGI) | Yes | Yes | Yes |
| Yale Brown Obsessive Compulsive Scale (Y‐BOCS) | Yes | Yes‐ RUPP 2005 | |
| Ritvo‐Freeman Real Life Rating Scale | Yes | Yes‐ RUPP 2005 | |
| Aberrant Behavior Checklist (ABC) | Yes | Yes | |
| Nisonger Child Behavior Rating Form (N‐CBRF) | Yes | ||
| Vineland Adaptive Behavior Scale | Yes‐ RUPP 2005 | ||
| Self‐injurious Behavior Questionnaire (SIB‐Q) | Yes | ||
| Visual Analog Scale‐ Clinician Rated (mood scale) | Yes | ||
| Visual Analog Scale‐Parent rated (most troublesome symptom) | Yes |
Validated scales
1. Clinical Global Impression Scale ‐CGI scale (Guy 1976)
This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven point scoring system is usually used with low scores showing decreased severity. Seven indicates 'very much worse', four 'no change' and one is 'very much improved.'
2. The Yale‐Brown Obsessive Compulsive Scale ‐ Y‐BOCS (Goodman 1989a, Goodman 1989b)
The Y‐BOCS (modified) is a measure of obsessions and compulsions. Each of the ten items is scored on a five point scale from 0 indicating "least symptomatic'" to 4 indicating "most symptomatic", so that the total Y‐BOCS score ranges from 0 to 40. The first five items of the Y‐BOCS are designed to assess the severity of repetitive thoughts, whereas the last 5 items determine the severity of repetitive behaviour.
3. Ritvo‐Freeman Real Life Rating Scale (Freeman 1986)
This is an observational measure of a variety of symptoms of autism comprising of 5 subscales: (I) sensory motor behaviours (e.g. hand flapping, rocking), (II) social relationship to people (e.g. appropriate responses to interaction attempts, initiating appropriate physical interactions), (III) affectual reactions (e.g. abrupt changes in affect, crying, temper outbursts), (IV) sensory responses (e.g. agitated by noises, rubbing surfaces, sniffing self or objects) and (V) language (e.g. communicative use of language, initiating appropriate verbal communication. Each is scored on a four point scale with 0 indicating "never" and 3 "almost always". A higher score indicates greater severity of autistic symptoms.
4. Aberrant Behavior Checklist‐ ABC (Aman 1986)
Consists of 58 items subdivided amongst 5 scales: irritability, lethargy and social withdrawal, stereotypic behaviour, hyperactivity/non compliance, and inappropriate speech. A score for each item ranged from 0 indicating "no problem" to 3 indicating "severe problem".
5. Nisonger Child Behavior Rating form ‐ N‐CBRF (Tasse 1996)
This consists of 60 items subdivided among 6 scales: conduct problem, insecure/anxious, hyperactive, self injury/stereotypic, self isolated/ritualistic, and overly sensitive. A score for each item ranged from 0 indicating "no problem" to 3 indicating "severe problem".
6. Vineland Adaptive Behavior Scales (Sparrow 1984)
The maladaptive behavior domain is divided into two subscales. Part I consists of 27 items, including mood and anxiety‐related symptoms along with hyperactivity, lying, cheating, bed‐wetting and others. Part II consists of nine items, with more severe symptoms such as psychosis, self‐injury, and property destruction. Each item is scored on a three point scale where 0 indicates "no, never", 1 indicates "sometimes or partially" and 2 signifies "yes, usually". Therefore the range of scores for part 1 is 0‐54 and the range for part 2 is 0‐18, and the range for the total score is 0‐72.
7. Self Injurious Behaviour Questionnaire ‐ SIB‐Q (Gualtieri 2002)
This is a 25‐item clinician‐ rated instrument developed by C. Thomas Gualtieri that assesses self injurious behaviour, physical aggression towards others, destruction to property and other maladaptive behaviour. Patients can receive a score of 0 indicating "not a problem" to 4, "severe problem" on each of the 25 items (range for total score 0 to 100).
Unvalidated scales
1. Visual Analog Scale (VAS) (used to measure clinicians' ratings of participants' behaviour)
A visual analog scale (VAS) was used in one included study (McDougle 1998). It was designed to help clinicians assess different mood states on a VAS scored on 100mm line where 0 indicates "not at all" and 100 indicates "most ever". The ten items included "anxious or nervous", "calm", "depressed", "eye contact", "happy", "irritable", "restless", "social interaction", "talkative" and "tired". This scale was not validated.
2. Visual Analog Scale (VAS) (used to measure parent/carer's ratings of severity of participants' 'troublesome' symptoms)
Shea 2004 used a visual analog scale for the parents. At the baseline visit the parent reported which symptom was the most troublesome. The severity of that symptom was recorded with a vertical mark on a 100‐mm line, with lower scores indicating a better condition.
3. Parent‐derived target symptoms scale
In RUPP 2002 parent defined symptoms eg tantrums, aggression and hyperactivity were rated by blinded clinical judges on a nine point scale. 1= normalised, 5=unchanged, 9=disastrous. The lower the score, the larger the improvement.
Excluded studies
34 studies were excluded, two of which were randomised (Hellings 2001; Zarcone 2005) but did not use the appropriate diagnostic inclusion criteria for our study. Two studies were excluded because they were run as open‐label studies which developed into randomised discontinuation studies Troost 2005, RUPP 2005. It was common for studies to be excluded because many were case studies and not randomised controlled studies (see Table of Excluded Studies for further information).
Risk of bias in included studies
See also 'Table of Characteristics of Included Studies'.
Sequence generation
Randomisation for the all three included studies was judged to be adequate. Where there was any doubt the authors were contacted (Shea 2005, Scahill 2005a) and were able to give more information. One study (RUPP 2002) produced a paper describing the methodology in more detail (Scahill 2001).
Blinding
In all three studies both the participants and those delivering the drug were blinded. The outcome assessors were either the parents or the clinicians or both and were thus also blinded. Therefore, all trials were assessed as adequate for this criterion.
Attrition/Intention‐to‐treat
All studies gave details of attrition and all reported using statistical methods to account for missing data.
For RUPP 2002, 21 of 101 children withdrew from the study early. Three children in the risperidone group withdrew as they did not find the treatment effective; one in the placebo group had a seizure and the rest withdrew from lack of efficacy (twelve), withdrawal of consent (one), nonadherence (one). Data were missing for a further three at followup (three). In Shea 2005 (n = 79) seven children withdrew, two from the treatment group due to adverse effects and the other due to insufficient response, and five from the placebo group: one because of adverse effects, two from 'insufficient response' and two following the withdrawal of consent. In McDougle 1998 (n= 31) seven did not complete the trial: one was withdrawn for agitation in the treatment group and four for agitation in the placebo group. One patient in the treatment group developed abnormal gait after four weeks and another had lack of significant improvement in symptoms.
