Abstract
Background:
Higher leptin and lower adiponectin levels have been linked to progressing systemic inflammation and diseases of aging. Among older adults with obesity and an inflammatory conditions, we quantified effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation on leptin, adiponectin, and the leptin-to-adiponectin ratio (LAR). We also examined associations among adipokine and cytokine levels.
Methods:
Using a randomized, double-blind, placebo-controlled design, participants (mean age 61.3 ± 2.1) received 1.5 g EPA + 1.0 g DHA (n = 14) or mineral oil (n = 18) daily. Plasma adipokine and cytokine levels were quantified by electrochemiluminescence at all study intervals.
Results:
While no between-group differences were detected, there was a reduction in the LAR (by 23%, p=.065) between weeks 4 and 8 among the EPA+DHA group. Adiponectin levels were negatively associated with IL-1β levels at week 4 (p=.02) and TNF-α levels at week 8 (p=.03).
Conclusion:
Potential benefits of EPA+DHA supplementation among aging populations warrant further study.
Keywords: Leptin, Adiponectin, Leptin-to-adiponectin ratio, Inflammation, EPA, DHA
1. Introduction
Chronic systemic inflammation has been linked to diseases of aging that introduce significant risk for morbidity and mortality [e.g., cardiovascular disease (CVD), diabetes mellitus (DM), depression] [1–5]. So strong is the relationship between aging and systemic inflammation that it inspired the term inflammaging [6]. Studies have begun to uncover multiple metabolic and immune-related antecedents to inflammaging [2,7,8], with findings of rising leptin and diminishing adiponectin levels increasingly implicated.
Adipose tissue (AT) stores excess energy as fat and also functions as an endocrine organ, producing leptin and adiponectin to help regulate feeding behavior, energy expenditure, and inflammation [8]. After secretion by adipocytes, leptin and adiponectin enter the peripheral circulation and act as regulatory hormones that communicate with other organs including the brain, liver, and immune system [7–9]. Excess AT, as seen in obesity and aging, leads to an imbalance in circulating levels of leptin and adiponectin, which also has significant immune implications [10–12]. Leptin’s actions are primarily proinflammatory and the leptin receptor (LEPR) is ubiquitously distributed in nearly all immune cells [10–14]. Conversely, adiponectin has been shown to counteract pro-inflammatory mediators [9,12,15–17], and up-regulate expression of anti-inflammatory mediators [9,15,17] (Fig. 1). Given the opposing actions of leptin and adiponectin, the leptin to adiponectin ratio (LAR) has shown particular promise as a biological reflection of increased disease risk [18–22].
Fig. 1.
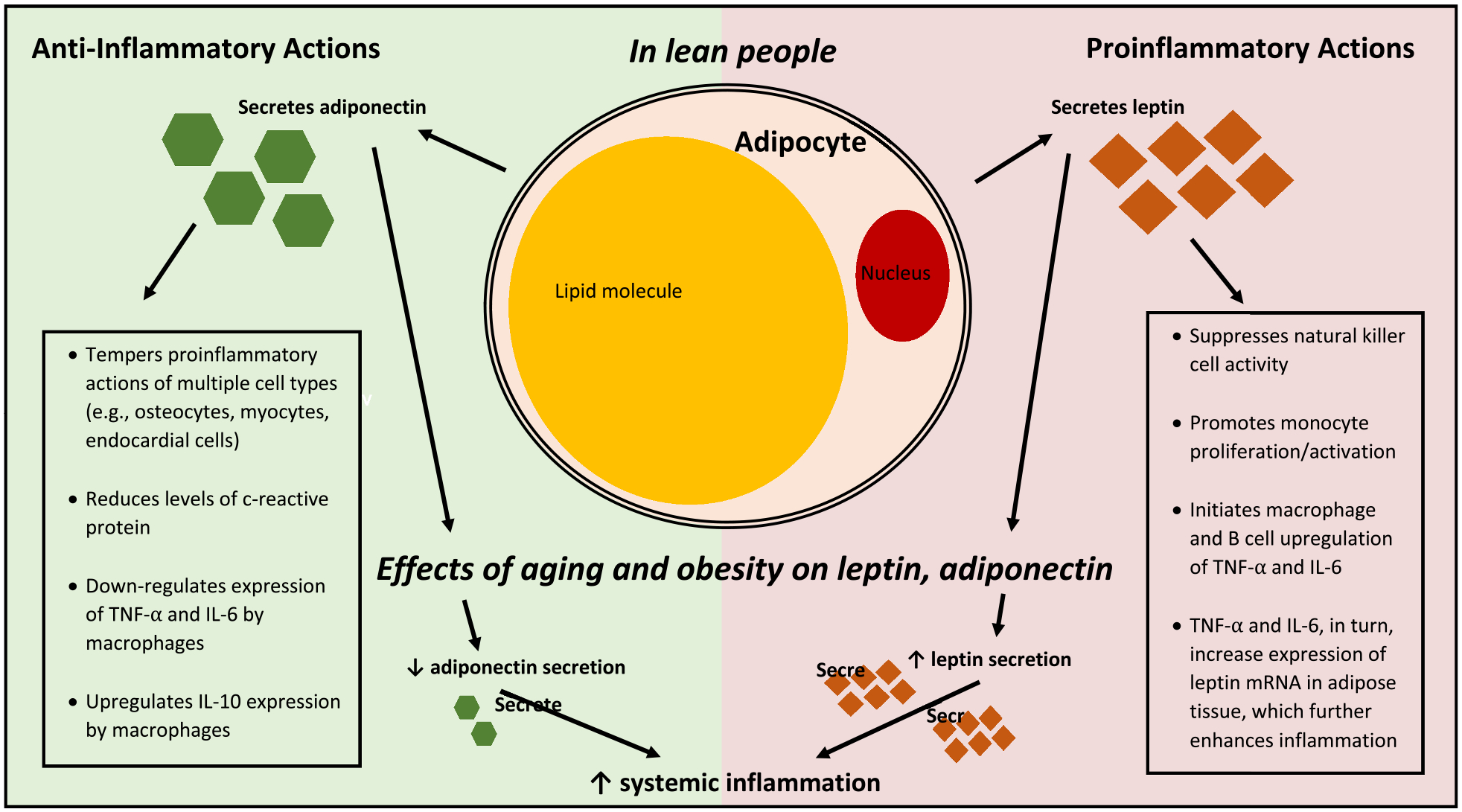
Role of leptin and adiponectin in inflammation. Leptin and adiponectin are primarily secreted by adipocytes; after entry into peripheral circulation they act as hormones, helping to regulate inflammatory responses. The figure depicts the anti-inflammatory effects of adiponectin on the left and the proinflammatory effects of leptin on the right in lean individuals. In aging and obesity, an imbalance in leptin and adiponectin is hypothesized to amplify systemic inflammation.
