Abstract
Background
Research suggests adherence to treatment recommendations is low. In type 2 diabetes, which is a chronic condition slowly leading to serious vascular, nephrologic, neurologic and ophthalmological complications, it can be assumed that enhancing adherence to treatment recommendations may lead to a reduction of complications. Treatment regimens in type 2 diabetes are complicated, encompassing life‐style adaptations and medication intake.
Objectives
To assess the effects of interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus.
Search methods
Studies were obtained from searches of multiple electronic bibliographic databases supplemented with hand searches of references.
Selection criteria
Randomised controlled and controlled clinical trials, before‐after studies and epidemiological studies, assessing changes in adherence to treatment recommendations, as defined in the objectives section, were included.
Data collection and analysis
Two teams of reviewers independently assessed the trials identified for inclusion. Three teams of two reviewers assessed trial quality and extracted data. The analysis for the narrative part was performed by one reviewer (EV), the meta‐analysis by two reviewers (EV, JW).
Main results
Twenty‐one studies assessing interventions aiming at improving adherence to treatment recommendations, not to diet or exercise recommendations, in people living with type 2 diabetes in primary care, outpatient settings, community and hospital settings, were included. Outcomes evaluated in these studies were heterogeneous, there was a variety of adherence measurement instruments. Nurse led interventions, home aids, diabetes education, pharmacy led interventions, adaptation of dosing and frequency of medication taking showed a small effect on a variety of outcomes including HbA1c. No data on mortality and morbidity, nor on quality of life could be found.
Authors' conclusions
Current efforts to improve or to facilitate adherence of people with type 2 diabetes to treatment recommendations do not show significant effects nor harms. The question whether any intervention enhances adherence to treatment recommendations in type 2 diabetes effectively, thus still remains unanswered.
Plain language summary
Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus
Twenty‐one studies assessing interventions to improve adherence to treatment recommendations, not to diet or exercise, in people with type 2 diabetes in different settings (outpatients, community, hospitals, primary care) were included. There were many outcomes evaluated in these studies and a variety of adherence measurement instruments was used. Nurse led interventions, home aids, diabetes education and pharmacy led interventions showed a very small effect on some outcomes including metabolic control. No data on mortality or morbidity, nor on quality of life could be found.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About', 'Cochrane Review Groups (CRGs)'). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
Type 2 diabetes mellitus is one of the most common chronic diseases. The number of people with type 2 diabetes mellitus is continuously increasing worldwide. Six percent of the population of the world suffers from this disease, and only half of them have been diagnosed (Amos 1997). Strict metabolic control (for micro‐vascular related outcomes) and blood pressure control (for micro‐ and macrovascular related outcomes) seem to be of importance in order to prevent vascular complications: macro‐ and micro‐vascular disease being the most substantial diabetes‐related causes of morbidity and mortality (Kinmonth 1999; UKPDS 33 1998; UKPDS 34 1998; UKPDS 34 1998; UKPDS 38 1998; Vermeire 1999; Wens 1999).
The potential benefits of any adequate treatment though are not similar for all cases. The risk of micro‐vascular complications increases with the plasma glucose concentration and the duration of diabetes, while the risk of macro‐vascular disease depends on age, gender, genetic factors, and life‐style (for example nutrition, exercise, smoking), as well as hyperglycaemia (Goyder 1998). Once diabetes has been diagnosed, diabetes patients are confronted with the need for life‐style adaptation, for example, weight reduction, adapted nutrition and more exercise. A treatment with oral hypoglycaemic agents or insulin is often unavoidable. In order to reduce diabetes‐related complications, blood pressure and blood lipids control as well as foot care are necessary. All this requires a substantial degree of treatment adherence from patients. Patient compliance or patient adherence to treatment has been acknowledged, irrespective of the type of disease, to be a major problem in health care, involving consumers and health care providers equally. Not one in two patients adheres to treatment to health care recommendations as proposed (Vermeire 2001).
Low compliance
Low compliance to prescribed medical interventions is an ever present and complex problem, especially for patients with a chronic illness. With increasing numbers of medications and other medical interventions shown to do more good than harm when taken or implemented as prescribed, low compliance is a major problem in health care. Three decades have passed since the start of compliance research. The enormous amount of quantitative research undertaken is of variable methodological quality, with no gold standard for the measurement of compliance and it is often not clear which type of non‐compliance is being studied. Medical non‐compliance imposes a considerable financial burden upon modern health care systems. This burden is estimated to be 100 billion dollars each year in the USA (Donovan 1992; Donovan 1995; Haynes 1997; Morris 1992). Compliance to treatment is a key link between process and outcome in medical care. Therefore poor compliance may have a major impact on clinical outcome.
From compliance to concordance
Compliance is a word with negative connotations. It suggests yielding and submission to prescriptions of doctors. In the context of health care, compliance has been defined as the extent to which a person's behaviour in terms of taking medication, following diets, or executing life‐style changes coincided with medical or health advice. Compliance can also be viewed in terms of the results of taking medication: the number of doses not taken or taken incorrectly that jeopardize the therapeutic outcome or the point below which the desired therapeutic result is unlikely to be achieved. These are process‐oriented definitions. Outcome‐oriented definitions differ from them because the emphasis is on the end‐result.
The process of seeking, receiving and following treatment and advice has many stages and many opportunities for non‐compliance. Different types of non‐compliance include: delay in seeking care, non‐participation in health programmes, breaking of appointments and failure to follow doctors' advice. Other types can be distinguished: receiving a prescription, but not having it picked up at a pharmacy, taking an incorrect dose, taking the medication at wrong times, forgetting one or more doses of the medication or stopping the treatment too soon. Furthermore, compliance may be intentional or unintentional. There is something morally and psychologically flawed in the very concept of compliance. Perhaps non‐compliance may be no more deviant than compliance. Non‐compliance can be defined as a person's informed decision not to adhere to a therapeutic treatment. The backbone of the concordance model is the patient as a decision maker, and a cornerstone is professional empathy. Concordance indicates the extent to which what the patient thinks about what is asked from him matches what the health care‐giver thinks the patient actually does. The term adherence has been proposed as an alternative to compliance and is growing in popularity. It is suggested that this term incorporates the broader notions of concordance, cooperation and partnership.
Description of the intervention
Compliance research
The lack of a valid method for measuring compliance has itself been a major barrier to compliance research. There are a number of traditional measures of patients' compliance in taking medication, including both direct and indirect measures. Direct measures involve the detection of a chemical in a body fluid. However, these are not available for all medications. In addition, they may not account for the variability of pharmacokinetic factors of medications and individuals. Direct observation is only possible in restricted situations. Indirect measures are more frequently used. They include process measures such as interviews, diaries, tablet counts, electronic devices, prescription filling dates and therapeutic and preventive outcome measures. Each of these has drawbacks. Patients can improve for reasons other than following the prescribed regimen and a person's condition can deteriorate or remain stable even when the medications are taken as prescribed.
To date, none of the suggested explanations of compliance or non‐compliance has accounted for more than a modest part of the observed variation in compliance. Almost 200 different doctor‐, patient‐ and encounter‐related variables have been studied but none of them has been found to be consistently related to compliance or to be fully predictive. Researchers designing clinical trials to evaluate a medical therapy may do so under two different settings: (i) under ideal experimental circumstances with treatments taken in the manner prescribed, or (ii) under the circumstances pertaining to usual medical practice. However, a true difference in efficacy may be diluted by differential compliance between treatment groups. The difficulty in measuring compliance hinders attempts to evaluate methods for enhancing compliance. Methods that have been investigated include short‐term regimens, fewer doses per day, lower medication costs, easy‐to‐use packaging, reminders, tailoring information for the individual patient, patient education, and patient satisfaction measurement. It is also important to assess the impact of side‐effects on compliance to that particular treatment. Non‐compliance with scheduled appointments may also have important effects on health outcomes. In order to enhance this type of compliance, different patient‐related, medical‐encounter‐related and care delivery system interventions have been assessed. Because one of the most commonly advocated ways to enhance compliance is the improvement of the doctor‐patient relationship, different aspects have been highlighted and assessed (Vermeire 2001). Other issues that have to be addressed are self‐management and self‐care. These terms focus on the fact that the patient himself takes care of a series of aspects of the medical treatment, in this case of the diabetes treatment. These can be, for example, the self‐monitoring of blood glucose and the adaptation of medication dosage to self‐measured glucose levels.
Enhancing adherence
On the theme of adherence‐enhancing interventions there exist two systematic reviews. One (Haynes 1997) summarises the results of randomised controlled trials of interventions to help patients follow prescriptions for medication, focusing on those trials that measured both adherence and intervention outcomes. The authors' conclusion is that although adherence and treatment outcomes can be improved by certain ‐ usually complex ‐ interventions, full benefits of medication cannot be realised at currently achievable levels of adherence. No study on diabetes was included. The second systematic review (Roter 1998) assesses the effectiveness of interventions, educational, behavioural and affective categories, to improve patient compliance with medical regimens and concludes that no simple strategy or programmatic focus showed any clear advantage with another. Comprehensive interventions combining different of the studied components were more effective than single‐focus interventions. There is also a Cochrane protocol on adherence to medication for controlling blood pressure (Schroeder 2002). With respect to diabetes, two Cochrane Review protocols plan the assessment of the effect of exercise, psychological and lifestyle interventions on the development of future diabetic complications (Boulé 2001; Ismail 2001). A recent review (Renders 2001) concludes that multifaceted professional interventions can enhance the performance of health professionals in managing patients with diabetes. Organisational interventions that improve regular prompted recall and review of patients can also improve diabetes management. The addition of patient‐oriented education, can lead to improved patient health outcomes.
Abundant research has pointed at low patient adherence with treatment and life‐style recommendations especially in the case of chronic diseases (Vermeire 2001). The management of diabetes is a complex, lifelong process requiring a great deal of effort on the part of the patient. The patient, more than any health care provider, is the key to successful management. Poor management can result in a number of serious complications. Therefore, non‐adherence with therapeutic regimens among diabetes patients has been a continuing problem for health care providers (Nagasawa 1990; Vermeire 2001).
Treatment goals
The screening for diabetes, the strict treatment, the follow‐up, the self‐care and the self‐management, and also the efforts to enhance adherence to treatment are to be considered equally important interventions, with the aim of preventing micro‐ and macro‐vascular complications and hence to improve the diabetes patients' quality of life.
Why it is important to do this review
There are no satisfactory data in the literature on the effects of adherence‐enhancing interventions on diabetes‐related morbidity and mortality, and on the obstacles and constraints patients experience. It has not been assessed, either, what the most effective strategies have in common. Therefore, we attempted to summarise all this information according to strict quality criteria.
Objectives
To assess the effects of interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled and controlled trials, before‐after and epidemiological studies assessing changes in adherence to treatment recommendations were included.
Types of participants
Inclusion criteria
Studies were included in the review if the participants had type 2 diabetes. This type was formerly called non‐insulin‐dependent or adult‐onset diabetes mellitus. To be consistent with changes in classification and diagnostic criteria of type 2 diabetes mellitus through the years the diagnosis should have been established using the standard criteria at the time of the beginning of the trial (ADA 1999; Foster 1994; IDF1999; NDDG 1979; Wallach 2000; WHO 1980; WHO 1985; WHO 1998).
Studies in primary care (services of primary health care), outpatient settings (outpatient clinics), community settings (public health services) and hospital settings were included.
Exclusion criteria
We excluded studies investigating:
type 1 diabetes patients (unless results were reported separately to those for the patients of interest);
patients that were hospitalised at the beginning of the study (unless results were reported separately to those for the patients of interest);
interventions to improve exercise or diet .
Types of interventions
(1) Interventions that were aimed at improving the adherence to treatment recommendations were included. (2) Interventions that were aimed at patients as well as at health care providers.
We considered studies assessing interventions such as:
education (for example, information, feedback);
incentives;
use of electronic devices, decision support systems;
use of facilitators;
facilitating of self‐recording or self‐management;
scheduling appointments;
health‐care organisation, specific diabetes services;
health‐care provider‐patient relationship.
Interventions were compared with each other or with no intervention. Trials were only included if the intervention was given for a minimum of three months or if the follow‐up period after the intervention was a minimum of three months.
Types of outcome measures
Primary outcomes
health outcomes: diabetes related morbidity (hypoglycaemia and cardiovascular, neurologic, ophthalmologic and nephrologic complications), total and diabetes related mortality (death from myocardial infarction, stroke, peripheral vascular disease, renal disease, hyper‐ or hypoglycaemia or sudden death), hospitalisation rate or readmission rates to hospital, and referral to specialised diabetes care providers (for example physicians, podologists, dieticians);
direct indicators: blood glucose level, urinary glucose level, glycated haemoglobin concentration, levels of a prescribed drug in the blood, weight, blood lipids (cholesterol, triglycerides), serum creatinine, blood pressure and smoking habits (if the intervention was also aimed at smoking cessation);
indirect indicators: pill counts, refill records.
Secondary outcomes
We also considered additional parameters:
subjective report: self‐reported or self‐monitored adherence, well‐being or perceived health quality or patient satisfaction or functional status measured using validated instruments;
utilisation: appointment making and keeping, use of preventive services1;
quality of life, well being, perceived health quality (ideally, measured using validated instruments);
patient satisfaction (ideally, measured using validated instruments);
functional status defined as the ability to perform daily life activities (for example, walking, preparing meals, communicating with others,...) (ideally, measured using validated instruments);
obstacles to adherence (for example, dosing, packaging, the treatment's complexity, the understanding of given instructions, issues related to the health care system)
costs;
adverse effects of the intervention.
Timing of outcome measurement
Outcomes were assessed in the short (3‐6 months), medium (7‐12 months) or long term (more than 12 months).
Search methods for identification of studies
Electronic searches
Studies were obtained from searches of multiple electronic bibliographic databases supplemented with hand searches of references and consultation with experts.
We planned to contact companies, but we consulted a number of companies' web sites and bibliographies of company‐based studies. Due to the absence of references of studies assessing adherence enhancement companies were not contacted.
The following databases were searched from the beginning of the database to January 2002:
The Cochrane Library (issue 1, 2002) including the Cochrane Controlled Trials Register (CENTRAL) and the Database of reviews of effectiveness (DARE) and the NHS Health Economics Database
the Metabolic and Endocrine Disorders Group Specialised Register
MEDLINE
EMBASE
PsycInfo
Eric
Dissertation and Sociological Abstracts
Cinahl
The meta Register of Controlled Trials (http://www.controlled‐trials.com)
Sum Search (http://sumsearch.uthscsa.edu/searchform4.htm) and
Google search engines (http://www.google.com) on the Internet
Search strategies were adapted from the Cochrane Handbook, the Collaborative Metabolic and Endocrine Disorders Review Group, the Dutch Cochrane Centre and earlier personal search strategies. The following MEDLINE search strategy will be adapted for use with the other databases.