All studies reported using intention to treat analyses to adjust for withdrawal of participants: McDougle and colleagues by using the method of 'last observation carried forward' (McDougle 1998); likewise Shea et al reported that : 'The primary population for safety assessments was the intention‐to‐treat (ITT) population, defined as all randomised subjects who received at least one dose of study medication. The primary population for efficacy assessments was the ITT‐efficacy population, defined as all randomised subjects who received at least one dose of study medication and for whom there was at least 1 post‐baseline efficacy assessment. The primary efficacy parameter was the change in irritability from baseline to study endpoint (e.g. the last observation...') (Shea 2004). Authors of RUPP 2002 reported simply that 'Data were analyzed according to the intent‐to‐treat principle'.
Sample size/ power calculation
Sample sizes varied but were small. The smallest sample size was 31 (McDougle 1998), and the largest study contained 101 participants (RUPP 2002).
Effects of interventions
Due to the small number of studies meeting the inclusion criteria for this review, the heterogeneity in populations (adults and children), the wide range of instruments used to measure outcomes, and the variety in presentation of results, meta‐analysis was possible within the current version of this review for three outcomes only (the ABC, the CGI, and weight gain). Most findings are therefore presented narratively, in the order of outcomes outlined in the protocol.
1. Core features of autism (social interaction and behavioural problems and stereotypy or obsessional behaviour)
The Yale‐Brown Obsessive Compulsive Scale ‐ Y‐BOCS (Goodman 1989a, Goodman 1989b) In McDougle 1998 the authors reported that repetitive behavior reduced with the use of risperidone. This effect began at week 4 and after 12 weeks the Y‐BOCS score for the risperidone group had reduced from 16.15 (SD 3.58) to 12.77 (SD 3.63) (p< 0.01). In the placebo group the change was minimal, with a mild worsening of symptoms ‐ 14.29 (SD 3.50) to 14.35 (SD 3.02) (p< 0.01). In McDougle 2005 (which reported further results from RUPP 2002) only the compulsion subscale was used. The mean score changed from 15.51 (SD 2.73) to 11.65 (SD 4.02) in the risperidone group compared to 15.18 (SD 3.88) to 14.21 (SD 4.81) in the placebo group. No significance values were reported.
Ritvo‐Freeman Real Life Rating Scale (Freeman 1986)
On the five aspects of this scale used in the McDougle study (McDougle 1998) there was statistically significant improvement in sensory motor behaviors (subscale I) and affectual reactions (subscale III). In subscale I the risperidone group reduced in score from 0.79 (SD 0.65) to 0.38 (SD 0.38) versus the placebo group which changed from 0.71 (SD 0.58) to 0.64 (SD 0.49) (p< 0.004). In subscale III affectual reaction symptoms were reduced from 1.02 (SD 0.39) to 0.35 (SD 0.37) in the risperidone group, whereas the placebo group changed from 0.78 (SD 0.49) to 0.82 (SD 0.57) (p < 0.001).
Other symptoms such as social relationship to people, sensory responses and language did not show significant improvement and the results for these were not published.
In the second analysis of the RUPP study (RUPP 2002; McDougle 2005) the investigators modified the scale to allow parents to rate the items. Just as in the McDougle 1998 study, there were improvements in the subscales of subscale I and III, and also subscale IV. A meta‐analysis was not performed as the two studies used different observers to measure change in the patients' scores (clinicians vs. parents). In the McDougle 2005 re‐analysis, further results were reported for outcomes not discussed in previous papers. In subscale I of the Ritvo‐Freeman RLRS, the risperidone group changed from 1.0 (SD 0.52) to 0.59 (SD 0.42), compared with placebo which changed from 0.93 (SD 0.58) to 0.91 (SD 0.6) (p = 0.002). In subscale II risperidone showed an improvement from 0.6 (SD 0.43) to 0.15 (SD 0.42) compared with 0.72 (SD 0.43) to 0.46 (SD 0.52) in the placebo group (n.s). In subscale III the baseline for the risperidone group was 1.68 (0.64) changing to 0.88 (SD 0.56) compared with placebo 1.84 (SD 0.64) to 1.6(0.71) (p < 0.001). In subscale IV risperidone improved sensory responses from 1.13 (SD 0.53) to 0.60 (SD 0.38) compared to placebo 1.21(SD 0.53) to 1.07 (SD 0.54) (p = 0.004). In subscale V the change was smaller, from 0.28 (SD 0.38) to 0.03(SD 0.29) compared with the placebo 0.46 (SD 0.42) to 0.34 (SD 0.41) (non‐significant).
Aberrant Behavior Checklist‐ ABC (Aman 1986)
For this outcome, meta‐analysis was possible as both Shea 2004 and RUPP 2002 used this measure throughout five subscales, albeit in different forms: Shea 2005 reporting changes scores and RUPP 2002, end point data. These were combined in a meta‐analysis using a random effects model and had the following results:
ABC Subscale 01: Irritability
Results for the ABC subscale concerning irritability yielded a mean score on treatment of 8.09 lower than on control (95% CI = ‐12.99, ‐3.19). The I2 for this outcome is = 77.7% (Analysis 2.1).
2.1. Analysis.
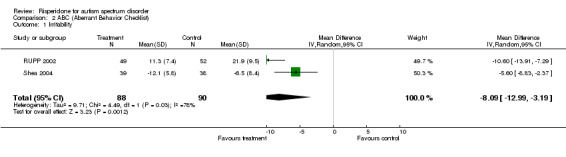
Comparison 2 ABC (Aberrant Behavior Checklist), Outcome 1 Irritability.
ABC Subscale 02: Social withdrawal/ Lethargy
Results for the ABC subscale concerning social withdrawal/ lethargy yielded a mean score on treatment of 3.00 lower than on control (95% CI = ‐5.03, ‐0.97). The I2 = 0% (Analysis 2.2).
2.2. Analysis.
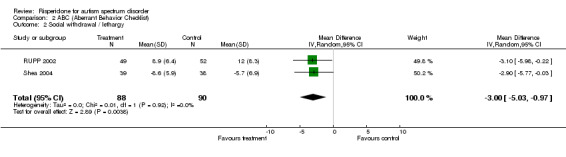
Comparison 2 ABC (Aberrant Behavior Checklist), Outcome 2 Social withdrawal / lethargy.