Some [23–27] but not all [28,29] randomized clinical trials (RCTs) report beneficial effects of omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation on leptin and adiponectin levels (Fig. 2). EPA and DHA are long known for their anti-inflammatory effects [25–33]. They are also thought to positively affect leptin and adiponectin levels by promoting a healthy weight [29,31,32], reducing the size of adipocytes [33,34], improving insulin sensitivity [33,35], reducing the expression of genes that encode for leptin, and increasing the expression of genes that encode for adiponectin [7,9,23,24,30] (Table 1). Discrepancies in trial findings may be related to differing doses or lengths of supplementation, with at least 2 g/d of EPA and DHA daily over at least 4 weeks appearing to be required to reap the expected benefit [31,32]. It also remains to be determined whether likelihood of benefit depends upon the demographics or clinical characteristics of the population under study.
Fig. 2.
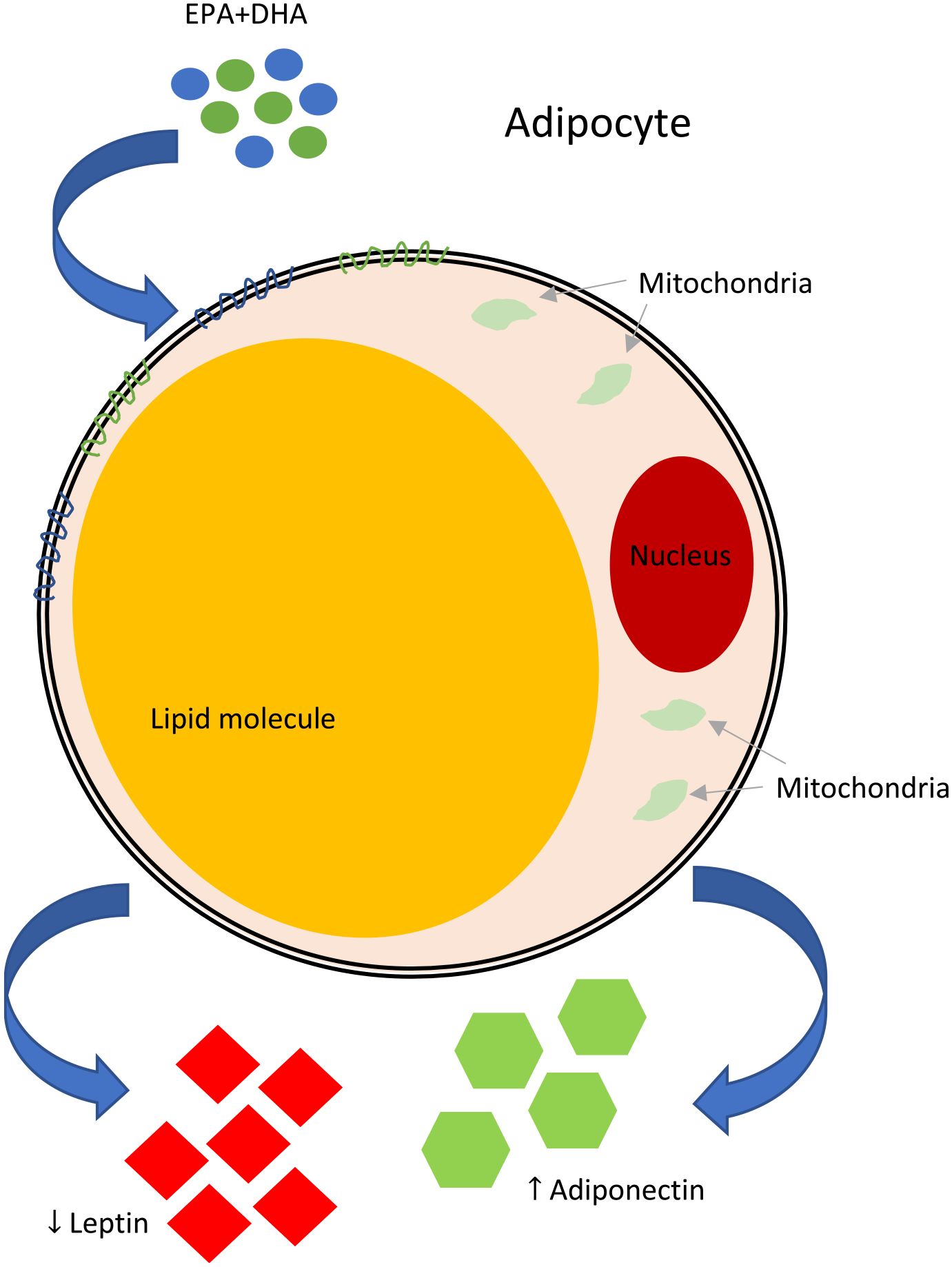
Hypothesized effects of EPA+DHA on leptin and adiponectin. Although the exact mechanisms remain unclear, it has been suggested that supplementing diets with EPA+DHA decrease leptin levels and increase adiponectin levels through four main hypotheses: 1) Direct or indirect alteration in expression of the genes that encode for leptin and adiponectin (Lep and Adipoq, respectively) [10,12, 30–32]; 2) Reduction of adipocyte size [33,34]; 3) Decrease in adiposity [29,31,32]; and, 4) Improvement of insulin sensitivity [33,35].