For details of the search strategies see Appendix 1.
Searching other resources
We tried to identify unpublished studies by contacting companies that produce aids for improving adherence (such as electronic devices) and experts who may have known about additional trials.
Studies published in any language were included.
Handsearching
In addition to searching electronic databases, bibliographic searches were performed.
Data collection and analysis
Selection of studies
To determine the studies to be assessed, two teams of independent researchers (EV and JW, PVR and YB) reviewed the titles, abstract sections and keywords of every record retrieved using a score list. The study was included if the information given suggested that the study:
included patients with type 2 diabetes mellitus;
assessed adherence to medical treatment, not to exercise nor to diet;
measured an outcome of an intervention enhancing adherence, and
used a design as described in the inclusion criteria for study design.
If there was any doubt regarding these criteria from the information given in the title and abstract, or if the abstract was absent or not clear enough to draw conclusions, the full article was retrieved for clarification. Studies were eliminated if both reviewers agreed that the study did not meet the criteria for considering studies for the review. Inter‐rater agreement was calculated using Cohen's kappa (Fleiss 1981) (kappa >0.7 (good agreement), between >0.5 and <0.7 (moderate agreement) and <0.5 (bad agreement)). Differences in opinion were resolved by discussion with a third party (PVR).
Data extraction and management
Three teams of two reviewers (EV and JW, PVR and YB, HH and AL) independently extracted data (the quality criteria, participant details, intervention details, outcome measures, baseline and post‐intervention results, and main conclusions), using structured forms for clinical trials and for observational studies. If there was missing information, it was decided that the authors of the article were not contacted. Differences in data extraction at item level were resolved by discussion and if consensus could not be reached, a referee (PVR) took a final decision.
Assessment of risk of bias in included studies
The risk of bias of included studies was assessed independently by three teams of two reviewers (EV and JW, PVR and YB, HH and AL).
To keep up with this process of including and excluding studies a minute description was kept in a log‐book. This resulted in a tree structure representation of this process (Moher 1999).
Observational studies are more prone to bias and confounding than experimental trials. Therefore, the methodological evaluation of observational research is not clear cut (Friedenreich 1994; Laupacis 1994; Levine 1994; Stoup 2000).
A set of criteria has been defined for case‐control and cohort study designs (Borhouts 1998; Harris 2001; Offringa 2000). Based on these criteria a 3‐category rating of 'good,' 'fair,' and 'poor' was applied. In general, a good study is one that meets all criteria well. A fair study is one that does not meet (or it is not clear that it meets) at least one criterion but that has no important limitation that could invalidate its results. A poor study has important limitations. These specifications are not meant to be rigid rules but rather are intended to be general guidelines, and individual exceptions, when explicitly explained and justified, can be made.
Cohort and patient‐control studies :
(1) were the groups to be compared clearly defined (setting, time, definition of exposition, definition of outcome or adverse effect, criteria for the selection in the cohorts or for the selection for patients and controls)? Good O Fair O Poor O (2) was selection bias excluded? (case control study) (controls should be a reflection of the source population) Good O Fair O Poor O (3) were all new cases included? (case control study) Good O Fair O Poor O (4) were exposure and outcomes equally and independently measured in both groups (information bias)? (case control study) Good O Fair O Poor O (5) loss‐to‐follow‐up? Good O Fair O Poor O (6) duration of follow‐up? Good O Fair O Poor O (7) had the analysis corrected for confounders? Good O Fair O Poor O (8) was there a dose‐response relation? Good O Fair O Poor O
Assessment of heterogeneity
We dealt with clinical and methodological heterogeneity to answer the question whether the combination of the different studies was meaningful.
Statistical heterogeneity was estimated by the visual appraisal of the forest plots. Heterogeneity was also tested by using the Z score and the Chi square statistic with significance set at P < 0.1. Statistical homogeneity was assessed using the I² test where I² values over 50% indicate moderate to high heterogeneity (Higgins 2003). If there was significant heterogeneity we had to decide whether the combination of the data was appropriate. If heterogeneity was important, more emphasis should be placed on the results of a random effects model, although use of this model does not overcome the problem of heterogeneity. Another way to handle this issue was to explore possible sources of heterogeneity by subgroup and sensitivity analyses or by employing meta‐regression.
Assessment of reporting biases
If there was a reasonable number of studies, a funnel plot would have been drawn to examine the possibility of small study bias. A funnel plot is a graphical display of sample size plotted against effect size. A gap on one side of the wide part of the funnel indicates that some studies have not been published or located or that another type of small study bias has arisen (Egger 1997).
Because the name 'funnel plot' is based on the fact that the precision in the estimation of the underlying treatment effect will increase as the sample size of component studies increases, effect estimates from small studies will therefore scatter more widely at the bottom of the graph, with the spread narrowing among larger studies. In the absence of bias, the plot will resemble a symmetrical inverted funnel (Sterne 2001).
Data synthesis
We undertook a descriptive review of all studies. Data would have been summarised statistically if they were available, of sufficient quality and sufficiently similar. We only would have pooled the effect size of trials with comparable interventions. If not, qualitative synthesis was performed.
We expected both event (dichotomous) data and continuous data. Dichotomous data would have been be expressed as odds ratios (OR). The relative risk (RR) would have been used as an alternative to the OR as interpretation is easier, especially if the outcome is a negative event. Continuous data would have been expressed as weighed mean differences (WMD) and an overall WMD would have been calculated if appropriate.
Subgroup analysis and investigation of heterogeneity
If there was a significant effect for one of the main outcome measures, we aimed to perform subgroup analyses to determine whether there were any systematic differences between groups of patients. The following subgroup analyses would have been considered:
age groups (below 60, over 60);
gender;
duration of diabetes (below or over five years);
presence of complications;
different comparison interventions;
type of treatment: oral hypoglycaemic agents, insulin, combination of treatments;
different settings (primary care, outpatient or community settings);
duration of intervention (short, medium, long term).
Sensitivity analysis
Once the data were analysed, we intended to consider how sensitive the results are to the way the analysis was done. We would have performed sensitivity analyses in order to explore the influence of the following factors on effect size :
repeating the analyses excluding unpublished studies (if there were any);
repeating the analyses excluding particular studies on for example combined interventions;
repeating the analyses excluding studies of the lowest quality (which would have been done already if there was a heterogeneity problem);
repeating the analyses excluding very long or large studies to establish how much they dominate the results;
repeating the analyses excluding studies using the following filters: diagnostic criteria, language of publication, source of funding, country, etc.;
repeating the analysis for single blinded studies.
The robustness of the results would have also been tested by repeating the analyses using different measures of effect size (risk difference, odds ratio, etc.) and different statistic models (fixed and random effects model).
Results
Description of studies
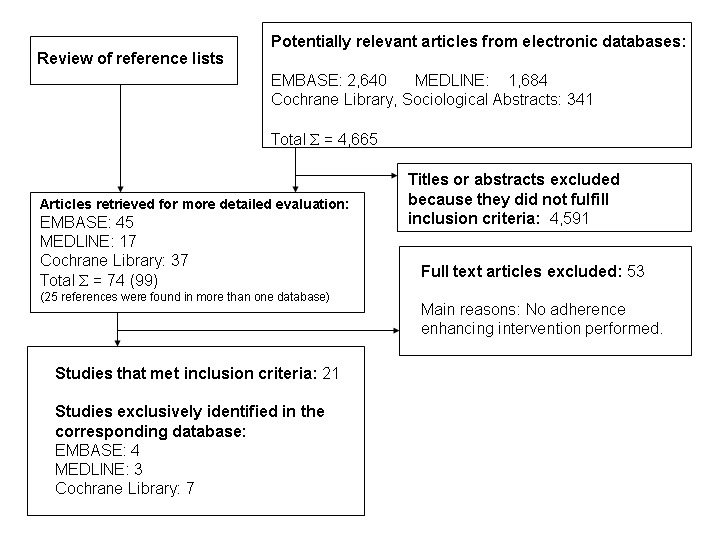
The search resulted in 4,665 abstracts. EMBASE contributed 2,640 articles, MEDLINE 1,684, and 341 other studies were added by searches in the Cochrane Central Register of Controlled Trials (CENTRAL), the National Library of Medicine Gateway and Sociological Abstracts (Figure 1). Internet search engines identified hundreds of hits that were informative on the subject, but not useful for the purpose of this review. On initial abstract review, the first apparent reason for exclusion was not meeting the most important inclusion criterion: only studies that assessed interventions aimed at improving the adherence to medical treatment recommendations were accepted. In a large number of studies the precise aim of the study appeared not to be explained clearly at all by the authors. Moreover, in a majority of articles, although adherence was mentioned to be the topic of interest, diabetes care in general or self‐care in particular were the core issues of the research reported on. This forced reviewers to careful consideration and led consequently to interrater agreements that improved, and were more consistent, as the selection process went on. Indeed, in many studies interventions aimed at influencing some aspects of diabetes care were, mistakenly and even misleadingly, called enhancing adherence concomitantly.
1.
Study flow diagram
One team of reviewers (PVR and YB) assessed MEDLINE searches and then other team (EV and JW) assessed EMBASE and the other search results. For the purpose of the inclusion, quality assessment and data‐extraction of studies an electronic grid was used. It was adapted from a grid originally designed by the Cochrane Metabolic Diseases and Endocrine Disorders Collaborative Review Group.
The interobserver agreement of the first team (PVR and YB) expressed as a kappa was 0.54 (95% CI 0.37 to 0.70), that of the second team (EV and JW) 0.83 (95% CI: 0.76 to 0.89). We identified 74 studies from which in a final round with the full text at hand 52 were excluded, and another one at the time of the analysis. The main reason for their exclusion was that, though authors mentioned in the abstract that the study was about adherence to medical treatment recommendations in type 2 diabetes, no adherence enhancing intervention was performed nor were changes in adherence to treatment recommendation measured properly.
Of the 21 included articles 13 were found in EMBASE, 12 in The Cochrane Library and eight in MEDLINE. Four studies were retrieved in EMBASE alone, seven only in The Cochrane Library and three uniquely in MEDLINE. Two articles were found in EMBASE, as well as in MEDLINE and in The Cochrane Library.
The included studies were: 14 randomised controlled trials (RCTs) (Canga 2000; Hopper 1984; Jaber 1996; Krier 1999; Matsuyama 1993; Mease 2000; Piette 2001; Pullar 1988; Rachmani 2002; Rosenkranz 1996; Simmons 2000; Skaer 1993; Smith 1986; White 1986) including one cross‐over study (Diehl 1985), one controlled trial (CT) (Davidson 2000), four controlled before and after studies (CBAs) (Bradshaw 1999; Clarke 2002; Coast‐Senior 1998; Jiang 1999) and finally one epidemiological study (Paes 1997).
The studies in this review encompassed a wide range of interventions including nurse interventions, home aids, diabetes education programmes, pharmacy based interventions, interventions comparing the effect of different dosing and frequency of medication intake. The 21 studies contain data on 4,135 patients.
Only one study evaluating economic aspects of adherence enhancement was identified (Skaer 1993).
Risk of bias in included studies
The methodological quality of each study is described in Characteristics of included studies and in the results section.
Overall judgement of methodological quality
From the 21 included studies three were considered of good, 13 of medium and five of poor methodological quality.
Groups equally provided with care
Groups were equally provided with care in 11 studies (Canga 2000; Davidson 2000; Diehl 1985; Hopper 1984; Krier 1999; Matsuyama 1993; Mease 2000; Rachmani 2002; Rosenkranz 1996; Skaer 1993; White 1986), in one study they were not (Jaber 1996) and data were missing in five studies (Jiang 1999; Piette 2001; Pullar 1988; Simmons 2000; Smith 1986).
Description of losses to follow‐up and intention‐to‐treat analysis
Six studies did not describe losses to follow‐up (Bradshaw 1999; Hopper 1984; Jiang 1999; Paes 1997; Rachmani 2002; Skaer 1993), fifteen adequately described losses (Canga 2000; Clarke 2002; Coast‐Senior 1998; Davidson 2000; Diehl 1985; Jaber 1996; Krier 1999; Matsuyama 1993; Mease 2000; Piette 2001; Pullar 1988; Rosenkranz 1996; Simmons 2000; Smith 1986; White 1986) and in eight studies it was certain that the authors performed an intention‐to‐treat analysis (Canga 2000; Davidson 2000; Mease 2000; Piette 2001; Rachmani 2002; Simmons 2000; Skaer 1993; White 1986).
Adequacy of length of follow up
All included study had a follow‐up period of at least three months. At the time of performing the analysis one study was excluded because of a follow‐up period of only four weeks (Tu 1993).
It is striking that not one author mentioned any data on the calculation of the statistical power of their study.
Allocation
In five randomised controlled trials an adequate randomisation procedure and concealment of allocation could be ascertained (Canga 2000; Diehl 1985; Matsuyama 1993; Piette 2001; Simmons 2000), in six an adequate randomisation but not concealment of allocation could be ascertained (Hopper 1984; Jaber 1996; Krier 1999; Mease 2000; Rachmani 2002; Smith 1986), and in four studies data were missing on both procedures in order to be able to judge properly (Pullar 1988; Rosenkranz 1996; Skaer 1993; White 1986).
Similarity at the start at baseline
At the start of the study groups were similar in 15 trials (Canga 2000; Coast‐Senior 1998; Davidson 2000; Diehl 1985; Hopper 1984; Jaber 1996; Krier 1999; Matsuyama 1993; Mease 2000; Piette 2001; Rachmani 2002; Simmons 2000; Skaer 1993; Smith 1986;White 1986) and in three articles data were missing (Jiang 1999; Pullar 1988; Rosenkranz 1996).
Blinding
In three studies there was adequate blinding of patients, administrators and outcome assessors (Canga 2000; Piette 2001; Simmons 2000), in two studies there was adequate blinding of patients, but not of administrators and outcome assessors (Krier 1999; Matsuyama 1993), in one study there was adequate blinding of patients, but unclear blinding of administrators or outcome assessors (Diehl 1985), in 11 studies data were missing on any blinding (Clarke 2002; Coast‐Senior 1998; Davidson 2000; Hopper 1984; Jaber 1996; Jiang 1999; Mease 2000; Pullar 1988; Rachmani 2002; Smith 1986; White 1986) and one study did definitely not apply any form of blinding (Rosenkranz 1996).
Effects of interventions
We did not find any data on quality of life, mortality or morbidity in the included studies, nor did we retrieve any information on cost‐benefits of adherence enhancing interventions.