ABC: Subscale 03: Hyperactivity
Results for the ABC subscale concerning hyperactivity yielded a mean score on treatment of 8.98 lower than on control (95% CI = ‐12.01, ‐5.94). The I2 = 19.80% (Analysis 2.3).
2.3. Analysis.
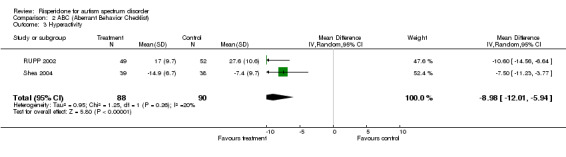
Comparison 2 ABC (Aberrant Behavior Checklist), Outcome 3 Hyperactivity.
ABC: Subscale 04: Stereotypy
Results for the ABC subscale concerning stereotypy yielded a mean score on treatment of 1.71 lower than on control (95% CI = ‐2.97, ‐0.45). The I2 = 0% (Analysis 2.4).
2.4. Analysis.
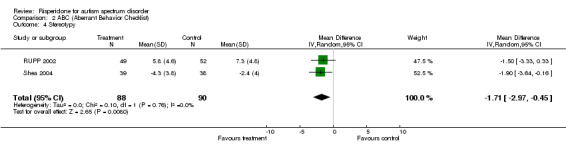
Comparison 2 ABC (Aberrant Behavior Checklist), Outcome 4 Stereotypy.
ABC: Subscale 05: Inappropriate speech
Results for the ABC subscale concerning inappropriate speech yielded a mean score on treatment of 1.93 lower than on control (95% CI = ‐3.79, ‐0.07). The I2 = 75.5% (Analysis 2.5).
2.5. Analysis.
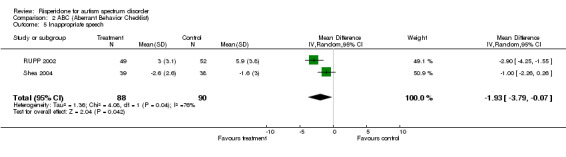
Comparison 2 ABC (Aberrant Behavior Checklist), Outcome 5 Inappropriate speech.
All results of the ABC outcome appear significantly to favour the intervention group, with the exception of inappropriate speech.
Nisonger Child Behavior Rating form ‐ N‐CBRF (Tasse 1996)
Shea 2004 reported benefit in the parent version of the Nisonger scale. The trial showed mean decreases of the study end‐point of 10.4 in the risperidone group versus 6.6 in the placebo (p <0.01). There were decreases in all the subscales secure/anxious, hyperactive, overly sensitive (statistically significant), self‐injury/stereotypic and self‐isolated/ritualistic (not statistically significant) subscales.
Vineland Adaptive Behavior Scales (VABS) (Sparrow 1984)
In McDougle 2005 (which reported further results from RUPP 2002) the total maladaptive behavior domain (part I and part II) was calculated . The results were 33.26 (SD 8.38) at baseline, to 20.34 (SD 7.93) at week 8 for risperidone, compared with 33.51 (SD 8.29) to 30.27(SD 8.87) in the placebo group (p < 0.001).
2. Non‐core behaviours such as sleep disturbance, self‐mutilation, aggression, attention and concentration problems, and gastrointestinal function
SIB‐Q
Only one separate symptom‐specific measure was used to measure self‐injury, the SIB‐Q (Gualtieri 2002) in McDougle 1998. In this, risperidone was reported to be superior to placebo in reducing self‐injurious behaviour, physical aggression to others and property destruction from the fourth week of administration and this benefit continued through week 8 end of treatment (24.2 to 9.5 in the treated group versus 32.8 to 15 in the control group (p < .001)).
3. Global impression of health
Clinical Global Impression Scale ‐CGI scale (Guy 1976)
As all three studies used this scale we were able to combine the results and perform meta‐analysis. The seven point scale was converted into binary data by all triallists using the scale (McDougle 1998; RUPP 2002; Shea 2004) in an identical way, comparing 'no improvement' to 'much improved/very much improved'. Seven indicates 'very much worse', four 'no change' and one is 'very much improved.' This showed an improvement in the CGI with a relative risk of improvement of 4.83 (95% CI = 2.21, 10.59). These results indicate a significant difference between groups, but heterogeneity appears substantial (I2 = 43%) (Analysis 1.1).
1.1. Analysis.
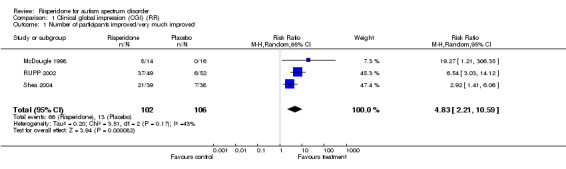
Comparison 1 Clinical global impression (CGI) (RR), Outcome 1 Number of participants improved/very much improved.
4. Adverse events
General
All three studies reported extractable adverse event data. In Shea 2004 100 per cent of participants in the risperidone group and 79.5 per cent of participants in the placebo group reported adverse events. The most frequent adverse events reported in the risperidone group were somnolence, upper respiratory tract infections, rhinitis and increased appetite. The most common events in the placebo group were aggressive reaction, fever, upper respiratory tract infection, insomnia, vomiting, diarrhoea and emotional lability. In the RUPP 2002 study 60 adverse events were recorded, 29 of which occurred in 5 percent or more of the children. The most common ones included increased appetite, nasal congestion, fatigue, enuresis and drowsiness. All of these adverse effects were present in both groups. In McDougle 1998, 87 per cent of risperidone treated patients had at least one adverse effect including sedation, dry mouth, agitation, weight gain, enuresis, dyspepsia, diarrhoea, constipation, abnormal gait and sialorrhea. In the placebo group the only reported adverse event was agitation.
Vital signs
The Shea 2004 study reported changes from baseline in vital signs. There was an increase in pulse and diastolic blood pressure in the risperidone group, which was not found in the placebo group. McDougle 1998 reported no clinically significant changes in blood pressure, pulse, respiratory rate and temperature but did not provide the data. The RUPP 2002 study also did not provide data but found no difference between the groups in blood pressure, pulse and routine laboratory tests but did not state whether any change occurred within the groups.
Weight gain
A meta‐analysis was possible for data from two trials involving similar age groups (RUPP 2002, Shea 2004) Risperidone subjects increased in weight by a mean of 2.7kg (95% CI = 1.15, 2.41) (Analysis 3.1) compared to 1.0 kg in the placebo (Shea 2004). In McDougle 1998 (a trial on adults) the authors reported that the weight gain observed was not to the same degree as previous studies on children, however no data were made available.