Table 1.
Hypothesized Mechanisms of action of EPA+DHA on leptin and adiponectin.
| Mechanism | Leptin | Adiponectin | References |
|---|---|---|---|
| Promote healthy weight | ↓ | ↑ | [25] |
| Reduce adipocyte size | ↓ | ↑ | [25,88] |
| Improve insulin sensitivity | ↓ | ↑ | [25,26] |
| Reduce Lep and increase Adipoq gene expression | ↓ | ↑ | [7,9,23,24,30] |
Lep and Adipoq are genes that encode for leptin and adiponectin, respectively. EPA = eicosapentaenoic acid, DHA = docosahexaenoic acid.
Using data from a randomized, double-blind, placebo-controlled trial that determined the effects of daily 1.5 g EPA + 1.0 g DHA supplementation on biomarkers of inflammation after 4 and 8 weeks of treatment among adults aged 50 years and older with obesity and a chronic inflammatory condition [33], we assessed the effects of the intervention on plasma levels of leptin, adiponectin, and the LAR at the same time points. To further our understanding of the role of leptin and adiponectin in inflammaging in this population, we also examined associations among plasma leptin, adiponectin, the LAR, and pro-inflammatory cytokine levels. We hypothesized that EPA+DHA supplementation would be associated with lower leptin levels, higher adiponectin levels, and a lower LAR at 4 weeks and 8 weeks compared to placebo. We hypothesized that plasma leptin levels and the LAR would be positively associated, and plasma adiponectin levels would be negatively associated with plasma pro-inflammatory cytokine levels at both assessments.
2. Participants and methods
The current study is a secondary analysis of data generated by a RCT conducted between August 2012 and July 2015 in accordance with the Ethical Principles for Medical Research involving Human Subjects outlined in the Declaration of Helsinki in 1975 (last updated in 2018, see https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medicalresearch-involving-human-subjects/).
2.1. Participant sample
The study population consisted of patients recruited from two outpatient wound clinics affiliated with a large Midwestern medical center. Eligible participants were adults aged 50 to 85 years diagnosed with at least one chronic inflammatory condition (nonhealing venous leg ulcers). All participants completed the study protocol during three study visits (weeks 0, 4, and 8) at the medical center-affiliated clinical research center, after signing an informed consent document approved by the facility’s Institutional Review Board. Forty participants consented to the study with almost equal allocation to the two intervention groups (n = 21 for EPA+DHA Group and n = 19 for Control Group). After accounting for attrition, the final sample of the parent study included 35 people. There were complete data regarding plasma levels of leptin and adiponectin for 32 of the 35 participants in the parent study that were analyzed for the current study (EPA+DHA Group (n = 14), Control Group (n = 18). A general summary of patient flow through the parent study and inclusion in this secondary analysis is presented in the CONSORT diagram shown in Fig. 3.
Fig. 3.