According to the nature of the interventions studies were grouped in a logical way. 'Nurse interventions' (Clarke 2002; Mease 2000; Piette 2001) compared nurse‐led interventions, mostly a telephone follow‐up, with usual care. In these studies a variety of outcomes were evaluated, but unfortunately they were heterogenous in the different studies: HbA1c, weight, diabetes related symptoms, appointment keeping, changes in medication taking, changes in uses of preventive services, and self‐care behaviour. 'Home aides' (Hopper 1984; Smith 1986) are mailed educational materials, appointment reminders or home health aides visits versus usual care, evaluating appointment keeping, prescription refill, screening percentage, eye clinic attendance and negative utilisation of services (emergency room visit, missed scheduled visits). 'Diabetes education programmes' (Bradshaw 1999; Jiang 1999; Krier 1999; White 1986) assessed the effect of a variety of education programmes on HbA1c, different metabolic parameters, blood pressure and self‐reported compliance. 'Pharmacy based interventions' (Coast‐Senior 1998; Davidson 2000; Jaber 1996; Matsuyama 1993; Skaer 1993) assessed the effectivity of pharmacist‐led interventions (pill count, MEMS, comprehensive care, treatment adjustments, prescription refill reminders) on self‐reported adherence, medication prescription refill, and some metabolic parameters. 'Dosing and frequency' (Paes 1997; Pullar 1988; Simmons 2000) interventions assessed the effect of calendar blister packs and once up to three times daily medication intake on compliance.
Besides these created categories there remain four solitary comparisons: standard consultation versus a patient participation programme (Rachmani 2002), oral chlorpropamide intake versus insulin injections (Diehl 1985), fundus photography shown and explained versus not shown nor explained (Rosenkranz 1996), and finally a nurse‐led education and counseling intervention on smoking cessation (Canga 2000).
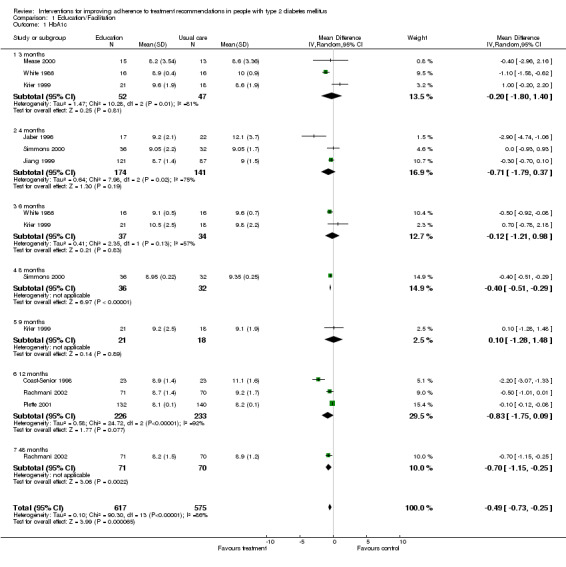
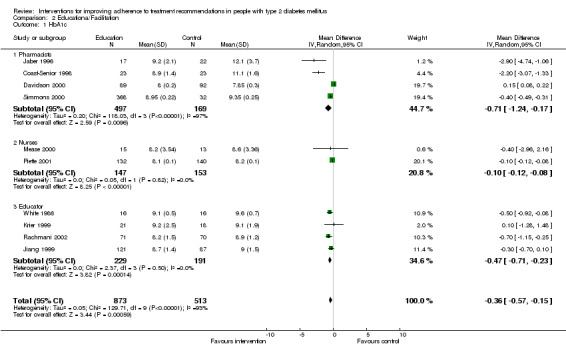
We pooled the studies assessing educational interventions: those facilitating adherence (Krier 1999; Piette 2001; Rachmani 2002; Simmons 2000; White 1986) and those offering diabetes education (Coast‐Senior 1998; Jiang 1999) versus usual care or a control. In all these studies HbA1c was a common outcome measure.
The results of the 21 included studies are reported narratively, though some of the comparisons fit for the calculation of pooled weighted mean differences. Because within each of these trials methods, patients and many other characteristics are unlikely to be identical, as would be implied by the use of a fixed effect meta‐analysis model, a random effects model was used. The educational intervention trials were pooled. The studies offering diabetes education with outcome measure HbA1c could not be used in a meta‐analysis because these studies were controlled before and after trials.
Nurse led interventions
The comparison of the effectiveness of education classes plus weekly nurse telemedicine 'home visit' versus usual care (Mease 2000) , over a period of three months, shows a statistically significant reduction in mean HbA1c level of 0.4%. The mean weight reduction is limited to a non significant 4%. Furthermore there were no significant changes on a Diabetes Quality of Life scale nor on the Medical Outcome Health Survey SF‐36 scale. Some metabolic parameters such as microalbuminuria, serum creatinine and serum lipids did not improve during the study period. Physicians and case managers considered telemedicine to have a high benefit, but technological problems were a major obstacle.
A 12 months telephone nurse‐led follow‐up intervention (Piette 2001) in which a nurse called patients weekly to talk about self‐care, medication adherence and symptoms, showed a small but statistically significant (P = 0.04) lowering of HbA1c. Moreover patients in the intervention group reported fewer (‐10%) diabetes related symptoms, while these increased with the same proportion in the control group.
Another 12 months telephone calls programme (Clarke 2002) focused on improving participants' understanding of their disease and the crucial importance of adhering to standards of care while providing support in helping patients to change their behaviour and lifestyle. Authors assessed subjective reports of adherence and measured utilisation of medical services. There were no differences in the mean change score of medicine taking, of taking recommended medical tests nor in the use of preventive health services. Patients in the intervention group had more frequently testing of HbA1c, low density lipoproteins, microalbuminuria and diabetic retinopathy.
Home aids interventions
Appointment keeping and prescription refills were the outcome measures of a 12 months' study of the effectiveness of mailed packets (physician phone numbers, visiting hours, list of early warning signs), a booklet on management of diabetes, appointment reminders, accompanied by an intense follow‐up of visit failures versus usual care (Smith 1986). After one year the intervention group averaged 12% more total contacts than the control group (5.8 versus 5.2, P = 0.01), due largely to an increase in kept scheduled visits (4.1 versus 3.6, P = 0.006). Visit failures were mainly reduced in high‐risk patients and those patients at higher risk of hospitalisation. The effect of the intervention did not diminish during the year of the study.
The impact of home health aides visits on diabetic control and health care utilisation was assessed on fasting blood glucose, eye clinic attendance and negative utilisation of medical services (Hopper 1984). Fasting blood glucose declined when compared to control group (10.1 mg/dl versus an increase of 5.1 mg/ dl). Missed clinic appointments and attendance of emergency room decreased slightly in the intervention group, and eye clinic attendance increased significantly ((1.3 versus 0.7, representing a change of 0.4 (95%CI 0.1 to 0.7) versus ‐0.02 (95%CI ‐0.3 to 0.3)).
Diabetes education interventions
A nine months study comparing quarterly visits of a diabetes educator versus usual care, assessed changes in HbA1c, weight and self‐reported medical compliance on a 4‐point Lickert scale (Krier 1999). Neither group showed any statistical significant change in their HbA1c. Weight increased in both intervention and control groups. There was a small increase in self‐reported medical compliance in both intervention and control groups.
Another RCT compared group management with an advice‐educational technique on its effect on HbA1c (White 1986). There was a 10% decline in HbA1c levels (P < 0.05) in the first three months of the study in both groups, However, little change occurred during the final three months of the study.
The effect of a five‐section education program during four months was compared to a basic course, and evaluated by measurement of fasting blood glucose, HbA1c, serum cholesterol, triglycerides, blood pressure and body weight (Jiang 1999). In both experimental and control groups the decline in HbA1c levels was statistically significant (9.4% to 8.7%, versus 9.3% to 9.0%). In the experimental group the decline in systolic blood pressure and body weight was statistically significant.
The effect of three structured educational programs (traditional, video, educator) assessed HbA1c, weight and mean change in levels of knowledge (Bradshaw 1999). The authors indicate that there was no effect on the levels of HbA1c or weight, but data are missing. There was a significant increase in percentage of correct answers on knowledge questions on diabetes in the group with educator.
Patient participation consultation
A four years study compared the effect of standard consultations with a patient participating programme in which patients shared therapeutic responsibilities (Rachmani 2002). HbA1c, blood pressure, LDL and the number of cardiovascular events was evaluated in both groups. All outcome measures declined in a statistically significant way in the intervention and control groups.
Pharmacy based interventions
During a four months intervention comparing adherence data from a medication event monitoring system (MEMS) with pill counts in assisting pharmacists in making recommendations regarding diabetes therapy (Matsuyama 1993) did not show any significant change in metabolic control. There were no significant changes in non‐adherence in both groups. In the MEMS group, 47% of the recommendations regarding diabetes therapy made by pharmacists were related to patient education compared to 12% in the control group.
In a four month period, a comprehensive pharmacist care model was compared to usual care (Jaber 1996). Patients in the intervention group were offered diabetes education, medication counseling, and evaluation plus adjustment of their hypoglycaemic regimen. Significant improvement of HbA1c levels was obtained (P = 0.003) in the intervention group. No significant changes in blood pressure control, lipid profile, renal function parameters, weight, or quality of life measures was noted between groups.
The effectiveness of mailed prescription‐refill reminders, specialised packaging, or a combination was assessed versus usual care in a one year study on the medication possession ratio (number of days' supply of medication obtained throughout 360 days of the trial) (Skaer 1993). Patients receiving mailed prescription‐refill reminders, unit‐of‐use packaging, or a combination of both interventions achieved a significant (P < 0.05) increase in the medication possession rate for sulphonylurea therapy relative to controls. No significant difference was discerned between groups receiving reminders or special packaging. Patients receiving both interventions experienced a significant decrease in the use of physician, laboratory and hospital services reaching 68 US dollars decrease per capita compared to the 3‐month time frame prior to the study. In the experimental group however, much more hypoglycaemic episodes occurred than in the control group (17 versus 2). The authors indicate that the systolic blood pressure improved, but the article does not allow to discern whether within or between group data are presented. HbA1c declined within and between groups. There was a higher decline in persons with a higher start level. There were no changes in body weight, serum lipids and renal function between or within groups. There were no data on self‐reported adherence to medication. There were also no changes in scores on validated Health Status and quality of life questionnaires.
The effect of a one year pharmacist treatment adjustments versus standard physician led care on HbA1c, appointment keeping, eye examination and refusal to make recommended medication adjustments was studied (Davidson 2000). The HbA1c levels fell significantly (‐0.8% versus ‐0.05%) in the intervention group more than in the control group (P = 0.03). In this study compared groups were not clearly defined and there are no data on losses to follow‐up.
The effectiveness of pharmacists initiating insulin treatment on HbA1c was evaluated after one year (Coast‐Senior 1998). HbA1c levels decreased by 22% (P < 0.05). On the contrary symptomatic hypoglycaemic episodes occurred in 35% of patients.
Oral antidiabetic drugs versus insulin
In a two times 24 weeks cross‐over study compliance to the oral intake of chlorpropamide was compared to insulin injections (Diehl 1985). Adherence to treatment was evaluated as the percentage of expected tablets or insulin used. There were no differences in adherence with the two medications in terms of percent of prescription used, proportion taking at least 80% of prescribed medication, self‐report of medication or diet compliance, or protocol dropout rates. However, treatment satisfaction was higher with chlorpropamide.
Dosing and frequency
The adherence to oral medication according to an intake of once, twice or three times a day was evaluated by tablet count and the detection of the level/dose phenobarbital ratio ‐ as a chemical marker of medication intake (Pullar 1988). Inadequate compliance was defined as either interview evidence of having missed some study tablets, a value for compliance by tablet count less than 85%, or a phenobarbital level dose ratio less than 85%. Mean compliance for once daily dosing was significantly higher than for twice a day (P < 0.05) or three times a day (P < 0.05).
HbA1c and blood pressure were evaluated after four and eight months in patients receiving calendar blister pack compared to the usual delivery of tablets (Simmons 2000). HbA1c was reduced by 0.95 ±0.22% in the calendar group and 0.15 ±0.25% in the control group (P = 0.026). Diastolic blood pressure decreased significantly, in contrast with systolic blood pressure that remained unchanged.
In an observational study (Paes 1997) adherence with dosing and medication prescription refill was measured with MEMS, pill counts and by analysis of pharmacy records. This study observed that compliance is influenced by the frequency of doses. The compliance, the percentage of doses taken measured by MEMS during the six months observation period in this study, was 98.7% (SD 18.6) in the case of a once daily dose and 65.8% (SD 30.1) in the case of a dose of three times daily. Refill compliance with prescribed regimen was 79.1% (SD 18.8) in the once daily regimen and 38.1% (SD 35.8) in the three times a day regimen.
Fundus photography
To investigate whether instantaneous Polaroid fundus photography during a patient consultation would effect patient's future screening behaviour, three groups of patients were evaluated: those to whom the picture was not shown, versus those to whom the picture was shown, explained and handed out, versus to whom the picture was shown, explained but not handed out (Rosenkranz 1996). The outcome measure was future appointment keeping with the ophthalmologist and was 51%, 80% and 68% respectively in described patient groups.
Smoking cessation
To assess the effectiveness of a six months nurse‐led education and counseling programme on smoking cessation, self‐reported and verified smoking cessation as well as the change in mean cigarettes per day was evaluated versus usual care (Canga 2000). Smoking cessation incidence was 17% in the intervention group compared with 2.3% in the usual care group, which was a difference of 14.7% (95%CI 8.2 to 21.3%). The mean number of cigarettes per day decreased from 20 to 15.5 (‐4.6 cig/d; 95%CI ‐3.3 to ‐6) at six months follow‐up for the experimental group versus from 19.7 to 18.1 for the control group (‐1.6 cig/d; 95% CI ‐0.4 to ‐2.8) (P < 0.01). Another study assessed the effect of three structured education programmes (traditional, video and educator) on patient reported smoking cessation (Bradshaw 1999). Of the smoking participants 41% reported they had ceased over the period of the study.
Discussion
Summary of main results
The aim of this review was to identify effective interventions or strategies to enhance adherence to treatment recommendations in people with type 2 diabetes in primary care, outpatient settings, community and hospital settings. A large number of studies was identified, but only 21 articles were included in the review. This is mainly due to the fact that though many authors stated that their study was about compliance or adherence the core issue was self‐care or some aspect of diabetes management. Many authors do not feel the need to define adherence, nor do they discuss in which way and to what extent the outcome measures used really reflect adherence! Only a few articles measured adherence directly or indirectly using questionnaires. For these reasons we had to adapt one of the inclusion criteria from 'assessment of an intervention to enhance adherence' in 'assessment of an intervention aimed at improving adherence'. Many studies seem to report on 'black box" research: doing an intervention and measuring HbA1c at the end of the study period. In many studies it remains unclear what really happened in those participants, what made metabolic parameters change or what made them remain unchanged? Besides, what way does adherence play a role if an outcome changes? Therefore it was not an easy task for the reviewers to decide on inclusion or exclusion. Moreover, this process was very time consuming.