3.1. Analysis.
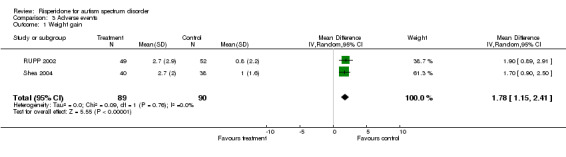
Comparison 3 Adverse events, Outcome 1 Weight gain.
Extra‐pyramidal effects (EP)
These were experienced in 27.5 per cent of the risperidone group compared to 12.8 per cent of the placebo group in one trial (Shea 2004). The RUPP 2002 used the Abnormal Involuntary Movement Scale (Guy 1976) and the Simpson Angus scale (Simpson 1970) and these showed no EPs in either group, however the parents did report extra‐pyramidal side effects of which tremor was most common. In the McDougle study there were no extra‐pyramidal symptoms other than the development of abnormal gait in one patient.
5. Quality of life for the individual or their carers
Investigators in the Shea 2004 study asked parents or carers to rate severity of participants' 'troublesome' behaviour; however, an unvalidated VAS was used.
The data from the RUPP 2002 study were used to measure changes in the symptoms of most concern to the parents or care giver. Risperidone was superior to placebo in reducing symptoms of most concern to parents and carers of autistic children with irritable behaviour. The most common symptoms identified by parents were tantrums, aggression and hyperactivity. Mean ratings at end point were 2.8 (SD 1.2) on risperidone compared with 4.5 (SD 1.3) on placebo.
6. Economic outcomes
No data on costs were reported within any of the studies included within this review.
Discussion
Our review suggests that risperidone may be of some benefit for behavioural problems in ASD, although findings must be interpreted with caution.
Meta‐analysis suggests significant improvements in some core aspects of ASD, namely irritability, social withdrawal, hyperactivity and stereotypical behaviours as well as in more global, 'non‐core symptom' assessment of severity . In results from a single study on adults with ASD, repetitive behaviour, sensory motor behaviours, affectual relations and self‐injurious behaviour also appeared to improve (McDougle 1998). Improvements were noted in the parent rated scales as well as the quality of life for carers in two studies involving children with ASD (RUPP 2002; Shea 2004). However, heterogeneity in outcomes and outcome measures within the included studies precluded more in the way of meta‐analysis, a problem which has been commented upon in other Cochrane work on autism (Sinha 2004; Williams 2005).
These results must be considered alongside the limitations of the studies. The included RCTs were few and small, the largest consisting of 101 participants, and ages of participants varied. A considerable proportion of participants left two of the studies (20% to 25% in McDougle 1998 and RUPP 2002) and long‐ term efficacy data were lacking. In addition, the drug dosage regimens were not consistent in the three included RCTs.
As well as these limitations, there was evidence of a variety of adverse effects, the most common and significant of which was weight gain. This was marked in children aged 5 to 17 (weight gain for adults was not reported).
Authors' conclusions
Implications for practice.
Although this review reports encouraging improvement in some behavioural aspects of autism spectrum disorder, carers and clinicians should be aware of the paucity of evidence in administering this drug in such a vulnerable group of people. This patient group will inevitably continue to present challenging behaviours over many years; therefore, it is vital to consider issues surrounding long‐term use. As the RCTs are of short duration it is impossible to evaluate the long term side effects and efficacy of risperidone, and this is particularly important .
Implications for research.
There were a wide number of different rating instruments used amongst the studies in this review. There is thus a need for a unified, reliable and valid rating scale to measure the different aspects of autism spectrum disorders. In trials considered within this review, even those which employed the same scales often modified them, making meta‐analysis difficult. This is a common problem in systematic reviews of ASD. Further, improvements in one or more multi‐symptom scales may not correlate with those that cause most concern to either patient or caregiver. This problem was addressed in one paper (Arnold 2003), using the same participants of the RUPP 2002 study, where the authors focused on the problems of greatest concern to the parents. This important aspect of the treatment of autism and its outcomes should be considered in further studies. Given that ASD is often diagnosed in early childhood and optimum duration for treatment is not established, long term followup is essential for patients, caregivers and clinicians.
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2009 | Amended | Typographical errors corrected and a sentence rephrased in adverse effects section of results to clarify presentation of analysis 3.1. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 8 November 2008 | Amended | Converted to new review format. |
| 18 October 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Jo Abbott, Jane Dennis, Julian Higgins, Geraldine Macdonald, Steve Milan, Julie Millener and Georgia Salanti of the Cochrane Developmental, Psychosocial and Learning Problems Group. Thanks also to Mark Fenton and Dr Christian Gold for helpful comments.
Data and analyses
Comparison 1. Clinical global impression (CGI) (RR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants improved/very much improved | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 4.83 [2.21, 10.59] |
Comparison 2. ABC (Aberrant Behavior Checklist).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Irritability | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐8.09 [‐12.99, ‐3.19] |
| 2 Social withdrawal / lethargy | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐5.03, ‐0.97] |
| 3 Hyperactivity | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐8.98 [‐12.01, ‐5.94] |
| 4 Stereotypy | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.97, ‐0.45] |
| 5 Inappropriate speech | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.79, ‐0.07] |
Comparison 3. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain | 2 | 179 | Mean Difference (IV, Random, 95% CI) | 1.78 [1.15, 2.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
McDougle 1998.
| Methods | Allocation: randomised, computer generated. No further details. Blindness: double‐blind, identical appearing capsules. Duration: 12 weeks (preceeded by 4 week absence of psychotropic drugs). 12 week open‐label extension of risperidone given to placebo group. | |
| Participants | Diagnosis: adults with autism or pervasive developmental disorder not otherwise specified (DSM IV). History: at least "moderate severity" on Clinical Global Impression. Y‐BOCS (compulsion scale >10), SIB‐Q >24 or a Ritvo‐Freeman Real‐Life Rating Scale overall score >0.20. Excluded if met DSMIV criteria for schizophrenia or had psychotic symptoms or if significant acute medical condition was identified. N=31 Age: 18‐43, mean 28.1 Sex: 9 women, 22 men. 7 participants were not mentally retarded. Location: 24 outpatient, 7 inpatients. | |
| Interventions | 1. Risperidone: commenced on 1mg/day increased up to 10mg/day. N= 15. 2. Placebo: 1mg/day increased to maximum of 10mg/day. N=16. Identical capsules given at night. | |
| Outcomes | 1. Leaving the study early. 2. Clinical Global Impression. 3. Ritvo‐Freeman Real‐life Rating Scale. 4. Visual Analog Scale 5. SIB‐Q 6. Physiological measures (sitting and standing blood pressure, pulse rate and weight). 7. Examination of extra pyramidal and other adverse effects. Unable to use Y‐BOCS (no data reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RUPP 2002.