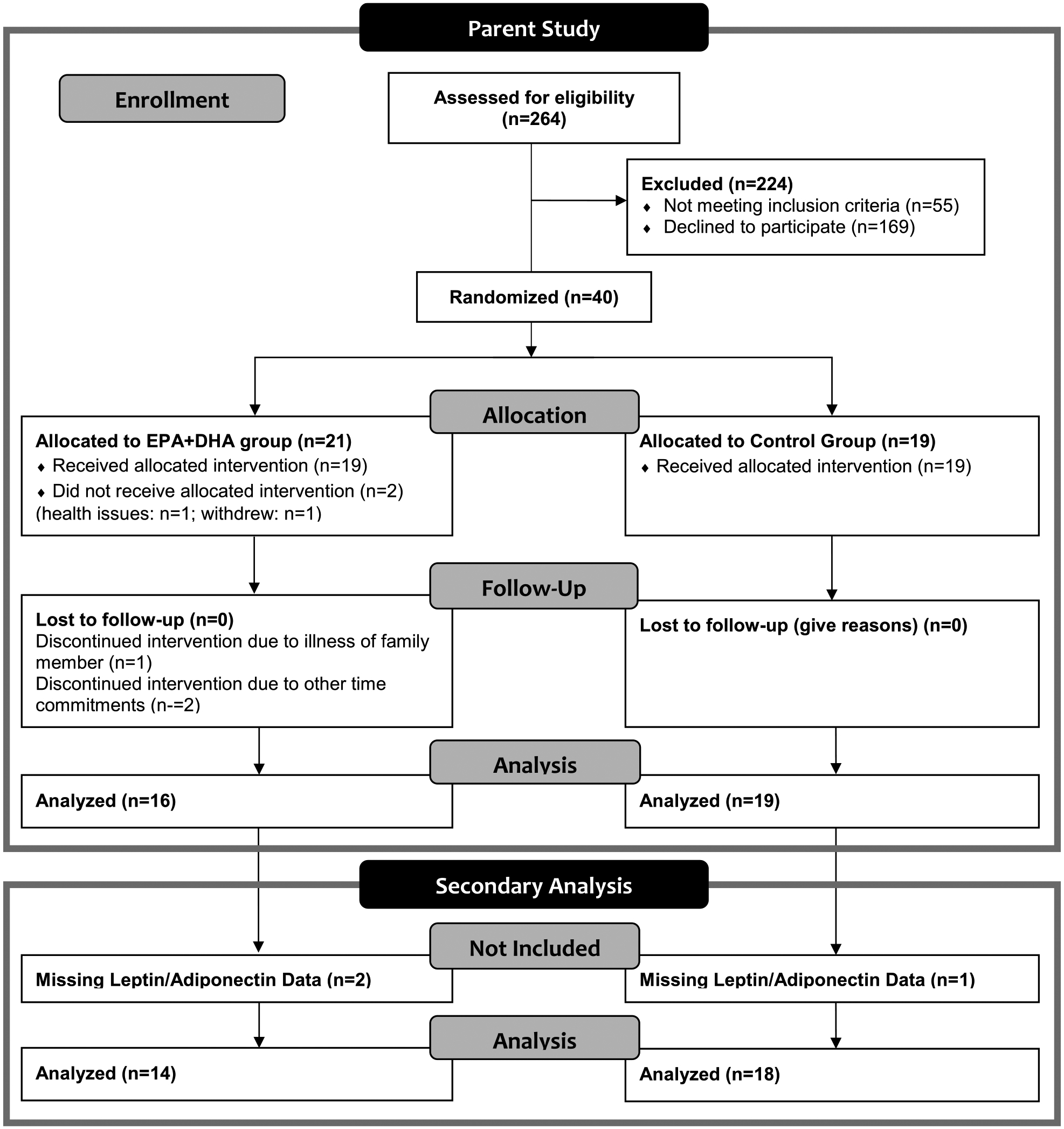
CONSORT diagram of participant flow through parent study and inclusion in secondary analysis. The CONSORT diagram depicts the flow of participants through the parent study from eligibility assessment through completion of the parent study and inclusion in secondary analysis. Of the 264 persons assessed for eligibility 40 participants met inclusion and exclusion criteria and were randomized into the two groups. Thirty-five of those 40 participants completed the entire parent study. Of the 35 participants that completed the parent study, only 32 had complete leptin and adiponectin data for all three study time points. As such, data from 32 participants were included in this secondary data analysis.
2.2. Study design
The parent study used a randomized, double-blind, placebo-controlled, repeated measures design to assess the effects of daily oral EPA+DHA supplementation versus placebo on levels of polyunsaturated fatty acid (PUFA)-derived inflammatory mediators, proinflammatory cytokines, and polymorphonuclear leukocytes in blood and wound fluid, and wound healing in older adults with chronic venous leg ulcers (CVLUs) (Trial registration: December 12, 2012 at ClinicalTrials.gov identifier NCT01754506). The results of the analyses of primary outcome measures have been previously published [26]. For the current study, existing data from the parent study were analyzed to determine the effects of daily oral EPA+DHA supplementation versus placebo on plasma levels of leptin, adiponectin, and the LAR across the study interval (weeks 0, 4 and 8), and to evaluate associations between leptin, adiponectin, LAR, and the proinflammatory cytokines interleukin (IL)–6, IL-1β, and tumor necroses factor (TNF)-α.
2.3. Study interventions
All soft gels (EPA+DHA or mineral oil) were identical in appearance, lemon-flavored, and compounded and packaged in like containers by J. R. Carlson Laboratories, Inc. (Arlington Heights, IL) in order to eliminate detection of allocated group. The contents of the soft gels for the intervention group are listed in Table 2. Participants of both groups were instructed to store the soft gels in the refrigerator, take five soft gels and one tablet containing 81 mg of acetylsalicylic acid (ASA) daily with their evening meal (ASA was administered because it has been found to enhance specialized pro-resolving mediators (e.g., lipoxins, resolvins) from EPA and DHA [48]. Participants were also instructed to bring the bottles containing any remaining soft gels and tablets to the next study visit to determine adherence to the study protocol, refrain from using anti-inflammatory drugs, if possible, and maintain their usual diet with the exception of excluding fish, seafood, algae, kelp, and other nutritional supplements containing fish oil. Participants in the EPA+DHA group consumed a total dose of 2.5 g of EPA+DHA (1.5 g of EPA and 1.0 g of DHA), which has been previously shown to significantly increase plasma EPA+DHA levels [34] and decrease some inflammatory biomarkers after four weeks of supplementation [35,36]. A dose of up to 3.0 g/d is considered safe and acceptable for the general public [37]. Participants in the control group consumed a total daily dose of 2.5 mL of light mineral oil which included 0 g of EPA and 0 g of DHA. Mineral oil is chemically inert, with the majority (98%) remaining unabsorbed in the feces; a dose of 2.5 mL daily has not been associated with any adverse events in prior studies [34,38].