The studies included in the review are heterogeneous in terms of interventions, participants, settings, countries and outcomes. The majority of studies used a set of outcome measures which do not lead to consistent and homogeneous results. Even interventions aimed at improving diabetes care in general do not result in homogeneous results for different aspects. It is clear that a rather 'simple' intervention as a diabetes education programme is not able do have consistent and homogeneous effects in a complex disease, as is the case for diabetes mellitus. It is indeed imaginable to conclude that an effective adherence enhancing intervention does not lead to satisfactory declining HbA1c values. Diabetes management is indeed much more than taking medication alone. Moreover, an effective intervention may lower some metabolic parameters, without affecting other parameters such as weight, blood pressure or renal function. Indeed, did UKPDS not confront researchers first with the paradox that tight glycaemic control induced an increase in HbA1c values and second with the conclusion that tight blood pressure control was more effective than tight glycaemic control in hypertensive diabetic persons in reducing diabetes related morbidity and mortality? Therefore, there is a need for better research discerning between adherence enhancing strategies and their effect on diabetes related outcomes.
The methodological quality was often limited. Besides the criteria for quality that are presented in the table with the characteristics of included studies, more remarks can be made. The most important question remains that of the validity of the studies because of low numbers of participants, lack of power calculations, poor or absent definition of adherence and the assumption that metabolic parameters such as HbA1c adequately reflect adherence. Moreover, regarding the educational interventions concerned, these were so poorly described that it was not possible to discern differences or similarities between programmes. In a majority of articles the quality of the reporting in the written articles was incomplete and inaccurate such as missing data, incomplete figures or tables and the absence of confidence intervals or standard deviations.
Therefore, it is difficult to draw general conclusions from this review. In general most results float around the zero line, which may be interpreted by saying that there are no effective interventions or, by saying that the quality of the research was so poor that it was not able to reveal any significant effect. HbA1c declined slightly in most of the studies, furthermore positive effects could be noted on appointment keeping, eye clinic attendance, improved knowledge of diabetes, and prescription refill adherence. Once daily dosing appeared to be effective in increasing oral medication intake.
Our conclusions on the effectiveness of educational interventions on metabolic control are concordant with those of a UK Health Technology Assessment systematic review (Loveman 2003). These authors conclude that education as part of intensification of treatment produces improvement in diabetic control in type 1 diabetes. In type 2 diabetes they find mixed results which means that no clear classification is possible as to what features of education may be beneficial. A diversity of educational programmes did not yield consistent results on measures of metabolic control, with studies of lower quality producing significant effects. Economic evaluations comparing education with usual care or other educational interventions in type 2 diabetes mellitus were not identified. A recent review on dietary advice was not able to find any effectivity, though the adoption of exercise appeared to improve glycated haemoglobin at six and twelve months (Moore 2004). Psychological interventions showed improvements in long‐term glycaemic control but not in weight control or blood glucose concentration. The pooled mean difference in HbA1c was ‐0.3 (95% CI ‐0.6 to 0.1) (Ismail 2004).
This review illustrates the necessity that all relevant databases should be consulted. MEDLINE, EMBASE and the Cochrane Controlled Trial Register contributed almost equally to the included studies (Wens 2004).
The majority of the authors drew positive conclusions while presenting statistically significant differences between intervention and control groups. In contrast, it is crucial to notice that those results were probably not very clinically relevant.
Because almost all HbA1c values floated around the zero line we decided not to perform sensitivity analyses in order to explore the influence on a number of factors on the overall effect size.
The problems encountered are not typical for this review. Recently, a systematic review was published on adherence improving to blood pressure lowering medication, in which authors described exactly the same problems and pitfalls. They were not able to draw general conclusions, only but the assumption that reducing the number of daily doses appears to be effective in enhancing adherence in ambulatory care (Schroeder 2004).
Regarding to the fact that there we just a few studies available, the funnel plot was not performed due to the lack of reliability in this case.
The results of this review are concordant with an earlier Cochrane Review that summarised the results of RCTs of interventions to help patients, without any specific medical condition, follow prescriptions for medication, focusing on those trials that measured both adherence and outcomes. No effective interventions were identified. The conclusions of an earlier narrative review of the compliance/adherence literature (Vermeire 2001) are concordant with those of this systematic review: though every health care professional considers adherence to be an crucial element in health care, though everyone recognises non‐adherence as a major health care problem, though every scientist ascertains that diabetes mellitus may be considered a new epidemic in the decades to come, the evidence base on adherence to treatment recommendations in diabetes is almost inexistent.
Authors' conclusions
Implications for practice.
Current efforts to improve or facilitate adherence of people with type 2 diabetes to treatment recommendations do not show significant effects nor harms. The question whether any intervention enhances adherence to treatment recommendations in type 2 diabetes effectively, thus still remains unanswered.
Implications for research.
In research on the effectiveness of interventions a number of, generally accepted but not generally applied, rules ought to be respected. With regard to adherence research methodological flaws mainly refer to the research question, the definition of and measure instruments for adherence. Quite often the research question is not clearly formulated, neither is adherence defined properly. If adherence is not well defined in the first place, how is it possible then to operationalise enhancement of adherence in the second place?
Besides, the measurement instruments for adherence should be apt to measure adherence. Mostly adherence is assessed indirectly, leaving the reader with the question how valid this research was.
This type of flawed and biased research does not appear to be very conclusive. In addition, different study findings cannot be compared easily. The question whether any intervention enhances adherence to treatment recommendations in type 2 diabetes effectively, thus still remains unanswered. Adherence should be defined explicitely, the measurement instruments should be as direct as possible, assuring that the established changes really reflect effects of the intervention on adherence itself. Indeed, researchers studying adherence should be careful not to be biased too easily by the results of 'black box' research in which any measured effect is assigned to an intervention that has being taken place before. There may not doubt about the causal relationship between the intervention and the outcome.
Next the encountered variety of educational interventions that has been tested, in the many excluded studies as well, reflects that the choice of components of education programmes is often not based on a theoretical or empirical rationale. Many authors say that education is the cornerstone of diabetes management, they study a programme they designed, leaving everyone in a state of uncertainty about which aspect of the education, hence which combination of components, and which duration are determinants for better adherence. Finally there is a need to know whether interventions have an effect on the short term, a long acting effect, or whether they need to be repeated periodically. In adherence research, evidence based economical evaluations are missing. When effective interventions will be revealed in the future, their impact on human and financial resources merits prime attention. In recent years attention is being paid to a shift from compliance to concordance and shared‐decision making. The quality of the health care provider ‐ patient encounter takes into consideration the patient's health beliefs, medication and treatment recommendations review. In concordance, the treatment regimen is tailored to what is manageable and attainable for this particular person. A robust research evidence based on this topic needs to be built in future research projects.
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the team of the Dutch Cochrane Centre for helping in performing the EMBASE literature search. Furthermore the realisation of this systematic review would not have been possible without the support of Hilary Hearnshaw and Antje Lindenmeyer from Warwick University (UK). For very practical reasons we are very grateful to Primary Care Diabetes Europe which granted our request for funding.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. TYPE 2 DIABETES MELLITUS 1. See Cochrane Metabolic and Endocrine Disorders Group search strategy. COMPLIANCE/ADHERENCE 2 Patient compliance [MeSH, all subheadings and categories included] 3 Health behavior [MeSH, all subheadings and categories included] 4 Health education [MeSH, all subheadings and categories included] 5 Self care [MeSH, all subheadings and categories included] 6 Patient education [MeSH, all subheadings and categories included] 7 Patient satisfaction [MeSH, all subheadings and categories included] 8 Educational status [MeSH, all subheadings and categories included] 9 Patient dropouts [MeSH, all subheadings and categories included] 10 Physician‐patient relations [MeSH, all subheadings and categories included] 11 Delivery of health care [MeSH, all subheadings and categories included] 12 (health NEAR (behavio*r or education*)) 13 (self‐care OR self‐management) 14 (complianc* OR adherenc*) 15 (patient* NEAR (education* OR satisfaction*)) 16 (relationship NEAR (physician* OR doctor*)) 17 patient* dropout* 18 treatment* refusal* 19 (empowerment* OR concordanc*) 20 delivery of health care 21 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR#18 OR #19 OR #20 RANDOMISED CONTROLLED CLINICAL TRIALS and CONTROLLED CLINICAL TRIALS 22 See Cochrane Metabolic and Endocrine Disorders Group search strategy SYSTEMATIC REVIEWS AND META‐ANALYSES 23 See Cochrane Metabolic and Endocrine Disorders Group search strategy ECONOMIC INFORMATION 24 See Cochrane Metabolic and Endocrine Disorders Group search strategy. TYPE 2 DIABETES AND COMPLIANCE/ADHERENCE AND DIFFERENT STUDY TYPES 25 #1 AND #21 AND (#22 OR #23 OR #24) |
Data and analyses
Comparison 1. Education/Facilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c | 9 | 1192 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.73, ‐0.25] |
| 1.1 3 months | 3 | 99 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.80, 1.40] |
| 1.2 4 months | 3 | 315 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.79, 0.37] |
| 1.3 6 months | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.21, 0.98] |
| 1.4 8 months | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.51, ‐0.29] |
| 1.5 9 months | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.28, 1.48] |
| 1.6 12 months | 3 | 459 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.75, 0.09] |
| 1.7 48 months | 1 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.15, ‐0.25] |
1.1. Analysis.
Comparison 1 Education/Facilitation, Outcome 1 HbA1c.
Comparison 2. Educationa/Facilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c | 10 | 1386 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.57, ‐0.15] |
| 1.1 Pharmacists | 4 | 666 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.24, ‐0.17] |
| 1.2 Nurses | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.12, ‐0.08] |
| 1.3 Educator | 4 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.71, ‐0.23] |
2.1. Analysis.
Comparison 2 Educationa/Facilitation, Outcome 1 HbA1c.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bradshaw 1999.
| Methods | ‐ Controlled before and after study ‐ Groups clearly defined ‐ Selection bias can be excluded ‐ Losses to follow‐up: not described ‐ Duration of follow‐up: clearly defined ‐ No correction for confounders ‐ Overall judgement of quality: medium | |
| Participants | ‐ 230 type 2 diabetes patients ‐ United Kingdom ‐ Urban ‐ Recruitement in primary care services ‐ Traditional (87), Video (86), Educator (57) ‐ Sex: M 52.2 % ‐ Age: mean 66.4, traditional (M: 66.7%), video (M: 46.8%), educator (M: 58.5%) ‐ Smoking: 22.2 %, traditional 28.7 %, video 23.3 %, educator 10.5% ‐ Weight: mean 77.9 kg ‐ HbA1c: mean 7.35, traditional 7.63, video 7.35, educator 6.91 | |
| Interventions | To assess the effectiveness of three structured educational programmes ( traditional, video and educator) on foot care, management and natural history of diabetes. | |
| Outcomes | Change in levels of knowledge, HbA1c, blood glucose, reported smoking cessation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Canga 2000.
| Methods | ‐ Randomised controlled trial ‐ Randomisation and concealment of allocation: adequate ‐ Adequate blinding of patients, administrators and outcome assessors ‐ description of losses to follow‐up and intention‐to‐treat analysis ‐ Groups similar at the start of the study ‐ Groups equally provided of care ‐ Overall judgement of quality: good | |
| Participants | ‐ 280 type 2 diabetes patients in outpatient settings in an urban area in Spain ‐ Multicentre study ‐ Sex: intervention (F: 22, M: 125), control (F: 18, M: 115) ‐ Age: intervention (54.4 +‐ 15), control (55.8 +‐ 14.9) ‐ Daily cigarettes: intervention (19.9 +‐ 11.9), control (19.8 +‐ 11.5) ‐ Years of smoking: intervention (36.4 +‐ 16), control (38 +‐ 15.2) ‐ BMI: intervention (27.0 +‐ 4.7), control (26.2 +‐ 4.4) ‐ treatment modality: insulin (intervention 47, control 47) | |
| Interventions | Nurse‐led education and counseling versus usual care for smoking cessation. | |
| Outcomes | Self‐reported and verified smoking cessation. Change in mean cigarettes per day. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
Clarke 2002.
| Methods | ‐ Controlled before and after study ‐ Blinding (patients, outcome assessors): no ‐ Losses to follow‐up: clear ‐ Duration of follow‐up: clear ‐ Overall judgement of quality: poor | |
| Participants | ‐ 748 type 2 diabetes patients ‐ Multicentre study, patients recruited in primary care services ‐ Sex: F: 386, M: 362 ‐ Age: mean 59 | |
| Interventions | To evaluate the effect of a Diabetes Healthways management programme. Behaviour and metabolic outcomes of patients enrolled are compared before and after. Nurse call system aimed at formulating health care goals and reinforcing behavioural changes. | |
| Outcomes | Change in medication taking, change in use of preventive services, HbA1c, LDL, diabetic retinopathy (DRE), microalbuminuria | |
| Notes | Of the 2982 patients enrolled , 748 were assessed in the survey. No baseline characteristics are shown for the enrolled population, though the survey sample is claimed to be representative. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Coast‐Senior 1998.
| Methods | ‐ Controlled before and after study ‐ No blinding ‐ Adequate description of losses to follow‐up ‐ Groups similar at the start of the study ‐ Overall judgement of quality: medium | |
| Participants | ‐ 23 type 2 diabetes patients ‐ Single centre study in an urban area in the USA ‐ Patients recruited in primary care services ‐ Sex: M: 23 ‐ Mean age: 65, SD 9.4 ‐ HbA1c: 11.1%, SD 1.6 ‐ Fasting blood glucose: 219 mg/dL 219, SD 45 mg/dl ‐ Random blood glucose: 236 mg/dl, SD 72 mg/dl ‐ Duration since diagnosis: 8.8 years, SD 4.2 ‐ Treatment modality: diet + oral hypoglycaemic agents + insulin: 23 | |
| Interventions | To determine the impact of clinical pharmacists, involved in direct patient care, on the management of patients with type 2 diabetes who require insulin. Patients were referred to clinical pharmacist for managment of diabetes: the intervention included an initial interview, initiating insulin treatment and adjusting insulin doses by pharmacists, and follow‐up by appointment or phone call. | |
| Outcomes | HbA1c, fasting blood glucose (FBG), random blood glucose | |
| Notes | Participants were veterans with multiple co‐morbidities (mean 4, range 2‐8) and on multiple medications (mean 5, range 2‐10) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Davidson 2000.