| Methods | Allocation: randomisation conducted by National Institute of Mental Health. Randomisation was blocked on anticonvulsant status and pubertal status within site. Blindness: double‐blind, multisite. Duration: 8 week (preceded by a drug free period for 7‐28 days). 4 month open‐label extension in those with positive response, followed by 2 month placebo‐controlled discontinuation phase. | |
| Participants | Diagnosis: 5‐17 year olds meeting criteria for autistic disorder DSM‐IV. Weight of at least 15 kg, mental age of at least 18 months, free of serious medical disorders and other psychiatric disorders requiring medication. History: at least "moderate severity" on Clinical Global Impression ‐ Severity (CGI‐S)scale. Score at least 18 on Irritability subscale of Aberrant Behavior Checklist. N=101 Age: 5‐17, mean 8.8. Sex: 82 boys, 19 girls. | |
| Interventions | 1. Risperidone: commenced on 0.5mg/day OD, increased to BD and by 0.5mg increments depending on weight of the child. Up to a maximum 2.5mg/day (20‐45 kg), 3.5mg/day (>45kg). N=49 2. Placebo. N=52. | |
| Outcomes | 1. Aberrant Behavior Checklist‐ Irritability subscale.
2. Clinical Global Impression Scale.
3. Medication dose.
4. Adverse events including extra pyramidal symptoms.
5. Withdrawal from study. 2005 study: 6. Ritvo‐Freeman Real life rating scale 7. Childrens Yale‐Brown Obsessive Compulsive scale 8. The maladaptive behavior domain of the VIneland Adaptive Behavior Scales. 9. Parent‐defined target symptoms rating scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shea 2004.
| Methods | Allocation: randomised, randomisation codes kept under lock and key in pharmacy. Blindness: double‐blind. Duration: 8 week multicenter study. | |
| Participants | Diagnosis: 5‐12 year olds with DSM‐IV for PDD. History: At least 30 on Childhood Autism Rating Scale (CARS). Excluded if diagnosed with schizophrenia or other psychotic disorders, clinically relevant nonneurologic disease, clinically significant laboratory abnormalities, or a seizure disorder for which receiving anticonvulsants, or seizure in the last 3 months. Also excluded if hypersensitivity to neuroleptics, tardive dyskinesia, neuroleptic malignant syndrome, drug or alcohol abuse or human immunodeficiency virus infection. N= 79. Age: 5‐12 years, mean 7.6. Sex: 61 male, 18 female. Location: outpatients. | |
| Interventions | 1. Risperidone (oral solution) 0.01mg/kg/day, maximum 0.06 mg/kg/day. 2. Placebo (oral solution) 0.01mg/kg/day. | |
| Outcomes | 1. Aberrant Behavior Checklist. 2. Clinical Global Impression change. 3. Nisonger Child Behavior Rating form (N‐CBRF). 4. Visual Analog Scale (VAS). 5. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2004 | Open label, 3 patients |
| Aman 2005 | Examined side effects, not efficacy |
| Anonymous 2002 | Discussion of RUPP trial, not a randomised controlled trial. |
| Anonymous 2003 | Discussion of RUPP trial, not a randomised controlled trial. |
| Bober 2005 | Case study |
| Broadstock 2003 | Review of literature, not a randomised controlled trial. |
| Caicedo 2002 | Discussion or RUPP study, not randomised controlled trial. |
| Canitano 2006 | Open label |
| Cohen 1998 | Not a randomised controlled trial |
| Dartnall 1999 | Not a randomised controlled trial |
| Di Martino 2001 | Not a randomised controlled trial. |
| Dinca 2005 | Systematic review |
| Findling 2004 | Not a randomised controlled trial |
| Gagliano 2004 | No control group |
| Gallucci 2006 | Combination of two drugs |
| Gilman 1995 | Review of literature, not randomised controlled trial. |
| Hellings 2001 | Allocation: randomised, double‐blind crossover study. Participants: 20 individuals with mental retardation, age 6‐65. Intervention: risperidone versus placebo, 22 week trial. Study did not used standardised diagnostic inclusion criteria and only looking at weight gain as the outcome. |
| Hellings 2005 | No behavioural outcomes, only prolactin levels |
| Horrigan 1997 | Not a randomised controlled trial |
| King 2003 | Observational study, not randomised controlled trial. |
| McAdam 2002 | Allocation: randomised, double‐blind crossover study. Participants: 20 individuals with mental retardation, age 6‐65. Intervention: risperidone versus placebo, 22 week trial. Study did not used standardised diagnostic inclusion criteria and only looking at consumer satisfaction and social validity as outcome measures. |
| McCartney 1999 | Case reports, not randomised controlled trial. |
| McCracken 2003 | Discussion of RUPP trial, not randomised controlled trial. |
| McDougle 1995 | Case report on 3 adults, not randomised controlled trial. |
| McDougle 2000 | Background rationale for RUPP trial, not a randomised controlled trial. |
| McDougle 2002 | Unable to obtain poster. |
| McDougle 2003 | Review of literature, not randomised controlled trial. |
| Mukaddes 2004 | Pilot study, no control group |
| Natsukari 2004 | Open label |
| RUPP 2000 | Research on assessment of autistic disorder symptoms. Not testing drug with outcome measure. |
| RUPP 2005 | Discontinuation trial |
| Troost 2005 | Discontinuation trial |
| Williams 2006 | No control group |
| Zarcone 2001 | Allocation: randomised. Participants: 20 individuals with mental retardation, age 6‐65. Intervention: risperidone versus placebo, 22 week trial. Study did not used standardised diagnostic inclusion criteria. |
Contributions of authors
Ora Jesner and Mehrnoosh Aref‐Adib wrote the protocol and planned the review with supervision and training from Esther Coren. The search strategy was developed by the reviewers in concert with CDPLPG trial search coordinators Eileen Brunt and Jo Abbott. The review was written by Ora Jesner and Mehrnoosh Aref‐Adib.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
McDougle 1998 {published data only}
- McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double‐blind, placebo‐controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Archives of General Psychiatry 1998;55(7):633‐641. [DOI] [PubMed] [Google Scholar]
RUPP 2002 {published data only}
- Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, Chuang SZ, Davies M, Hollway J, Aman MG, Cronin P, Koenig K, Kohn AE, McMahon DJ, Tierney E. Parent‐defined target symptoms respond to risperidone in RUPP Autism Study: Customer approach to clinical trials. Journal of the Academy of Child and Adolescent Psychiatry 2003;42(12):1443‐1450. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough JJ, Ritz L, Vitiello B. Risperidone for the core symptom domains of autism: results from the study by the Autism Network of the Research Unit on Pediatric Psychopharmacology. American Journal of Psychiatry 2005;162(6):1142‐1148. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold E, Lindsay R, Nash P, Holloway J, McDougle CJ, Posy D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D. Risperidone in children with autism and serious behavioral problems. The New England Journal of Medicine 2002;347(5):314‐321. [DOI] [PubMed] [Google Scholar]
Shea 2004 {published data only}
- Light M, Dunbar F, Shea S. Efficacy and safety of risperidone in the treatment of children with autistic and other pervasive developmental disorders: a randomised double‐blind placebo‐controlled trial. the international journal of neuropsychopharmacology. 2004 (22nd June).
- Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, Dunbar F. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114(5):644‐641. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 2004 {published data only}
- Alexander RT, Michael DM, Gangadharan SK. The use of risperidone in adults with Asperger syndrome. British Journal of Developmental Disabilities 2004;50(2):109‐115. [Google Scholar]
Aman 2005 {published data only}
- Aman MG, Arnold MDLE, McDougle CJ, et al. Acute and Long‐Term Safety and Tolerability of Risperidone in Children with Autism. Journal of Child and Adolescent Psychopharmacology 2005;15(6):869‐84. [DOI] [PubMed] [Google Scholar]
Anonymous 2002 {published data only}
- Anonymous. Children with autism may benefit from risperidone. The Pharmaceutical Journal 2002;269(7210):184. [Google Scholar]
Anonymous 2003 {published data only}
- Anonymous. The atypical neuroleptic risperidone for autistic children with severe behavioral problems [Risperidon bei autistischen Kindern mit schweren Verhaltensproblemen]. Deutsche Apotheker Zeitung 2003;143(14):36‐37. [Google Scholar]
Bober 2005 {published data only}
- Bober D. Risperidone in a Very Young Child With PDD. Journal‐American Academy of Child and Adolescent Psychiatry 2005;44(8):725‐7. [DOI] [PubMed] [Google Scholar]
Broadstock 2003 {published data only}
- Broadstock M, Doughty C. The effectiveness of pharmacological therapies for young people with autism spectrum disorder: a critical appraisal of the literature. Christchurch, NZ: New Zealand Health Technology Assessment, 2003. [Google Scholar]
Caicedo 2002 {published data only}
- Caicedo C, Williams SH. Risperidone improves behavior in children with autism. Journal of Family Practice 2002;51 (11):915. [PubMed] [Google Scholar]
Canitano 2006 {published data only}
- Canitano R. Self injurious behavior in autism: clinical aspects and treatment with risperidone. Journal of Neural Transmission 2006;113(3):425‐31. [DOI] [PubMed] [Google Scholar]
Cohen 1998 {published data only}
- Cohen SA, Ihrig K, Lott RS, Kerrick JM. Risperidone for Aggression and Self‐Injurious Behavior in Adults with. Journal of Autism and Developmental Disorders 1998;28(3):229‐33. [DOI] [PubMed] [Google Scholar]
Dartnall 1999 {published data only}
- Dartnall NA, Holmes JP, Morgan SN, McDougle CJ. Brief Report: Two‐Year Control of Behavioral Symptoms with Risperidone. Journal of Autism and Developmental Disorders 1999;29(1):87‐91. [DOI] [PubMed] [Google Scholar]
Di Martino 2001 {published data only}
- Martino A, Zuddas A. Long‐term risperidone treatment in pervasive developmental disorders: efficacy, safety and putative mechanism of action. Biolgical basis and clinical perspectives in autism; consensus in child neurology. 2001.
Dinca 2005 {published data only}
- Dinca O, Paul M, Spencer NJ. Systematic review of randomized controlled trials of atypical antipsychotics and selective serotonin reuptake inhibitors for behavioural problems associated with pervasive developmental disorders. Journal of Psychopharmacology 2005;19(5):521‐32. [DOI] [PubMed] [Google Scholar]
Findling 2004 {published data only}
- Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications (Review) (127 refs). Journal of Clinical Psychiatry 2004;65(Suppl 6):30‐44. [PubMed] [Google Scholar]
Gagliano 2004 {published data only}
- Gagliano A, Germano E, Pustorino G, et al. Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. Journal of Child & Adolescent Psychopharmacology 2004;14(1):39‐47. [DOI] [PubMed] [Google Scholar]
Gallucci 2006 {published data only}
- Gallucci G, Duncan C, Hackerman F. Combination Use of Atomoxetine and Risperidone for Hyperactivity and Impulsivity in Autistic Disorder. Mental Health Astpects of Developmental Disabilities 2006;9(1):23‐5. [Google Scholar]
Gilman 1995 {published data only}
- Gilman JT, Tuchman RF. Autism and associated behavioral disorders: pharmacotherapeutic intervention. Pediatric Psychiatry 1995;29 (1):47‐56. [DOI] [PubMed] [Google Scholar]
Hellings 2001 {published data only}
- Hellings JA, Zarcone JR, Crandall K, Wallace D, Schroeder SR. Weight gain in a controlled study of risperidone in children, adolescents and adults with mental retardation and autism. Journal of Child and Adolescent Psychopharmacology 2001;11(3):229‐238. [DOI] [PubMed] [Google Scholar]
Hellings 2005 {published data only}
- Hellings JA, Zarcone JR, Valdovinos MG, Reese RM, Gaughan E, Schroeder SR. Risperidone‐induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. Journal of Child & Adolescent Psychopharmacology 2005;15(6):885‐92. [DOI] [PubMed] [Google Scholar]
Horrigan 1997 {published data only}
- Horrigan JP, Barnhill LJ. Risperidone and explosive aggressive autism. Journal of Autism and Developmental Disorders 1997;27(3):313‐323. [DOI] [PubMed] [Google Scholar]
King 2003 {published data only}
- King B, Zwi K, Nunn K, Longworth J, Dossetor D. Use of risperidone in a paediatric population: an observational study. Journal of Paediatrics and child health 2003;39(7):523‐527. [DOI] [PubMed] [Google Scholar]
McAdam 2002 {published data only}
- McAdam DB, Zarcone JR, Hellings J, Napolitano DA, Schroeder SR. Effect of risperidone on aberrant behavior in persons with developmental disabilities: social validity measures. American Journal of mental retardation 2002;107(4):261‐269. [DOI] [PubMed] [Google Scholar]
McCartney 1999 {published data only}
- McCartney KN, Calvert GJ. Successful use of risperidone in adults with autism and pervasive developmental disorders. Advances in Therapy 1999;16(4):158‐163. [Google Scholar]
McCracken 2003 {published data only}
- McCracken JT, McGough J, Shah B, Scahill L, Hymen S. Risperidone was safe and effective for short term treatment of children with autism and serious behavioral disturbances. Evidence Based Medicine 2003;8(1):22. [Google Scholar]
McDougle 1995 {published data only}
- McDougle CJ, Broadkin BS, Yeung PP, Naylor ST, Cohen DJ, Price LH. Risperidone in adults with autism or pervasive developmental disorder. Journal of child and adolescent psychopharmacology 1995;5(4):273‐282. [DOI] [PubMed] [Google Scholar]
McDougle 2000 {published data only}
- McDougle CJ, Scahill L, McCracken JT, Aman MG, Tierney E, Arnold LE, Freeman BJ, Martin M, McGough JJ, Cronin P, Posey DJ, Riddle MA, Ritz L, Swiezy NB, Vitiello B, Volkmar FR, Votolato NA, Walson P. Research units on pediatric psychopharmacology (RUPP) autism network: Background and rationale for an initial controlled study of risperidone. Child and adolescent psychiatric clinics of North America 2000;9(1):201‐224. [PubMed] [Google Scholar]
McDougle 2002 {published data only}
- McDougle CJ, Aman M, McCracken JT, Scahill L, Tierney E, Vitello B. Risperidone treatment of autistic disorder: longer term benefits and blinded discontinuation after 6 months. 41st Anuual meeting of the American College of Neuropsychopharmacology, San Juan. 2002 (December 8‐12th).