Table 2.
Fatty acids in study supplement as determined by independent analysis.
| Fatty acid | Common name | Mg/capsule | % of total fatty acids |
|---|---|---|---|
| C6:0 | Caproic Acid | 0.10 | 0.0 |
| C8:0 | Caprylic Acid | 0.08 | 0.0 |
| C14:0 | Myristic Acid | 1.04 | 0.1 |
| C16:0 | Palmitic Acid | 21.00 | 2.0 |
| C16:1 | Palmitoleic Acid | 7.94 | 0.8 |
| C18:0 | Stearic Acid | 34.25 | 3.4 |
| C18:1 | Oleic Acid | 98.17 | 9.7 |
| C18:2n6 | Linoleic Acid | 30.09 | 3.0 |
| C18:3n6 | Gamma-linolenic Acid | 1.45 | 0.2 |
| C18:3n3 | Alpha-linolenic Acid | 7.27 | 0.7 |
| C18:4n3 | Stearidonic Acid | 20.46 | 2.1 |
| C20:0 | Arachidic Acid | 9.99 | 1.0 |
| C20:1 | Eicosenoic Acid | 36.80 | 3.7 |
| C20:2n6 | Eicosadienoic Acid | 2.99 | 0.3 |
| C20:3n6 | Dihomo-gamma-linolenic Acid | 2.48 | 0.3 |
| C20:4n6 | Arachidonic Acid | 20.32 | 2.1 |
| C20:3n3 | Eicosatrienoic Acid | 2.70 | 0.3 |
| C20:4n3 | Eicosatetraenoic Acid | 17.41 | 1.8 |
| C20:5n3 | Eicosapentaenoic Acid (EPA) | 324.67 | 33.4 |
| C21:5n3 | Heneicosapentaenoic Acid | 16.59 | 1.51 |
| C22:0 | Behenic Acid | 4.43 | 0.5 |
| C22:1 | Cetoleic Acid | 55.86 | 5.7 |
| C22:2n6 | Docosadienoic Acid | 1.32 | 0.1 |
| C22:4n6 | Adrenic Acid | 3.56 | 0.4 |
| C22:5n6 | Docosapentaenoic Acid (n-6) | 6.83 | 0.6 |
| C22:5n3 | Docosapentaenoic Acid (n-3) | 47.05 | 4.4 |
| C22:6n3 | Docosahexaenoic Acid (DHA) | 217.78 | 21.2 |
| C24:0 | Lignoceric Acid | 0.73 | 0.1 |
| C24:1 | Nervonic Acid | 5.45 | 0.6 |
| Total fatty acids 998.81 100.0 |
2.4. Assessments
Study visits were completed at week 0 (baseline), week 4, and week 8. At week 0, participants self-reported sociodemographic data via an electronic questionnaire and medication and comorbidity data via interviews by study nurses; all data were recorded using Research Electronic Data Capture (REDCap) [39]. Sociodemographic data included gender, age, race, marital status, employment status, income, highest level of education completed, and the total number of people in their household. Medical records were reviewed by study staff to confirm health history data. Fasting blood samples were collected at all three study time points. Levels of serum leptin and adiponectin were determined by electrochemiluminescence using Human Leptin and Adiponectin kits and the Meso QuickPlex SQ 120 (Meso Scale Discovery, Rockville, MD). Per the manufacturer, the specificity or lower limit of detection for leptin and adiponectin are 0.00578 and 0.0013 ng/ml, respectively [40,41]. Plasma PUFA levels were analyzed by the well-established gas chromatography method [42]. Levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were quantified using Invitrogen (1600 Faraday Avenue Carlsbad, CA, 92,008) Human ELISAs for IL-1β (cat# KHC0014), IL-6 (cat# KHC0061) and TNFα (cat# KHC3011) [33].
2.5. Statistical methods
Congruent with the RCT design of the parent study, we conducted an intent-to-treat analysis. We analyzed data from the participants for whom samples had been collected at all three time points for leptin and adiponectin plasma levels. We used descriptive statistics to summarize sample characteristics. Both histograms and Kolmogorov-Smirnov tests revealed that the leptin, adiponectin, and LAR data were not normally distributed. Therefore, we conducted transformation using a two-step process [43]. The first step requires transforming the variable into a percentile rank, resulting in a uniform distribution. In the second step, an inverse-normal transformation is applied to the results from the first step guided by the mean and standard error of the original variable to achieve normal distribution with the same mean and standard error. Two-sample T-tests and Chi-square statistics were used to check the balance of study groups in baseline measures. Paired t-tests were used to examine within-group change from baseline across the study time points for each intervention group. Independent sample t-tests were conducted to compare mean levels of leptin, adiponectin, and LARs between treatment groups at each time point. We also compared the change from baseline in levels of leptin, adiponectin, and LARs between treatment groups at week 4 and week 8 using two-sample t-tests. Pearson r correlations were conducted to assess the relationship between the following variables: leptin, adiponectin, and LAR to IL-6, IL-1β, and TNF-α. All tests were two-sided at a significance level of 0.05.