| Methods | ‐ Controlled, not randomised trial ‐ No blinding of patients or administrators, blinding of outcome assessment unclear ‐ Intention‐to‐treat‐analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Single centre study in primary care services in the USA ‐ Particpants: 181 type 2 diabetes patients (intervention: 89, control: 92) ‐ Sex: intervention (F: 42, M: 47), control (F: 46, M: 46) ‐ Mean age: intervention (54.8 years), control (51.8 years) ‐ Ethnicity: intervention (Afro‐American 14.6%, Caucasian 14.6%, Hispanic 69.7%), control (Afro‐American 17.4%, Caucasian 15.2%, Hispanic 64.1%, other 2.2%, undetermined 1.1%) ‐ Hypertension: intervention (67.4%), control (45.6%) ‐ HbA1c: intervention (8.8% (0.2) n=79), control (7.9 % (0.2), n=73) ‐ Retinopathy: intervention (n=29), control (n=14) ‐ Neuropathy: intervention (n=21), control (n=8) ‐ Nephropathy: intervention (n=35), control (n=13) ‐ Diabetic foot: intervention (n=15), control (n=1) ‐ Macrovascular complications (coronary artery disease or cerebrovascular disease): intervention (n=5), control (n=8) ‐ Treatment modality: diet alone (intervention 0, control 8), oral hypoglycaemic agents (intervention: 38, control: 51), insulin: (intervention: 20, control: 19), diet+oral agents+insulin: (intervention: 31, control 14) | |
| Interventions | To evaluate an evidence‐based process in a free medical clinic in patients referred to pharmacists using detailed algorithms versus standard physician led care. | |
| Outcomes | Change in HbA1c, refusal to make the recommended medication adjustements, appointment keeping, eye examination. | |
| Notes | Of the 89 participants in the intervention group 12 had type 1 diabetes, as did 9 persons in the control group. The intervention group has been referred is 'sicker' than the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Diehl 1985.
| Methods | ‐ Cross‐over study ‐ Adequate randomisation ‐ Adequate patient blinding, unclear blinding of administrators, and data missing on blinding of outcome assessors ‐ Adequate description of losses to follow‐up ‐ No intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Single centre study in the USA ‐ 77 type 2 diabetes patients recruited in outpatient settings ‐ Participants: intervention (chlorpropamide 40), control (insulin 37) ‐ Sex: intervention (F: 29, M: 11), control (F: 26, M: 11) ‐ Age: intervention (50.6 yrs, SD 9.5), control (51.2 yrs, SD 9.3) ‐ Ethnicity: intervention (95% Mexican‐American), control (91.9% Mexican‐American) ‐ Glycaemia: intervention (236.3 mg/dl, SD 81.5), control (243.6 mg/dl, SD 73.3) ‐ Treatment modality (prior to study): diet alone: intervention (40), control (37) | |
| Interventions | To compare compliance with chlorpropamide versus insulin among persons newly treated for type 2 diabetes. | |
| Outcomes | Percentage of expected medication taken, estimation of medication taken by patients, dropout rates, description of adherence on a 4 point scale, participants' attitudes and satisfaction. | |
| Notes | ||
Hopper 1984.
| Methods | ‐ Randomised controlled trial ‐ Randomisation adequate. ‐ Concealment of allocation: data missing ‐ Blinding of patients and administrators: data missing ‐ Blinding of outcome assessors: data missing ‐ Losses to follow‐up: data missing ‐ Intention‐to‐treat analysis: no ‐ Groups similar at the start of the study ‐ Groups equally provided of care ‐ Overall judgement of quality: poor | |
| Participants | ‐ 227 type 2 diabetes patients ‐ Outpatients in a city in the USA ‐ Participants: intervention (114), control (113) ‐ Sex: intervention (F: 83, M: 31), control (F: 84, M: 29) ‐ Mean age: intervention (59), control (57) ‐ Ethnicity: intervention (Black 90), control (Black 79) ‐ Blood glucose: intervention (226 95%CI 214‐238), control (221 95% CI 209‐233) ‐ Duration since diagnosis: intervention (11 years), control (12) ‐ Treatment modality: oral hypoglycaemic agents: intervention (19%), control (23%) ‐ Treatment with insulin: intervention (81%), control (77%) | |
| Interventions | Home health aids | |
| Outcomes | Fasting blood glucose, eye clinic attendance, negative utilisation (emergency room visits, missed visits) | |
| Notes | ||
Jaber 1996.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation ‐ No blinding of patients and administrators, unclear blinding of outcome assessment ‐ Losses‐to‐follow‐up adequately described ‐ No intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were not equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Single centre study in a city in the USA ‐ Outpatients ‐ 39 type 2 diabetes patients (intervention 17, control 22) ‐ Sex: intervention (F: 12, M: 5), control (F: 15, M: 7) ‐ Mean age: intervention (59, SD 12), control (65, SD 12) ‐ Ethnicity: intervention and control groups: 100% African American ‐ Mean weight: intervention (93 kg, SD 22), control (88 kg, SD 19) ‐ BMI: intervention (34, SD 7), control (33, SD 7) ‐ BMI > 27: intervention (13), control (18) ‐ Systolic blood pressure: intervention (140 mmHg, SD 20), control (143, SD 23) ‐ Diastolic blood pressure: intervention (82, SD 10), control (88, SD 9) ‐ Hypertension: intervention (14), control (17) ‐ Lipid abnormalities: intervention (10), control (11) ‐ HbA1c: intervention (11.5%, SD 2.9), control (12.2%, SD 3.5) ‐ Fasting blood glucose: intervention (11.1 mmol/l, SD 4), control (12.7 mmol/l, SD 4.7) ‐ Duration since diagnosis: intervention (6.8 years, SD 6.5), control (6.2 yrs, SD 4.8) ‐ Treatment modality: diet + oral hypoglycaemic agents: intervention (17), control (22) ‐ Exclusion criteria: IDDM, renal dysfunction, history of non‐attendance | |
| Interventions | To determine the impact of a comprehensive pharmaceutical care model (pharmacist provided care) versus standard care on treatment outcomes of NIDDM urban African Americans. Specific items of care were: evaluation and adjustment of doses, education regarding diabetes and its complications, training on recognition of hypoglycaemia and hyperglycaemia, instructions on diet and exercise, training in self‐monitorig of blood glucose. Treatment was titrated to targets. | |
| Outcomes | Blood glucose control, self‐recorded adherence, and self‐monitoring blood glucose logs | |
| Notes | Self‐monitoring mentioned, but not specified and no data were presented. | |
Jiang 1999.
| Methods | ‐ Controlled before and after study ‐ Patient , administrator of treatment, and outcome assessment blinding: data missing. ‐ Descritpion of losses to follow‐up are missing ‐ No intention to treat analysis ‐ Similarity of groups at the start of the study: not mentioned ‐ Groups equally provided of care: data missing ‐ Overall judgement of quality: poor | |
| Participants | ‐ Multicenter study in urban areas in Taiwan. ‐ 217 type 2 diabetes patients in an outpatient setting. ‐ Inclusion criteria: aged between 35 and 70 years old, able to read, HbA1c level >/=8.0% and with stable metabolic control. ‐ Sex: intervention (F 61, M 69), control (F 49, M 38) ‐ Age: intervention (52.3 +‐ 6.7), control (52.9 +‐ 7.4) ‐ Body Mass Index: intervention (25.2 +‐ 3.5), contol (25.6 +‐ 3.2) ‐ Duration of disease since diagnosis: intervention (8.0 years +‐ 8.0), control 6.7 yrs +‐ 5.3) ‐ Treatment: Diet and oral hypoglycaemic agents: intervention 121, control 87 | |
| Interventions | A 5 section education program was offered to type 2 diabetes patients. Diabetes self‐care was assessed before and 4 months after attending a diabetes education programme including basic knowledge, dietary control, blood glucose monitoring, management of hypoglycemia, medication compliance, foot care and exercise. A total of 121 attended four to five sections and were called the intervention group, and the 87 who only received the basic section were called the control group. | |
| Outcomes | Fasting plasma glucose, HbA1c, total cholesterol, triglycerides, systolic and diastolic blood pressure, body weight, and waist‐hip ratio. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Krier 1999.
| Methods | ‐ Randomised Controlled Trial ‐ Randomisation adequate ‐ Patients are blinded, but not the administrator of treatment, and on blinding of outcome assessment data are missing. ‐ Losses to follow‐up described. ‐ Intention‐to‐treat analysis: unclear ‐ Groups were similar at the start of the study. ‐ Groups were equally provided of care. ‐ Overall judgement of quality: medium | |
| Participants | ‐ 39 type 2 diabetes patients living in a city in the United States. ‐ 21 people were allocated to the intervention group and 18 to the control group. ‐ Sex: intervention (F: 9, M: 5), control (F: 10, M: 4). ‐ Age: intervention (54.2 +‐ SD 5.6), control (56.2 +‐ SD 9). ‐ Ethnicity: intervention (6 Afro Americans), control (7 Afro Americans). ‐ Smoking: intervention (3), control (3). ‐ Weight: intervention (97.4 kg +‐ 23.9), control (93.8 +‐ 19.4). ‐ Locus of control: intervention (4.5 +‐1), control (4.0 +‐ 1.1). ‐ Knowledge test: intervention (11.3 +‐ 3.8), control (14.2 +‐ 3.6). ‐ HbA1c: intervention (9.6% +‐ 1.9), control (10.3% +‐ 2.3). ‐ Treatment modality: oral hypoglycemic agents (intervention 8, control 7), insulin (intervention 5, control 5), combination (intervention 1, control 2). | |
| Interventions | To evaluate the effect of quarterly visits of a diabetes educator versus usual care. | |
| Outcomes | HbA1c, 4‐point Lickert scale on compliance with diet and medication. | |
| Notes | ||
Matsuyama 1993.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation and concealment of allocation ‐ Blinding of patients and adminstrators of treatment ‐ No blinding of outcome assessors. ‐ Losses to follow described ‐ No intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ 32 type 2 diabetes outpatients (USA veterans) ‐ Single centre study in an urban area in the USA ‐ Participants: intervention: 15, control: 17 ‐ Sex: intervention (M: 15), control (F: 1, M: 16) ‐ Age: intervention (mean 64 years, SD 7), control (mean 64 yrs, SD 8) ‐ HbA1c: intervention (12.7%, SD 1.9), control (12.1 %, SD 2.6) ‐ Glycaemia: intervention (12.1 mmol/l, SD 1.9; 167.81 mg/dl, SD 35.57 ), control 11.1 mmol/l, SD 2.9; 147.63 mg/dl, SD 38.57) ‐ Duration since diagnosis: intervention (6.7 years, SD 7.6 yrs), control (8.5 years, SD 8.5 yrs) ‐ Treatment modality: oral hypoglycaemic agents: intervention 15, control 16 | |
| Interventions | To compare adherence data from a medication event monitoring system (MEMS) with pill counts in assisting pharmacists in making recommendations regarding diabetes therapy. | |
| Outcomes | MEMS and pill count adherence rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
Mease 2000.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation ‐ Unclear blinding of patients, administrators or outcome assessors ‐ Adequate description of losses to follow‐up ‐ Intention‐to‐treat analysis ‐ Groups were similar at the start of the study and were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Single centre study in the USA ‐ 28 Type 2 diabetes patients recruited in primary care services ‐ Inclusion: HbA1c >8.0% | |
| Interventions | To determine whether telemedicine can improve self‐care for type 2 diabetes: education classes and weekly telemedicine 'visits' versus education only. | |
| Outcomes | HbA1c and weight. | |
| Notes | There are no data on adherence, although the intervention included 'reinforcement of medication compliance'. | |
Paes 1997.
| Methods | ‐ Observational study ‐ Groups clearly defined ‐ Selection bias can be excluded ‐ Losses to follow‐up: not described ‐ Duration of follow‐up: clear ‐ Confounders: not mentioned and not corrected for ‐ Overall judgement of quality: good | |
| Participants | ‐ 91 type 2 diabetes patients ‐ Multicentre study in an urban area in the Netherlands ‐ Patients recruited in general practice ‐ Sex: F 59.6% ‐ Age: F 70.1 +‐ 10.7, M: 67.8 +‐ 11.2 ‐ Treatment modality: oral hypoglycaemic agents | |
| Interventions | To evaluate the impact of dosage frequency ‐ once, twice or three times a day ‐ on the compliance of patients who receive their medicines from community pharmacies. | |
| Outcomes | Compliance with dosage and with prescribed regimen measured with MEMS, pill counts, pharmacy records. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Piette 2001.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation and concealment of allocation ‐ Blinding of patients and administrators: adequate ‐ Adequate blinding of outcome assessment ‐ Description of losses to follow‐up and intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ No information on control group care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Type 2 diabetes patients recruited in an outpatient setting in a mixed rural and urban area. ‐ Participants: intervention (132), control (140) ‐ sex: intervention (M: 126), control (M: 138) ‐ mean age: intervention (60 yrs, SD 10), control (61 yrs, SD 10) ‐ ethnicity: inter vention (Black: 32, Hispanic 18, Other 11), control (Black 17, Hispanic 16, Other 15) ‐ BMI: intervention (31, SD 7), control (31, SD 6) ‐ HbA1c: intervention (8.2%, SD 1.7), control (8.1, SD 1.7) ‐ serum glucose: intervention (188 mg/dl, SD 94), control (168 mg/dl, SD 68) ‐ all complications: intervention (1), control (0.7) ‐ vascular symptoms: intervention (0.7, SD 0.8), control (0.6, SD 0.8) ‐ treatment modality: insulin: intervention (39), control (31) | |
| Interventions | To evaluate automated telephone disease management with telephone nurse follow‐up versus usual care, as a strategy for improving diabetes treatment processes and outcomes. | |
| Outcomes | All diabetes related symptoms, hypo‐ and hyperglycaemic symptoms, vascular symptoms, HbA1c, serum glucose, self‐monitoring frequency, foot inspection, weight monitoring, podiatry visits, ophtalmology visits, diabetic clinic visits, cholesterol tests. | |
| Notes | Inclusion criteria: veterans aged younger than 75, with a life expectancy of more than 12 months, not newly diagnosed type 2 diabetes, and having a touch tone telephone a home. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
Pullar 1988.
| Methods | ‐ Randomised controlled trial ‐ Concealment of allocation: data missing ‐ Blinding (patient, administrator, outcome measurement): data missing ‐ losses to follow‐up: described ‐ intention‐to‐treat analysis: unclear ‐ similarity of groups at the start of the study: not mentioned ‐ groups equally provided of care: unclear ‐ overall judgement of quality: poor | |
| Participants | ‐ 179 type 2 diabetes outpatients ‐ Single centre study in a city in the UK. ‐ Age: group 1 (64.9 years +‐ 8.6), group 2 (63.5 years +‐ 10.5), group 3 (63.6 years +‐ 10.1) ‐ Treatment modality: all patients on diet and oral hypoglycaemic agents | |
| Interventions | Comparison of compliance with tablets prescribed once, twice, or three times a day. | |
| Outcomes | Tablet count, phenobarbital LDR (level/dose ratios). | |
| Notes | ||
Rachmani 2002.