McDougle 2003 {published data only}
- McDougle CJ, Stigler KA, Posey DJ. Treatment of aggression in children and adolescents with autism and conduct disorder. Journal of clinical psychiatry 2003;64(4):16‐25. [PubMed] [Google Scholar]
Mukaddes 2004 {published data only}
- Mukaddes NM, Abali O, Gurkan K Notes: EXCLUDE EXCLUDE OPEN. Short‐term efficacy and safety of risperidone in young children with autistic disorder (AD). World Journal of Biological Psychiatry 2004;5(4):211‐214. [DOI] [PubMed] [Google Scholar]
Natsukari 2004 {published data only}
- Natsukari I, Sugiura M, Okada A, Nakaoka T. Risperidone treatment of children and adolescents with behavioral and affective disruptive disorders excluding schizophrenia. Japanese Journal of Child and Adolescent Psychiatry 2004;45(1):31‐52. [Google Scholar]
RUPP 2000 {published data only}
- Resaerch Units in Pediatric Psychopharmacology. Arnold LE, Aman MG, Martin A, Collier‐Crespin A, Vitiello B, Tierney E, Asarnow R, Bell‐Bradshaw F, Freeman BJ, Gates‐Ulanet P, Klin A, McCracken JT, McDougle CJ, McGough JJ, Posey DJ, Scahill L, Swiezy NB, Ritz L, Volkmar F. Assessment in multisite randomized clinical trials of patients with autistic disorder: the autism RUPP network. Journal of autism and developmental disorders 2000;30(2):99‐111. [DOI] [PubMed] [Google Scholar]
RUPP 2005 {published data only}
- Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: Longer‐term benefits and blinded discontinuation after 6 months. American Journal of Psychiatry 2005;162:1361‐1369. [DOI] [PubMed] [Google Scholar]
Troost 2005 {published data only}
- Troost PW, Lahuis BE, Steenhuis MP, Ketelaars CEJ, Buitelaar JK, England H, Scahill L, Minderaa RB, Hoekstra PJ. Long‐term effects of risperidone in children with autism spectrum disorders : A placebo discontinuation study. Journal of the American Academy of Child & Adolescent Psychiatry 2005;44(11):1137‐1144. [DOI] [PubMed] [Google Scholar]
Williams 2006 {published data only}
- Williams SK, Scahill L, Vitiello B, et al. Risperidone and Adaptive Behavior in Children With Autism. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(4):431‐439. [DOI] [PubMed] [Google Scholar]
Zarcone 2001 {published data only}
- Zarcone JR, Ellings JA, Crandall K, Reese RM, Marquis J, Fleming K, Shores R, Williams D, Schroeder SR. Effect of risperidone on aberrant behavior of persons with developmental disabilities: a double‐blind crossover study using multiple measures. Americal journal on mental retardation 2001;106(6):525‐538. [DOI] [PubMed] [Google Scholar]
Additional references
Aman 1986
- Aman M, Singh N. Aberrant Behaviour Checklist; Manual. New York: Slosson Educational Publications, 1986. [Google Scholar]
Anderson 1984
- Anderson LT, Campbell M, Grega DM, Perry R, Small AM, Green WH. Haloperidol in the treatment of infantile autism: effects on learning and behavioural symptoms. Americal Journal of Psychiatry 1984;141:1195‐1202. [DOI] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM IV). Fourth. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Arnold 2003
- Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, Chuang SZ, Davies M, Hollway J, Aman MG, Cronin P, Koenig K, Kohn AE, McMahon DJ, Tierney E. Parent‐defined target symptoms respond to risperidone in RUPP Autism Study: Customer approach to clinical trials. Journal of the Academy of Child and Adolescent Psychiatry 2003;42(12):1443‐1450. [DOI] [PubMed] [Google Scholar]
Astra Zeneca 2004
- Medical Information Desk. Personal communication (phone call from MA) 2004 (11th November).
BNF 2003
- British Medical Association. British National Formulary. Royal Pharmaceutical Society of Great Britain, 2003. [Google Scholar]
BNF 2005
- Joint Formulary Committee. British National Forumlary. March 2005. London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, March 2005. [Google Scholar]
Bryson 2003
- Bryson B, Rogers D, Fombonne E. Autism Spectrum disorders: early detection, intervention, education and psychopharmacological management. Canadian Journal of Psychiatry 2003;48(8):506‐516. [DOI] [PubMed] [Google Scholar]
Campbell 1997
- Campbell M, Armenteros JL, Malone LP, Adams PB, Eisenberg ZW, Overall JE. Neuroleptic‐ related dyskinesias in autistic children: a prospective longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry 1997;17:640‐655. [DOI] [PubMed] [Google Scholar]
Campbell 1999
- Campbell M, Rapport JL, Simpson GM. Anitpsychotics in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38:537‐545. [DOI] [PubMed] [Google Scholar]
Croen 2002
- Croen LA, Grether JK, Hoogstrate J, Selvin S. Changes in the prevalence of autism. Journal of Autism 2002;32(3):207‐215. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315 (7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eli Lilley 2004
- Medical Information desk. Personal communication (phone call from MA) 2004 (11th November).