Due to the pilot nature of the study, the small sample size may not provide sufficient power to detect statistical significance. Therefore, we calculated Cohen’s d for the between-group comparisons. The cutoffs of 0.2, 0.5, and 0.8 indicate small, medium, and large effect sizes, respectively. Statistical Package for the Social Sciences version 25.0 for Windows was used for all data analyses (SPSS, Chicago, IL).
3. Results
3.1. Participant characteristics
The majority of participants included in this secondary analysis were older (age: M±SD = 61.3 ± 2.1), white (62.5%), males (62.5%), who were obese (BMI: M±SD = 41.5 ± 2.1) and had an average of two comorbidities in addition to a CVLU diagnosis. Our sample of 32 participants included patients diagnosed with multiple chronic inflammatory conditions in addition to CVLUs, such as CVD (n = 25), DM (n = 16), arthritis (n = 15), and depression (n = 8). At baseline, the two treatment groups were balanced based on the descriptive characteristics (Table 3).
Table 3.
Baseline characteristics of participants included in secondary analyses.
| Factors | Total Sample | EPA+DHA Group | Control Group | |
|---|---|---|---|---|
| n/M (%/SE) | n/M (%/SE) | n/M (%/SE) | p-value* | |
| Participants | 32 (100) | 14 (43.8) | 18 (56.2) | 0.48 |
| Age in Years | 61.3 ± 2.1 | 60.8 ± 3.5 | 61.6 ± 2.8 | 0.85 |
| Race | 0.96 | |||
| White | 23 (71.9) | 9 (71.4) | 11 (72.2) | |
| African American | 9 (28.1) | 4 (28.6) | 5 (27.8) | |
| Sex | 0.85 | |||
| Male | 20 (62.5) | 9 (64.3) | 11 (61.1) | |
| Female | 12 (37.5) | 5 (35.7) | 7 (38.9) | |
| Marital Status | 1.00 | |||
| Married/Living w/Someone | 16 (50) | 7 (50) | 9 (50) | |
| Widow/Divorced/Single | 16 (50) | 7 (50) | 9 (50) | |
| Education | 0.58 | |||
| Some High School | 1 (3.1) | 0 (0) | 1 (5.6) | |
| High School Graduate | 10 (31.3) | 5 (35.7) | 5 (27.8) | |
| Some College | 12 (37.5) | 4 (28.6) | 8 (44.4) | |
| College Graduate or > | 9 (28.1) | 5 (35.7) | 4 (22.2) | |
| Employment Status | 0.43 | |||
| Disabled/Unemployed | 28 (87.5) | 13 (92.9) | 15 (83.3) | |
| Employed Part-time | 2 (6.3) | 0 (0) | 2 (11.1) | |
| Employed Full-time | 2 (6.3) | 1 (7.1) | 1 (5.6) | |
| Annual Income | 0.83 | |||
| < $10,000 | 7 (21.9) | 4 (28.6) | 3 (16.7) | |
| $10,000 – $24,999 | 11 (34.4) | 4 (28.6) | 7 (38.9) | |
| $25,000 – $44,999 | 4 (12.5) | 2 (14.2) | 2 (11.1) | |
| > $45,000 | 10 (31.3) | 4 (28.6) | 6 (33.3) | |
| Smoking History | 0.20 | |||
| Current Smoker | 6 (18.8) | 4 (28.6) | 2 (11.1) | |
| Former Smoker | 14 (43.8) | 7 (50) | 7 (38.9) | |
| Never Smoker | 12 (37.5) | 3 (21.4) | 9 (50) | |
| BMI (kg/m2) | 0.64 | |||
| BMI | 41.5 (2.1) | 40.4 (2.1) | 42.4 (3.3) | |
| Comorbidities | ||||
| CVD | 25 (78.1) | 11 (78.6) | 14 (77.8) | 0.96 |
| Diabetes | 16 (50.0) | 9 (64.3) | 7 (38.9) | 0.29 |
| Arthritis | 15 (46.9) | 8 (57.1) | 7 (38.9) | 0.31 |
| Depression | 8 (25) | 3 (21.4) | 5 (27.8) | 0.68 |
EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; M = mean; SE = standard error; BMI = body mass index; CVD = cardiovascular disease; Meds = medications; L/A = leptin and/or adiponectin.
p-value represents the between group comparison.
3.2. Effects of treatment
3.2.1. Plasma levels of epa+dha
The mean plasma levels of EPA+DHA at the study time points for both groups are displayed in Fig. 4. The EPA+DHA Group had significantly higher plasma levels of EPA+DHA at week 4 (M = 5.01, SE = 0.56) and at week 8 (M = 5.48, SE = 0.53) compared to week 0 (M = 2.02, SE = 0.18), t(13) = −5.12, p < .001, t(13) = −7.44, p < .001, respectively. Conversely, there were no significant differences in plasma levels of EPA+DHA across time in the Control Group (week 0: M = 1.95, SE = 0.11; Week 4: M = 1.92, SE = 0.13; week 8: M = 1.92, SE = 0.14), t(17) = 0.438, p = .67, t(17) = 0.437, p = .67, respectively. At week 0, the mean plasma levels of EPA+DHA between the EPA+DHA Group (M = 2.02, SE = 0.18) and Control Group (M = 1.95, SE = 0.11) were not significantly different, t(30) = 0.37, p = .71, d = 0.12. However, the between-group analyses showed that the plasma levels of EPA+DHA were significantly higher in the EPA+DHA Group at week 4 (M = 5.01, SE = 0.56) and at week 8 (M = 5.48, SE = 0.53) compared to the Control Group (week 4: M = 1.92, SE = 0.13; week 8: M = 1.92, SE = 0.14), t(30) = 5.997, p < .001, d = 2.01; t(30) = 7.218, p < .001, d = 2.42, respectively.