| Methods | ‐ Randomised controlled trial ‐ Randomisation adequate ‐ Blinding of patients or administrators of treatment: data missing ‐ Unclear blinding of outcome assessors ‐ Data missing on losses to follow‐up ‐ Intention‐to‐treat analysis ‐ Groups were similar at the start of the study and were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐Single centre study in Israel ‐ Not clear whehter the patients were recruted in a rural or an urban area. ‐ 142 type 2 diabetes patients: intervention (71), control (70) ‐ Sex: intervention (F: 35, M: 36), control (F: 37, M: 33) ‐ Mean age: intervention (57.4 years), control (56.8 years) ‐ BMI: intervention (28.4), control (28.7) ‐ Systolic blood pressure: internvention (162 mmHg, SD 7.3), control (160 mmHg, SD 6.9) ‐ Diastolic blood pressure: intervention (96 mmHg, SD 2.4), control (95 mmHg, SD 2.0) ‐ High Density Lipoproteines: (intervention 38 mg/dL, SD 3), control (39 mg/dL, SD 4) ‐ Trigycerides: intevention (236 mg/100 ml, SD 42), control (243 mg/100ml, SD 36) ‐ Low Density Lipoproteines: intervention (146 mg/dL, SD 10), control (146 mg/dL, SD 9) ‐ HbA1c: intervention (9.5 %, SD 1.6), control (9.6 %, SD 1.9) ‐ Retinopathy: intervention (11), control (10) ‐ Treatment modality: diet alone (intervention 18, control 16), diet + oral hypoglycaemic agent (intervention 43, control 46), diet + insulin (intervention 10, control 8) | |
| Interventions | To examine whether in comparison to a standard consultation, sharing the therapeutic responsability with patients (involving them in the process of their management by providing them with tools to monitor and supervise the effects of therapy) will improve the outcome. | |
| Outcomes | Total mortality, non‐fatal vascular events, cerebrovascular death, non‐fatal acute myocardial infarction, non‐fatal stroke, coronary artery bypass surgery, HbA1c, BMI, albumin/creatinine ratio, blood pressure, retinopathy and glomerular filtration rate. | |
| Notes | ||
Rosenkranz 1996.
| Methods | ‐ Randomised controlled trial ‐ Randomisation adequate ‐ Blinding (patients, administrators, outdome assessors): no ‐ Losses to follow‐up: clearly described ‐ Intention‐to‐treat analysis: unclear ‐ Similarity of groups at the start of the study: not mentioned ‐ Groups were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ 113 type 2 diabetes patients ‐ Single centre study with outpatients in a mixed rural and urban area in Germany. ‐ Group A (37), group B (35), group C (31) ‐ Sex: group A (F: 14, M: 23), group B (F: 14, M: 21), group C (F: 12, M: 19) ‐ Duration since diagnosis: group A (<5 years 15, 5‐10y: 10, 11‐20y: 6, > 20y: 6); group B (<5y: 5, 5‐10y: 10, 11‐20y: 12, >20y:6); group C: <5 y: 6, 5‐10y: 17, 11‐20y: 6, >20: 2 | |
| Interventions | To investigate whether instantaneous Polaroid fundusphotgraphy during a patient consultation would affect future ophtalmological screening behaviour: picture not shown (A) versus picture shown, explained and handed out (B) versus picture shown, explained but not handed out (C). | |
| Outcomes | Compliance to ophtalmologist consultation recommendations. | |
| Notes | ||
Simmons 2000.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation and concealment of allocation ‐ Blinding (patients, administrators, outcome assessors): adequate ‐ Description of losses to follow‐up: adequate ‐ Intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care: unclear ‐ Overall judgement of quality: good | |
| Participants | ‐ 68 type 2 diabetes patients in a multi‐centre study in an urban area in New‐Zealand ‐ Sex: intervention (F: 25, M: 11), control (F: 23, M: 9) ‐ Age: intervention (55 years +‐ 11), control (53 years +‐ 11) ‐ Ethnicity: local (intervention 26, control 25) ‐ Weight: intervention (100,4 kg +‐ 24.3), control (104.1 kg +‐ 26.6) ‐ BMI: intervention (37.3 +‐ 8), control (38.5 +‐ 10.2) ‐ Systolic blood pressure: intervention (141 mmHg +‐ 16), control (142 mmHg +‐ 18) ‐ Diastolic blood pressure: intervention (83 mmHg +‐ 10), control (80 mmHg +‐ 9) ‐ HbA1c: intervention (9.9% +‐ 2.2), control (9.5 % +‐ 1.7) ‐ Insulin treated: intervention (2), control (5) | |
| Interventions | To assess the impact of calendar blister pack use versus usual delivery of tablets. | |
| Outcomes | HbA1c, blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
Skaer 1993.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisation ‐ Blinding of patients, administrators and outcome assessors: inadequate ‐ No description of losses to follow‐up ‐ Intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care ‐ Overall judgement of quality: medium | |
| Participants | ‐ Single centre study in the USA ‐ 258 Type 2 diabetes patients recruited from primary care services ‐ Participants : intervention I1 (79), I2 (53), I3 (48), control (78) ‐ Sex: I1 (F: 54, M: 25), I2 (F: 31, M:22), I3 (F:29, M: 19), control (F: 49, M: 29) ‐ Mean age: I1 (53.6 yrs, SD 7.7), I2 (51.8 yrs, SD 8.6), I3 (52.3 yrs, SD 8.4), control (51.7 yrs, SD 7.8) ‐ Treatment modality: oral hypoglycaemic agents: I1: 79, I2: 53, I3: 48, control: 78 | |
| Interventions | To discern the effect of mailed prescription‐refill reminders (I1), specialised packaging (I2), or a combination of both (I3) on prescription refill compliance and health service utilisation. | |
| Outcomes | MPR (medication prescription refill), number of days' supply obtained over 360 days of the trial. | |
| Notes | Previously untreated type 2 diabetes patients, prescribed glyburide bd, no utilisation of nurse care for prior 3 months. | |
Smith 1986.
| Methods | ‐ Randomised controlled trial ‐ Randomisation: adequate ‐ Blinding (patient, administrator, outcome assessor): unclear ‐ Losses to follow‐up: described ‐ Intention‐to‐treat analysis: unclear ‐ Similartity of groups: clear ‐ Groups equally provided of care: unclear ‐ Overall judgement of quality: medium | |
| Participants | ‐ 859 type 2 diabetes outpatients ‐ Single centre study in the USA ‐ Sex: intervention (F: 316, M: 113), control (F: 323, M: 107) ‐ Mean age: intervention (59.3 years +‐12.5), control (59.0 yrs +‐ 13.2) ‐ Ethnicity: intervention (301 Black), control (300 Black) ‐ Systolic blood pressure: intervention (139 mmHg +‐22), control (138 mmHg +‐ 22) ‐ Diastolic blood pressure: intervention (80 mmHg +‐ 12), control (79 mmHg +‐ 11) ‐ Random glycaemia: intervention (209 mg/dL +‐ 94), control (216 mg/dL +‐ 94) ‐ Treatment modality: oral hypoglycemic agents (intervention: 97, control: 120), insulin (intervention: 328, control: 304), combination (intervention: 4, control: 6) | |
| Interventions | To determine the effect of mailed packets, mailed appointment reminders and intense follow‐up of visit failures. | |
| Outcomes | Appointment keeping, prescription refill, walk‐in visits, total visits and failure rate. | |
| Notes | ||
White 1986.
| Methods | ‐ Randomised controlled trial ‐ Adequate randomisztion ‐ Concealment of allocation: inadequate ‐ Blinding of patient, administrator of treatment, and of outcome assessment: data missing ‐ Losses to follow‐up clearly described and intention‐to‐treat analysis ‐ Groups were similar at the start of the study ‐ Groups were equally provided of care ‐ Overall judgement of quality: poor | |
| Participants | ‐ 32 type 2 diabetes outpatients ‐ Inclusion criteria: less than satisfactory control (FBG >140 mg/dl), infrequent hypoglycaemic reactions (<1/mo), no history of ketoacidosis, body weight >15% above the mean value for height, no history of alcohol abusus or severe personality disorder, and no current use of glucocorticoids. ‐ Sex: men only ‐ Age: intervention (62.4 years +‐ 5.5), control (60.7 years +‐ 6.4) ‐ Percentage of weight excess: intervention (36.3% +‐ 21.0), control (44.3% +‐ 21.0) ‐ Duration since diagnosis: intervention (10.2 years +‐ 12.9), control (13.6 years +‐ 9.6) | |
| Interventions | To compare the effect of group management versus an advice‐educational technique. | |
| Outcomes | HbA1c | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1990 | No measurement of adherence. |
| Arauz 2001 | No assessment of adherence. |
| Barcelo 2001 | Assessment of dietary adherence only. |
| Basa 1994 | No assessment of adherence. |
| Benjamin 1999 | Assessment of doctors' adherence with standards of care, not of patients with treatment. |
| Brown 1999 | No assessment of adherence. |
| Cabrera‐Pivaral 2000 | The effectiveness of an educational program aimed at modifying human behaviors in dietetic habits. |
| Campbell 1996 | Assessment of treatment intensity, not of adherence. |
| Coleman 1998 | No assessment of adherence. |
| Cooper 1998 | No data on adherence. |
| d'Eramo‐Melkus 1992 | No assessment of medication adherence. |
| Day 1992 | Asessment of a new integrated system of diabetes care with diabetes nurses, no assessment of adherence to treatment recommendations. |
| de Grauw 2001 | No assessment of an intervention intended to enhance adherence. |
| de Sonnaville 1997 | No assessment of adherence adherence. |
| Domenech 1995 | Judgement of the overall treatment quality, but no assessment of medication adherence. |
| Estey 1990 | Self‐monitoring of blood glucose does not count as a medical recommendation. |
| Fontbonne 1989 | No assessment of adherence. |
| Fritsche 1999 | No assessment of adherence. |
| Greenfield 1994 | No measurement of adherence. |
| Guillausseau 2001 | No data on adhernce to treatment recommendations. |
| Halbert 1999 | Not clear whether patients had type 2 diabetes. No real control group. |
| Hiss 2001 | Adherence was not specifically the aim of the intervention, no assessment of adherence. |
| Hoskins 1988 | No assessment of interventions to enhance adherence. |
| Jirkovska 2000 | No assessment of adherence. |
| Jones 1989 | Judging from mean age and number of years since diagnosis: type 1 diabetes patients. |
| Karter 2001 | No intervention. Only characteristics of adherent and non‐adherent people were assessed. |
| Kim 1996 | Effect on knowledge, self‐control of eating behaviour and coping with stress. No assessment of adherence. |
| Kinmonth 1998 | No assessment of adherence. |
| Kirkman 1994 | Although nurse calls are designed to encourage compliance, no specific measurement is taken. No focus on adherence to medication. Adherence is measured to diet, exercise and quitting smoking only. |
| Kronsbein 1988 | Adherence to glucosuria monitoring instructions. |
| Lo 1996 | No assessment of adherence. |
| Mazzuca 1986 | Authors assessed compliance by questionnaire, but they do not report the results. |
| McDermott 2001 | No assessment of adherence. |
| Morgan 1988 | No assessment of adherence. |
| Muchmore 1994 | Self‐monitoring blood glucose and dietary adherence. |
| Mulrow 1987 | Effectiveness of educational intervention in relation to compliance beliefs. |
| Neumann 1989 | No intervention neither a measurement of adherence. |
| Oi 1998 | No assessment of adherence. |
| Ovhed 2000 | No assessment of adherence. |
| Pieber 1995 | No assessment of adherence. |
| Pill 1998 | No assessment of adherence. |
| Ridgeway 1999 | Assessment of changes in medication requirements, not of adherence. |
| Rubin 1991 | Assessment of self‐care, not of adherence. |
| Rutten 1990 | No assessment of an intervention aimed at improving adherence, a protocol was studied instead. |
| Smith 1987 | Duplicate publication of Smith DM 1986 (included) whithout any additional information on adherence, but only more data on non‐elective hospitalisations. |
| Tamai 1995 | No extractable data. |
| Trento 2001 | No assessment of adherence. |
| Tu 1993 | Study duration only four weeks. |
| van den Arend 2000 | Self‐care behaviour was assessed by a self‐report questionnaire without any question on medication adherence. |
| Wedman 1987 | No assessment of an intervention aimed at enhancing adherence to treatment recommendations. |
| Weinberger 1995 | Although nurse calls are designed to encourage compliance, no specific measurement for adherence is taken. No focus on adherence to medication. |
| Whitlock 2000 | No assessment of adherence. |
| Wilson 1987 | No assessment of adherence. |
Contributions of authors
ETIENNE VERMEIRE: protocol development, literature searches, inclusion/exclusion of studies, quality assessment of trials, data extraction, data analysis, development of final review, corresponding author
JOHAN WENS: protocol development, inclusion/exclusion of studies, quality assessment of studies, data extraction, data analysis, development of final review
PAUL VAN ROYEN: protocol development, inclusion/exclusion of studies, quality assessment and data extraction, co‐drafting of the review
HILARY HEARNSHAW: quality assessment and data extraction , co‐drafting of review
ANTJE LINDENMEYER: quality assessment and data extraction
YVES BIOT: inclusion/exclusion of studies, quality assessment and data extraction
Sources of support
Internal sources
University of Antwerp, Belgium.
External sources
Centre for Primary Health Care Studies, University of Warwick, UK.