Findling 1997
- Findling RL, Maxwell K, Wiznitzer M. An open clinical trial of risperidone monotherapy in young children with autistic disorder. Psychopharmacology Bulletin 1997;33(1):155‐159. [PubMed] [Google Scholar]
Fombonne 1999
- Fombonne E. The epidemiology of autism: a review. Psychological Medicine 1999;29:769‐786. [DOI] [PubMed] [Google Scholar]
Freeman 1986
- Freeman BJ, Ritvo ER, Yokota A. A scale for rating symptoms of patients with the syndrome of autism in real life settings. Journal of the American Academy of Child Psychiatry 1986;25:130‐136. [DOI] [PubMed] [Google Scholar]
Goodman 1989a
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischman R, Hill C, Heninger GR, Charney DS. The Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS), I: development, use and reliability. Archives of General Psychiatry 1989;46:1006‐1011. [DOI] [PubMed] [Google Scholar]
Goodman 1989b
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS), II: Validity. Archives of General Psychiatry 1989;46:1012‐1016. [DOI] [PubMed] [Google Scholar]
Gringas 2000
- Gringas P. Practical paediatric psychopharmacological prescribing in autism. Autism 2000;4(3):229‐247. [Google Scholar]
Gualtieri 2002
- Gualtieri CT. Psychopharmacology of brain injured and mentally retarded patients. Philadelphia: Lippincott Williams and Wilkins, 2002. [Google Scholar]
Guy 1976
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. National Institute Mental Health (NIMH publication DHEW). Bethesda MD: NIMH, 1976:76‐338. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library, Issue 3. Chichester UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Janssen‐Cilag 2004
- Livingston B. Personal communication (letter to OJ) 2004 (14th October).
Kanner 1943
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217‐250. [PubMed] [Google Scholar]
Katona 2000
- Katona C, Robertson M. Psychiatry at a glance. Second Edition. Oxford: Blackwell Science, 2000. [Google Scholar]
Krug 1980
- Krug DA, Arick J, Almond P. Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. Journal of Child Psychology and Psychiatry 1980;21:221‐229. [DOI] [PubMed] [Google Scholar]
Lissauer 2002
- Lissauer T, Claydon G. Illustrated textbook of paediatrics. Second Edition. London: Mosby, 2002. [Google Scholar]
Lord 1989
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism Diagnostic Observation Schedule (ADOS). Journal of Autism and Developmental Disorders 1989;19(2):185‐212. [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview ‐ Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24:659‐685. [DOI] [PubMed] [Google Scholar]
Lundbreck 2004
- Medical Information Desk. Personal communication (phone call from MA) 2004 (11th November).
McDougle 2005
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough JJ, Ritz L, Vitiello B. Risperidone for the core symptom domains of autism: results from the study by the Autism Network of the Research Unit on Pediatric Psychopharmacology. American Journal of Psychiatry 2005;162(6):1142‐1148. [DOI] [PubMed] [Google Scholar]
Novartis 2004
- Dunne A. Personal Communication (letter to OJ and MA) 2004 (27th September).
Orion Pharma 2004
- Wearn V. Personal communication (email to OJ) 2004 (28th September).
Posey 2001
- Posey DJ, McDougle CJ. Pharmacotherapeutic management of autism. Expert Opinion on Pharmacotherapy 2001;2(4):587‐600. [DOI] [PubMed] [Google Scholar]
Potenza 1999
- Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open‐labelled pilot study. Journal of Clinical Psychopharmacology 1999;19:37‐44. [DOI] [PubMed] [Google Scholar]
S‐Synthelabo 2004
- Fryer S. Personal communication (letter to OJ and MA) 2004 (27th September).
Scahill 2001
- Scahill L, McCracken J, McDougle CJ, Aman M, Arnold LE, Tierney E, Cronin P, Davies M, Ghuman J, Gonzalez N, Koenig K, Lindsay R, Martin A, McGough J, Posey DJ, Swiezy N, Volkmar F, Ritz L, Vitiello B. Methodological issues in designing a multisite trial of risperidone in children and adolescents with autism. Journal of child and adolescent psychopharmacology 2001;11(4):377‐388. [DOI] [PubMed] [Google Scholar]
Scahill 2005a
- Scahill L. Personal communication (email to M.Aref‐Adib about randomisation, allocation and in‐press copies) 2005 (28th April).
Scahill 2005b
- Scahill L. Personal communication (email to M.Aref‐Adib regarding in‐press paper) 2005 (3rd May).
Schopler 1988
- Schopler E, Reichler R, Renner BR. Child Autism Rating Scale. Los Angeles: Western Psychological Services, 1988. [Google Scholar]
Shea 2004b
- Shea S. Personal communication (email to M.Aref‐Adib explaining data from conference now in paper) 2004 (14th December).
Shea 2005
- Shea S. Personal communication (email to M.Aref‐Adib explaining randomisation) 2005 (27th April).
Simpson 1970
- Simpson GN, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;212 (Suppl 44):11‐19. [DOI] [PubMed] [Google Scholar]
Sinha 2004
- Sinha Y, Silove N, Wheeler D, Williams K. Auditory integration training and other sound therapies for autism spectrum disorders. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003681.pub2] [DOI] [PubMed] [Google Scholar]
Skuse 2004
- Skuse D, Warrington R, Bishop D, Chowdhury U, Lau J, Mandy W, Place M. The Developmental, Dimensional and Diagnostic Interview (3di): A Novel Computerized Assessment for Autism Spectrum Disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):548‐558. [DOI] [PubMed] [Google Scholar]
Sparrow 1984
- Sparrow S, Balla D, Cicchetti D. Vineland Scales of Adaptive behavior, Survey Form Manual. Circle Pines, MN: American Guidance Service, 1984. [Google Scholar]
Tasse 1996
- Tasse MJ, Aman MG, Hammer D, Rojahn J. The Nissonger Behavior Rating Form: age and gender effect and norms. Research in Developmental Disabilities 1996;17:59‐75. [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organisation. The ICD‐10 Classification of Mental and Behaviour Disorders: Diagnostic Criteria for Research. Geneva: World Health Organisation, 1993. [Google Scholar]
Williams 2005
- Intravenous secretin for autism spectrum disorder. Williams KW, Wray JJ, Wheeler DM. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003495.pub2] [DOI] [PubMed] [Google Scholar]
Wing 1999
- Wing L. Diagnostic Interview for Social and Communication Disorders. Tenth. London: The National Autistic Society, 1999. [Google Scholar]
Zarcone 2005
- Zarcone J. Personal communication (email to M.Aref‐Adib explaining allocation, inclusion criteria and in press copy‐ unable to obtain) 2005 (13th May).