Fig. 4.
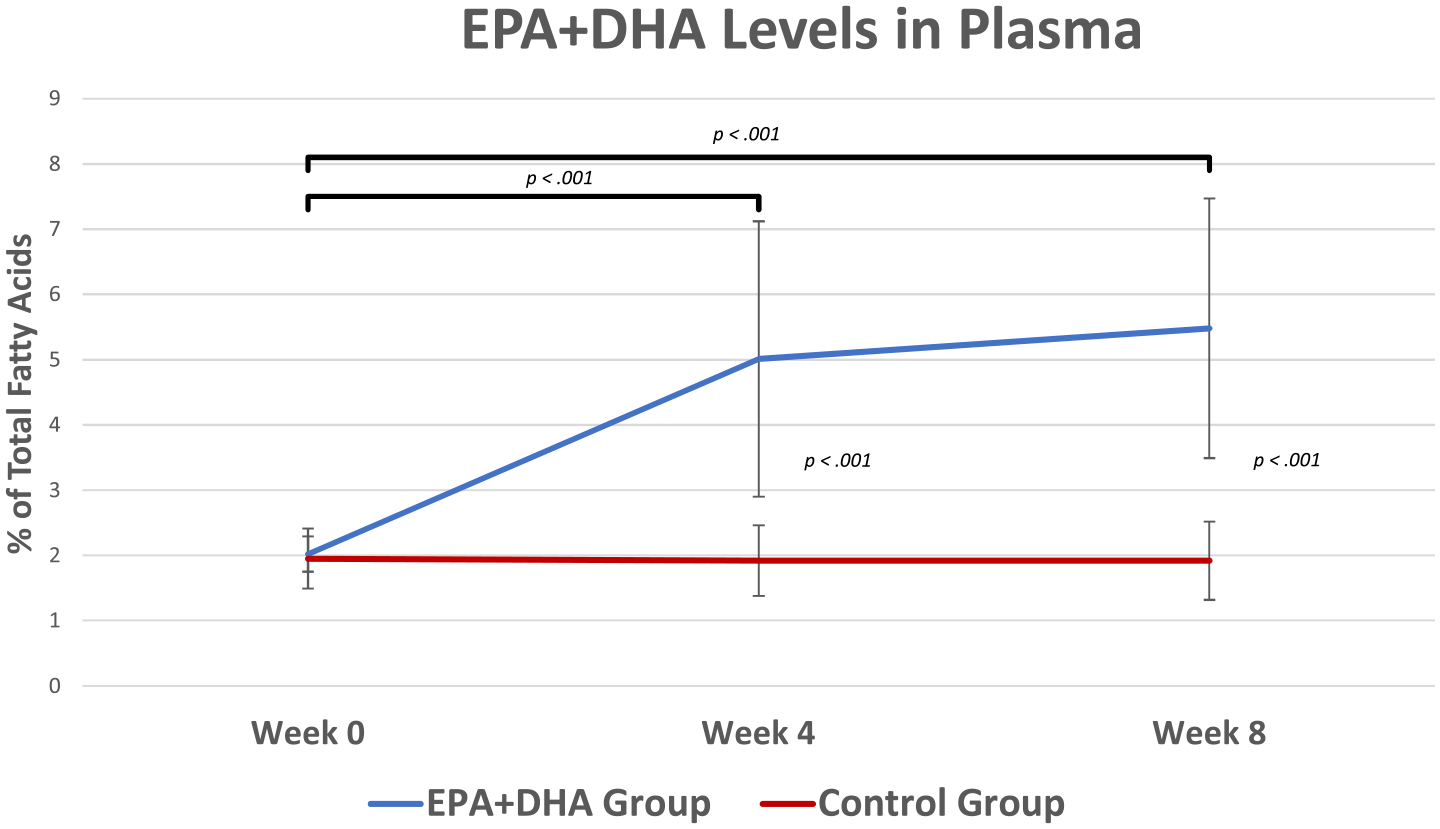
Plasma Levels of EPA+DHA by Treatment Groups at Study Time Points. At baseline, both groups had similar levels of plasma EPA+DHA (EPA+DHA group M = 2.02, SE = 0.18 and Control group M = 1.95, SE = 0.11, t(30) = 0.37, p = .71, d = 0.12). The EPA+DHA group had significantly higher plasma levels of EPA+DHA at week 4 (M = 5.01, SE = 0.56) and at week 8 (M = 5.48, SE = 0.53) compared to week 0 (M = 2.02, SE = 0.18), t(13) = −5.12, p < .001, t = −7.44, p < .001, respectively. alternatively, the Control group did not show significant changes in plasma levels of EPA+DHA across time (week 0: M = 1.95, SE = 0.11; Week 4: M = 1.92, SE = 0.13; week 8: M = 1.92, SE = 0.14), t(17) = 0.438, p = .67, t(17) = 0.437, p = .67, respectively. The between-group analysis showed that the plasma levels of EPA+DHA were significantly higher in the EPA+DHA group at week 4 (M = 5.01, SE = 0.56) and at week 8 (M = 5.48, SE = 0.53) compared to the Control group (week 4: M = 1.92, SE = 0.13; week 8: M = 1.92, SE = 0.14), t(30) = 5.997, d = 2.01, p < .001, t(30) = 7.218, p < .001, d = 2.42, respectively. This indicates that both groups adhered to the study protocol and that the treatment dose was adequate to significantly change plasma levels of EPA+DHA. EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid. Error bars represent ±SEs. Between group analyses revealed significant differences at Week 4 and Week 8, p < 0.001, < 0.001, respectively.