Primary Care Diabetes Europe, Belgium.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bradshaw 1999 {published data only}
- Bradshaw C, McColl E, Eccles M, Bryce C, Sampson R. Can we improve the education for type 2 diabetes patients in general practice?. Practical Diabetes International 1999;16(8):241‐5. [EndNote 96] [Google Scholar]
Canga 2000 {published data only}
- Canga N, Irala J, Vara E, Duaso MJ, Ferrer A, Martinez‐Gonzalez MA. Intervention study for smoking cessation in diabetic patients. Diabetes Care 2000;23(10):1455‐60. [EndNote 97] [DOI] [PubMed] [Google Scholar]
Clarke 2002 {published data only}
- Clarke J, Crawford A, Nash DB. Evaluation of a comprehensive diabetes disease management program: progress in the struggle for sustained behavior change. Disease management 2002;5(2):77‐86. [EndNote 94] [Google Scholar]
Coast‐Senior 1998 {published data only}
- Coast‐Senior EA, Kroner BA, Kelley CL, Trilli LE. Management of patients with type 2 diabetes by pharmacists in primary care clinics. The Annals of Pharmacotherapy 1998;32:636‐41. [EndNote 34] [DOI] [PubMed] [Google Scholar]
Davidson 2000 {published data only}
- Davidson MB, Karlan VJ, Hair TL. Effect of a pharmacist‐managed diabetes care program in a free medical clinic. American Journal of Medical Quality 2000;15(4):137‐42. [EndNote 60] [DOI] [PubMed] [Google Scholar]
Diehl 1985 {published data only}
- Diehl AK, Sugarek NJ, Bauer RL. Medication compliance in non‐insulin‐dependent diabetes: a randomized comparison of chlorpropamide and insulin. Diabetes Care 1985;8(3):219‐23. [EndNote 19] [DOI] [PubMed] [Google Scholar]
Hopper 1984 {published data only}
- Hopper SV, Miller JP, Birge C, Swift J. A randomized study of the impact of home health aides on diabetic control and utilization patterns. American Journal Public Health 1984;74(6):600‐2. [EndNote 64] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaber 1996 {published data only}
- Jaber LA, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmaceutical care model on diabetes management. The Annals of Pharmacotherapy 1996;30:238‐43. [EndNote 36] [DOI] [PubMed] [Google Scholar]
Jiang 1999 {published data only}
- Jiang Y‐D, Chuang L‐M, Wu H‐P, Shiau Q‐J, Wang C‐H, Lee Y‐J, Juang J‐H, et al. Assessment of the function and effect of diabetes education programs in Taiwan. Diabetes Research and Clinical Practice 1999;46:177‐82. [EndNote 72] [DOI] [PubMed] [Google Scholar]
Krier 1999 {published data only}
- Krier BP, Parker RD, Grayson D, Byrd G. Effect of diabetes education on glucose control. The Journal of the Louisiana State Medical Society : official organ of the Louisiana State Medical Society 1999;151:86‐92. [EndNote 54] [PubMed] [Google Scholar]
Matsuyama 1993 {published data only}
- Matsuyama JR, Mason BJ, Jue SG. Pharmacists' interventions using an electronic medication‐event monitoring device's adherence data versus pill counts. The Annals of Pharmacotherpay 1993;27:851‐5. [EndNote 2] [DOI] [PubMed] [Google Scholar]
Mease 2000 {published data only}
- Mease A, Whitlock WL, Brown A, Moore K, Pavlascak, Dingbaum A, Lacefield D, Buker K, Xenakis S. Telemedicine improved diabetic management. Military Medicine 2000;165(8):579‐84. [EndNote 76] [PubMed] [Google Scholar]
Paes 1997 {published data only}
- Paes AHP, Bakker A, Soe‐Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care 1997;20(10):1512‐7. [EndNote 3] [DOI] [PubMed] [Google Scholar]
Piette 2001 {published data only}
- Piette JD, Weinberger M, Kraemer FP, McPhee SJ. Impact of automated calls with nurse follow‐up on diabetes treatment outcomes in a department of veteran affairs health care system. Diabetes Care 2001;24(2):202‐8. [EndNote 92] [DOI] [PubMed] [Google Scholar]
Pullar 1988 {published data only}
- Pullar T, Birtwell AJ, Wiles PG, Hay A, Feely MP. Use of a pharmocologic indicator to compare compliance with tablets prescribed to be taken once, twice, or three times daily. Clinical pharmacology and therapeutics 1988;44:540‐5. [EndNote 41] [DOI] [PubMed] [Google Scholar]
Rachmani 2002 {published data only}
- Rachmani R, Levi Z, Slavachevski I, Avin M, Ravid M. Teaching patients to monitor their risk factors retards the progression of vascular complications in high‐risk patients with Type 2 diabetes mellitus ‐ a randomized prospective study. Diabetic Medicine 2002;19:385‐92. [EndNote 55] [DOI] [PubMed] [Google Scholar]
Rosenkranz 1996 {published data only}
- Rosenkranz S. Polaroid‐Fundusfotografie erhöht die Patientencompliance beim Screening auf diabetische Retinopathie. Diabetes und Stoffwechsel 1006;5:69‐75. [EN 29] [Google Scholar]
Simmons 2000 {published data only}
- Simmons D, Upjohn M, Gamble GD. Can medication packaging improve glycemic contol and blood pressure in type 2 diabetes?. Diabetes Care 2000;23(2):153‐6. [EndNote 93] [DOI] [PubMed] [Google Scholar]
Skaer 1993 {published data only}
Smith 1986 {published data only}
- Smith DM, Norton JA, Weinberger M, McDonald CJ, Katz BP. Increasing prescribed office visits. A controlled trial in patients with diabetes mellitus. Medical Care 1986;24(3):189‐99. [EndNote 62] [PubMed] [Google Scholar]
White 1986 {published data only}
- White N, Carnahan J, Nugent CA, Iwaoka T, Dodson MA. Management of obese patients with diabetes mellitus: comparison of advice education with group management. Diabetes Care 1986;9(5):490‐6. [EndNote 91] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1990 {published data only}
- Allen BT, DeLong ER, Feussner JR. Impact of glucose self‐monitoring on non‐insulin‐treated patients with type II diabetes mellitus. Diabetes care 1990;11(10):1044‐50. [EndNote 53] [DOI] [PubMed] [Google Scholar]
Arauz 2001 {published data only}
- Arauz AG, Sanchez G, Padilla G, Fernandez M, Rosello M, Guzman S. A communtiy diabetes educational intervention at the primary‐care level [Intervencion educativa comunitaria sobre la diabetes en el ambito de la atencion primaira]. Pan Am J Public Health 2001;9(3):145‐3. [EndNote 31] [DOI] [PubMed] [Google Scholar]
Barcelo 2001 {published data only}
- Barcelo A, Robles S, White F, Jadue L, Vega J. An intervention to improve diabetes control in Chile [Una intervencion para mejorar el control de la diabetes en Cile]. Pan American journal of public health 2001;10(5):328‐33. [EndNote 33] [PubMed] [Google Scholar]
Basa 1994 {published data only}
- Basa RP, McLeod B. Evaluation of a diabetes specialty centre: structure, process and outcome. Patient Education Counseling 1995;25:23‐9. [EndNote 79] [DOI] [PubMed] [Google Scholar]
Benjamin 1999 {published data only}
- Benjamin EM, Schneider MS, Hinchey KT. Implementing practice guidelines for diabetes care using problem‐based learning. Diabetes Care 1999;22(10):1672‐8. [EndNote 43] [DOI] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown SA, Hanis CL. Culturally competent diabetes education for Mexican Americans: the Starr County Study. Diabetes Educator 1999;25(2):226‐36. [EndNote 45] [DOI] [PubMed] [Google Scholar]
Cabrera‐Pivaral 2000 {published data only}
- Cabrera‐Pivaral CE, Gonzalez‐Perez G, Vega‐Lopez G, Gonzalez‐Hita M, Centeno‐Lopez M, Gonzalez‐Ortiz M, et al. Effects of behavior‐modifying education in the metabolic profile of the Type 2 diabetes mellitus patient. Journal of Diabetes and its Complications 2000;14:322‐6. [EndNote 46] [DOI] [PubMed] [Google Scholar]
Campbell 1996 {published data only}
- Campbell AM, Redman S, Moffitt PS, Sanson‐Fisher RW. The relative effectiveness of educational and behavioral instruction programs for patients with NIDDM: a randomized trial. The Diabetes Educatore 1996;22(4):379‐86. [EndNote 49] [DOI] [PubMed] [Google Scholar]
Coleman 1998 {published data only}
- Coleman R, Gill G, Wilkinson D. Noncommunicable disease management in resource‐poor settings: a primary care model from rural South Africa. Bulletin of the World Health Organization 1998;76(6):633‐40. [EN 35] [PMC free article] [PubMed] [Google Scholar]
Cooper 1998 {published data only}
- Cooper HC, Lorains JW. Managing poor control in type 2 diabetes. Practical Diabetes International 1998;15(6):173‐7. [EndNote 37] [Google Scholar]
d'Eramo‐Melkus 1992 {published data only}
- d'Eramo‐Melkus GA, Wylie‐Rosett J, Hagan JA. Metabolic impact of education in NIDDM. Diabetes Care 1992;15(7):864‐9. [EndNote 52] [DOI] [PubMed] [Google Scholar]
Day 1992 {published data only}
- Day JL, Metcalfe J, Johnson P. Benefits provided by an integrated education and clinical diabetes centre: a follow‐up study. Diabetic Medicine 1992;9:855‐9. [EndNote 71] [DOI] [PubMed] [Google Scholar]
de Grauw 2001 {published data only}
- Grauw WJC, Lisdonk EH, Gerwen WHEM, Hoogen HJM, Weel C. Insulin therapy in poorly controlled type 2 diabetic patients: does it affect quality of life?. B J Gen Pract 2001;51:527‐32. [EN 7] [PMC free article] [PubMed] [Google Scholar]
de Sonnaville 1997 {published data only}
- Sonnaville JJJ, Bouma M, Colly LP, Devillé W, Wijkel D, Heine RJ. Sustained good glycaemic control in NIDDM patients by implementation of structured care in general practi: 2‐year follow‐up study. Diabetologia 1997;40:1334‐40. [EndNote 78] [DOI] [PubMed] [Google Scholar]
Domenech 1995 {published data only}
- Domenech MI, Assad D, Mazzei ME, Kronsbein P, Gagliardino JJ. Evaluation of the effectiveness of an ambulatory teaching/trteatment programme for non‐insulin dependent (type 2) diabetic patients. Acta diabetologica 1995;32:143‐7. [EndNote 8] [DOI] [PubMed] [Google Scholar]
Estey 1990 {published data only}
- Estey AL, Tan MH, Mann K. Follow‐up intervention: its effect on compliance behavior to a diabetes regimen. Diabetes Educator 1990;16(4):291‐5. [EndNote 9] [DOI] [PubMed] [Google Scholar]
Fontbonne 1989 {published data only}
- Fontbonne A, Billault B, Acosta M, Percheron C, Varenne P, Besse A, et al. Is glucose self‐monitoring beneficial in on‐insulin‐treated diabetic patients? Results of a randomized comparative trial. Diabète & Métabolisme 1989;15:255‐60. [EndNote 98] [PubMed] [Google Scholar]
Fritsche 1999 {published data only}
- Fritsche A, Stumvoll M, Goebbel S, Reinauer KM, Schmülling RM, Häring HU. Long term effect of a structurend inpatient diabetes teaching and treatment programme in type 2 diabetic patients: influence of mode of follow‐up. Patient Education Counseling 1999;46:135‐41. [EndNote 67] [DOI] [PubMed] [Google Scholar]
Greenfield 1994 {published data only}
- Greenfield S, Kaplan SH, Sillman RA, Sullivan L, Manning W, D'Agostino R, et al. The uses of outcomes research for medical effectiveness, quality of care, and reimbursement in type II diabetes. Diabetes Care 1994;17(Suppl 1):32‐9. [EndNote 17] [PubMed] [Google Scholar]
Guillausseau 2001 {published data only}
- Guillausseau PJ, Greb W. 24‐hour glycemic profile in type 2 diabetic patients treated with gliclazide modified release once daily. Diabetes Medidine 2001;27:133‐7. [EndNote 13] [PubMed] [Google Scholar]
Halbert 1999 {published data only}
- Halpert RJ, Leung K‐M, Nichol JM, Legorreta AP. Effect of multiple patient reminders in improving diabetic retinopahty screening. Diabetes Care 1999;22(5):752‐5. [EndNote 84] [DOI] [PubMed] [Google Scholar]
Hiss 2001 {published data only}
- Hiss RG, Gillard ML, Armbruster BA, McClure LA. Comprehensive evaluation of community‐based diabetic patients. Effect of feedback to patients and their physicians: a randomized controlled trial. Diabetes Care 2001;24(4):690‐4. [EndNote 57] [DOI] [PubMed] [Google Scholar]
Hoskins 1988 {published data only}
- Hoskins PL, Alford JB, Handelsman DJ, Yue DK, Turtle JR. Comparison of different models of diabetes care on compliance with self‐monitoring of blood glucose by memory glucometer. Diabetes Care 1988;11(9):719‐24. [DOI] [PubMed] [Google Scholar]
Jirkovska 2000 {published data only}
- Jirkovska A, Havlova V, Hrachoviniva T, Fejfarova V, Hosova J, Maskova J, et al. The treatment effectg of education on the metabolic status of type 2 diabetic patients [Lecebny ucinek ambulantniho edukacniho kurzu na metabolicky syndrom u diabetiku 2 typu]. Praktický lékar 2000;80(2):95‐8. [EndNote 38] [Google Scholar]
Jones 1989 {published data only}
- Jones PM. Use of a course on self‐control behavior techniques to increase adherence to prescribed frequency for self‐monitoring blood glucose. Diabetes Educator 1989;16(4):296‐303. [EndNote 95] [DOI] [PubMed] [Google Scholar]
Karter 2001 {published data only}
- Karter AJ, Ackerson LM, Darbanian JA, D'Agostino RB, Ferrara A, Liu J. Self‐monitoring of blood glucose levels and glycemic control: the Northern California Kaiser permanent diabets regystry. The American journal of medicine 2001;111:1‐9. [EndNote 15] [DOI] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim WS, Sakano Y. Cognitive‐behavioral intervention to chronic disease patients. Jpn J Psychosom Med 1996;36:27‐32. [EndNote 23] [Google Scholar]
Kinmonth 1998 {published data only}
- Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ, the Diabetes Care from Diagnosis Research Team. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. BMJ 1998;317:1202‐8. [EndNote 82] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkman 1994 {published data only}
- Kirkman MS, Weinberger M, Landsman PB, Samsa GP, Shortliffe EA, Simel DL. A telephone‐delivered interevention for patients with NIDDM. Diabetes Care 1994;17(8):840‐6. [EndNote 1] [DOI] [PubMed] [Google Scholar]
Kronsbein 1988 {published data only}
- Kronsbein P, Mühlhauser I, Venhaus A, Jörgens V, Scholz V, Berger M. Evaluation of a structured treatment and teaching programme on non‐insulin‐dependent diabetes. Lancet 1988:1407‐10. [EndNote 68] [DOI] [PubMed] [Google Scholar]
Lo 1996 {published data only}
- Lo R, Lo B, Wells E, Chard M, Hathaway J. The develoment an d evaluation of a computer‐aided diabetes education progam. Australian Journal of Advanced Nursing 1996;13(4):19‐27. [EndNote 63] [PubMed] [Google Scholar]
Mazzuca 1986 {published data only}
- Mazzuca SA, Moorman NH, Wheeler ML, Norton JA, Fineberg NS, Vinicor F, et al. The Diabetes Education Study: a controlled trial of teh effects of diabetes patient education. Diabetes Care 1986;9(1):1‐10. [EndNote 18] [DOI] [PubMed] [Google Scholar]
McDermott 2001 {published data only}
- McDermott RA, Schmidt BA, Sinha A, Mills P. Improving diabetes care in the primary healthcare setting: a randomised cluster trial in romote indigenous communities. MJA 2001;174:497‐502. [EndNote 59] [DOI] [PubMed] [Google Scholar]
Morgan 1988 {published data only}
- Morgan BS, Littell DH. A closer look at teaching and contingency contracting with type II diabetes. Patient Education Counseling 1988;12:145‐58. [EndNote 39] [Google Scholar]
Muchmore 1994 {published data only}
- Muchmore DB, Springer J, Miller M. Self‐monitoring of blood glucose in overweight type 2 diabetic patients. Acta diabetologica 1994;31:215‐9. [EndNote 58] [DOI] [PubMed] [Google Scholar]
Mulrow 1987 {published data only}
- Mulrow C, Bailey S, Sönksen PH, Slavin B. Evaluation of an audiovisual diabetes education program: negative results of a randomized trial of patients with non‐insulin‐dependent diabetes mellitus. Journal of general internal medicine 1987;2:215‐219. [EndNote 74] [DOI] [PubMed] [Google Scholar]
Neumann 1989 {published data only}
- Neumann L, Weitzman S, Gross J. Improved knowledge of diabetic patients through education of primary care staff. Health Policy 1989;13:103‐8. [EndNote 16] [DOI] [PubMed] [Google Scholar]
Oi 1998 {published data only}
- Oi K, Komori H. Escape phenomenon with pravastatin during long‐term treatment of patients with hyperlipidemia associaqted with diabetes mellitus. Current Therapeutic Research 1998;59(2):130‐8. [EndNote 87] [Google Scholar]
Ovhed 2000 {published data only}
- Ovhed I, Johansson E, Odeberg H, Rastam L. A comparison of two different team models for treatment of diabetees mellitus in primary care. Scandinavian journal of caring sciences 2000;14:253‐8. [EndNote 88] [PubMed] [Google Scholar]
Pieber 1995 {published data only}
- Pieber TR, Holler A, Brunner GA, Schattenberg S, Schnedl WJ, Semlitsch B, et al. Diabetes treatment in Austira 5 years after the St. Vincent Declaration [Diabetesbetreuung in Osterreich 5 Jahre nach der St. Vincent‐Erklarung]. Wien Klin Wochenschr 1995;107(15):451‐6. [EndNote 89] [PubMed] [Google Scholar]
Pill 1998 {published data only}
- Pill R, Stott NCH, Rollnick SR, Rees M. A randomized controlled trial of an intervention designed to improve the care given in general practice to type II diabetic patients: patient outcomes and professional ability to change behaviour. Family Practice 1998;15(3):229‐35. [EndNote 83] [DOI] [PubMed] [Google Scholar]
Ridgeway 1999 {published data only}
- Ridgeway NA, Harvill DR, Harvill LM, Falin TM, Forester GM, Gose OP. Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic. Southern Medical Journal 1999;92(7):667‐72. [EndNote 47] [DOI] [PubMed] [Google Scholar]
Rubin 1991 {published data only}
- Rubin RR, Peyrot M, Saudek CD. Differential effect of diabetes education on self‐regulation and life‐style behaviors. Diabetes Care 1991;14(4):335‐8. [EndNote 75] [DOI] [PubMed] [Google Scholar]
Rutten 1990 {published data only}
- Rutten G, Eijck J, Nobel E, Beek M, Velden H. Feasibility and effects of a diabetes type II protocol with blood glucose self‐monitoring in general practice. Family Practice 1990;7(4):273‐8. [EndNote 4] [DOI] [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith DM, Weinberger M, Katz BP. A controlled trial to increase office visits and reduce hospitalizations of diabetic patients. Journal of General Internal Medicine 1987;2:231‐7. [EN 90] [DOI] [PubMed] [Google Scholar]
Tamai 1995 {published data only}
- Tamai H, Kubota S, Nozaki T. A psychosomatic approach to the treatment of diabtic patients who have difficulty in adhering to a self‐care regimen. Shinshin‐Igaku 1995;35:33‐9. [EndNote 28] [Google Scholar]
Trento 2001 {published data only}
- Trento M, Passera P, Tomalino M, Bajardi M, Pomero F, Allione A, Vaccari P, Molinatti GM, Porta M. Group visits improve metabolic control in type 2 diabetes. Diabetes Care 2001;24(6):995‐1000. [EndNote 86] [DOI] [PubMed] [Google Scholar]
Tu 1993 {published data only}
- Tu K‐S, McDaniel G, Templeton J. Diabetes self‐care knowledge, behaviors, and metabolic control of older adults ‐ The effect of a posteducational follow‐up program. Diabetes Educator 1993;19(1):25‐30. [EndNote 51] [DOI] [PubMed] [Google Scholar]
van den Arend 2000 {published data only}
- Arend IJM, Stolk RP, Rutten GEHM, Schrijvers GJP. Education integrated into structured general practice care for type 2 diabetic patients results in sustained improvement of disease knowledge and self‐care. Diabetic Medicine 2000;17:190‐7. [EndNote 27] [DOI] [PubMed] [Google Scholar]
Wedman 1987 {published data only}
- Wedman B, Kahan RS. Diabetes praphic aids used in counseling improve patient compliance. The American dietetic association 1987;87(12):1672‐4. [EN 70] [PubMed] [Google Scholar]
Weinberger 1995 {published data only}
- Weinberger M, Kirkman MS, Samsa GP, Shortliffe EA, Landsman PB, Cowper PA, et al. A nurse‐coordinated intervention for priamry care patients with non‐insulin‐dependent diabetes mellitus: impact on glycemic control and health‐related quality of life. Journal of general internal medicine 1995;10(2):59‐66. [EndNote 26] [DOI] [PubMed] [Google Scholar]
Whitlock 2000 {published data only}
- Whitlock WL, Brown A, Moore K, Pavliscsak H, Lanufeld D, Buker K, et al. Telemedicine improved diabetic management. Military medicine 2000;165(8):579‐84. [EndNote 76] [PubMed] [Google Scholar]
Wilson 1987 {published data only}
- Wilson W, Pratt C. The impact of diabetes education and peer support upon weight and glycemic control of elderly persons with noninsulin dependent diabetes mellitys (NIDDM). American Journal of Public Health 1987;77:634‐5. [EndNote 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. [DOI] [PubMed] [Google Scholar]
Amos 1997
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Medicine 1997;14(Suppl.5):S1‐85. [PubMed] [Google Scholar]
Borhouts 1998
- Borhouts JAJ, Koes BW, Bouter LM. The clinical course and prognostic factors of non‐specific neck pain: a systematic review. Pain 198, (77):1‐13. [DOI] [PubMed] [Google Scholar]
Boulé 2001
- Boulé N, Haddad E, Sigal R, Kenny G. Exercise for type 2 diabetes mellitus (Protocol for a Cochrane review). The Cochrane Library 2001, Issue 2. [Google Scholar]
Bouter 1991
- Bouter L, Dongen MCJM. Epidemiological research. Study design and interpretation. [Epidemiologisch onderzoek. Opzet en interpretatie]. Houten/Antwerpen: Bohn Stafleu Van Loghum, 1991:103‐9. [Google Scholar]
Donovan 1992
- Donovan JL, Blake DR. Patient non‐compliance: deviance or reasoned decision‐making?. Social Science and Medicine 1992;34:507‐13. [DOI] [PubMed] [Google Scholar]
Donovan 1995
- Donovan JL. Patient decision making. The missing ingredient in compliance research. International Journal of Technology Assessment in Health Care 1995;11:443‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997, (315):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleiss 1981
- Fleiss JL. Statistical methods for rates and properties. 2nd Edition. New‐York: Wiley, 1981:217‐37. [Google Scholar]
Foster 1994
- Foster DW. Diabetes Mellitus. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin BM, Fauci AS, Kasper DL editor(s). Harrison's Principles of Internal Medicine. 13th Edition. McGraw‐Hill, 1994:1979‐99. [Google Scholar]
Friedenreich 1994
- Friedenreich CM, Brant RF, Riboli E. Influence of methodological factors in a pooled analysis of 13 case‐control studies of colorectal cancer and dietary fibre. Epidemiology 1994;5:66‐79. [DOI] [PubMed] [Google Scholar]
Goyder 1998
- Goyder E, Irwig L. Screening for diabetes: what are we really doing?. BMJ 1998, (317):1644‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2001
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the U.S. Preventive Services Task Force. A review of the process. American Journal of Preventive Medicine 2001, (20):21‐35. [DOI] [PubMed] [Google Scholar]
Haynes 1997
- Haynes RB, McKibbon KA, Kanani R, Brouwers MC, Oliver T. Interventions to assist patients to follow prescriptions for medications. The Cochrane Database of Systematic Reviews 1997. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
IDF1999
- International Diabetes Federation Europe. A desktop guide to type 2 diabetes. 1999.
Ismail 2001
- Ismail K, McGuire H, Winkley K. Psychological interventions for improving glycaemic control in patients with diabetes mellitus (Protocol for a Cochran Review). The Cochrane Database of Systematic Reviews 2001, Issue 2. [Google Scholar]
Ismail 2004
- Ismail K, Winkley K, Rabe‐Hesketh S. Systematic review and meta‐analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363(9421):1589‐1597. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavagham DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996, (17):1‐12. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG editor(s). Systematic reviews in health care. Meta‐analysis in context. London: BMJ Books, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinmonth 1999
- Kinmonth AL, Griffin S, Wareham NJ. Implications of the United Kingdom Prospective Diabetes Study for general practice care of type 2 diabetes. British Journal of General Practice 1999:692‐4. [PMC free article] [PubMed] [Google Scholar]
Laupacis 1994
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guide to the medical literature. V. How to use an article about prognosis. Evidence‐Based Medicine Working Group. JAMA 1994;272:234‐7. [DOI] [PubMed] [Google Scholar]
LeBlanc 2001
- LeBlanc ES, Janowsky J, Nelson HD. Hormone replacement therapy and cognition. Systematic review and meta‐analysis. JAMA 2001;285(11):1489‐99. [DOI] [PubMed] [Google Scholar]
Levine 1994
- Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V. User's guide to the medical literature. IV. How to use an article about harm. Evidence‐Based Medicine Working Group. JAMA 1994;271:1615‐9. [DOI] [PubMed] [Google Scholar]
Loveman 2003
- Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N. Nice Technology Assessment Report. Vol. 7, Health Technology Assessment, 2003. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Lancet 1999, (354):1896‐900. [DOI] [PubMed] [Google Scholar]
Moore 2004
- Moore H, Summerbell C, Hooper L, Cruicksbank K, Vyas A, Johnstone P, et al. Dietary advice for treatment of type 2 diabetes mellitus in adults. The Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Morris 1992
- Morris LS, Schulz RM. Patient compliance: an overview. Journal of Clinical Pharmacy and Therapeutics 1992;17:183‐95. [DOI] [PubMed] [Google Scholar]
Nagasawa 1990
- Nagasawa N, Smith MC, Barnes JH, Fincham JE. Meta‐analysis of correlates of diabetes patients' compliance with prescribed medications. Diabetes Educator 1990;16(3):192‐200. [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐957. [DOI] [PubMed] [Google Scholar]
Offringa 2000
- Offringa M, Assendelft WJJ, Scholten RJPM. Inleidng in evidence‐based medicine. Klinisch handelen gebaseerd op bewijsmateriaal. Houten/Diegem: Bohn Stafleu Van Loghum, 2000. [ISBN 90 313 30663] [Google Scholar]
Renders 2001
- Renders CM, Valk GD, Griffin S, Wagner EM, Eijck JThM, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. The Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roter 1998
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient noncompliance: a meta‐analysis. Care 1998;36(8):1138‐61. [DOI] [PubMed] [Google Scholar]
Schroeder 2002
- Schroeder K, Fahey T, Ebrahim S. Interventions used to improve the adherence with treatment in patients with high blood pressure in ambulatory settings (Protocol for a Cochrane Review). The Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroeder 2004
- Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure‐lowering medication in ambulatory care? Systematic review of randomized controlled trials. Archives of internal medicine 2004;164(7):722‐32. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulze KF, Chalmers I, Hayens RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, (273):408‐12. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Smith GD, Altman DG editor(s). Systematic reviews in health care. Meta‐analysis in context. 2nd Edition. London: BMJ Books, 2001:189‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoup 2000
- Stoop DF, Berlin JA, Morton SC, Olkin I, Willianson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology. A proposal for reporting. JAMA 2000;283:2008‐12. [DOI] [PubMed] [Google Scholar]
UKPDS 33 1998
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, (352):837‐53. [PubMed] [Google Scholar]
UKPDS 34 1998
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, (352):854‐65. [PubMed] [Google Scholar]
UKPDS 38 1998
- UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and rsik of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. British Medical Journal 1998, (317):703‐13. [PMC free article] [PubMed] [Google Scholar]
Verhagen 2001
- Verhagen AP, Vet HCV, Bie RA, Boers M, Brandt PA. The art of quality assessment of RCTs included in systematic reviews. Journal of Clinical Epidemiology 2001;54:651‐4. [DOI] [PubMed] [Google Scholar]
Vermeire 1999
- Vermeire E. Diabetes care: concordance versus compliance [Diabeteszorg: concordance versus compliance]. Minerva (Huisarts Nu) 1999;28(3):120‐1. [Google Scholar]
Vermeire 2001
- Vermeire E, Hearnshaw H, Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics 2001;26:331‐42. [DOI] [PubMed] [Google Scholar]
Wallach 2000
- Wallach J. Interpretation of diagnostic tests. 2nd Edition. Philadelphia: Lippincott, Williams and Wilkins, 2000. [Google Scholar]
Wens 1999
- Wens J, Vermeire E, Driel M. The United Kingdom Prospective Diabetes Study: study design [The United Kingdom Prospective Diabetes Study: onderzoeksopzet]. Minerva (Huisarts Nu) 1999;28(3):122‐4. [Google Scholar]
Wens 2004
- Wens J, Vermeire E, Royen P, Hearnshaw H. A systematic review of adherence with medications for diabetes: response to Cramer. Diabetes Care 2004;27(9):2284. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva: World Health Organization, 1980. [PubMed] [Google Scholar]
WHO 1985
- World Health organization. Diabetes Mellitus: Report of a WHO Study Group. Technical Report Series No 727. Geneva: WHO, 1985. [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]