3.2.2. Leptin, adiponectin, and LAR
Table 4 shows the transformed leptin, adiponectin, and LAR data at weeks 0, 4, and 8, by treatment group.
Table 4.
Plasma leptin, adiponectin, and LAR data at Weeks 0, 4, and 8 by treatment group.
| Transformed Measure | Week | EPA+DHA Group | Control Group | ||
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | p-value | ES | ||
| Leptin | |||||
| 0 | 56.2 (14.2) | 79.1 (16.9) | 0.34 | 0.37 | |
| 4 | 72.6 (10.8) | 83.4 (17.2) | 0.60 | 0.18 | |
| 8 | 60.3 (13.1) | 77.2 (15.7) | 0.44 | 0.29 | |
| 4 vs 0 | 16.4 (11.3) | 4.3 (8.3) | 0.38 | 0.32 | |
| 8 vs 0 | 4.1 (5.5) | −1.9 (7.5) | 0.55 | 0.23 | |
| ADN | |||||
| 0 | 17.2 (8.7) | 27.2 (6.8) | 0.37 | 0.33 | |
| 4 | 16.5 (8.6) | 29.2 (6.5) | 0.24 | 0.43 | |
| 8 | 10.7 (8.8) | 32.9 (5.2)*†† | 0.03 | 0.8 | |
| 4 vs 0 | −0.7 (5.8) | 2.1 (5.1) | 0.72 | 0.13 | |
| 8 vs 0 | −6.5 (5.5) | 5.8 (4.9) | 0.11 | 0.60 | |
| LAR | |||||
| 0 | 6.6 (1.5) | 5.5 (1.1) | 0.53 | 0.23 | |
| 4 | 7.3 (1.2) | 5.9 (1.6) | 0.52 | 0.24 | |
| 8 | 5.6 (1.5)† | 5.3 (1.3) | 0.86 | 0.08 | |
| 4 vs 0 | 0.6 (1.0) | 0.4 (0.5) | 0.56 | 0.21 | |
| 8 vs 0 | −1.0 (1.3) | −0.2 (0.5) | 0.50 | 0.25 | |
Original leptin and adiponectin data were not normally distributed. Data in this table has been transformed following a two-step process. Step-one transforms original data into a percentile rank, which results in a uniform distribution. Step-two applies an inverse-normal transformation to the result from step-one, resulting in a normally distributed dataset. The mean and standard deviation of the original variables are used during the transformation in to keep the same mean and standard deviation in the final resulting variable. Templeton (2011) details about the use of this process. EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; ES = effect size; SE = standard error; LAR = leptin-to-adiponectin ratio.
Between groups: * p = .03; Within groups: † EPA+DHA group from Week 4 to Week 8, p = .065; †† Control group from Week 4 to Week 8, p = .09.
The within-group analyses showed no significant differences in mean plasma levels of leptin over the study interval in the Control group or in the EPA+DHA group. The between-group analyses showed that the mean plasma levels of leptin of the EPA+DHA and Control groups were not significantly different (week 0: M = 56.21, SE=14.16 vs. M = 79.07, SE = 16.94; week 4: M = 72.62, SE = 10.76 vs. M =; week 8: M = 60.26, SE = 13.12 vs. M = 83.37, SE = 17.21). However, a small-to-medium effect size of the between-group difference was identified at week 0 (d = 0.37) and week 8 (d = 0.29).
Due to differences in mean leptin levels at baseline, the changes in mean levels of leptin were calculated and between-group analyses were conducted. Although small difference in means was identified, the mean change of leptin was not statistically significantly different between groups from week 0 to week 4, week 4 to week 8, or total change week 0 to week 8, t(29) = 0.89, p = .38, d = 0.32, t(29) = −0.66, p = .52, d = 0.23, t(29) = 0.60, p = .55, d = 0.23, respectively.
The within-group analyses of adiponectin levels showed no significant changes in the Control group when comparing week 0 (M = 27.15, SE = 6.76) to week 4 (M = 29.21, SE = 6.46), or week 0 to week 8 (M = 32.92, SE = 5.23), t(16) = −0.41, p = .69, t(16) = −1.17, p = .26, respectively. However, the mean plasma levels of adiponectin in the Control group were higher at week 8 (M = 32.92, SE = 5.23) compared to week 4 (M = 29.21, SE = 6.46), t(16) = 1.8, p = .09. The within-group analysis of the EPA+DHA group showed no significant changes in mean plasma levels of adiponectin over the study interval (week 0: M = 17.22, SE = 8.73; week 4: M = 16.48, SE = 8.61; week 8: M = 10.73, SE = 8.76).
The mean plasma levels of adiponectin in the EPA+DHA group vs. the Control group were not significantly different at week 0 (M = 17.22, SE = 8.73 vs. M = 27.15, SE = 6.76) or week 4 (M = 16.48, SE = 8.61 vs. M = 29.21, SE = 6.46). However, the between-group differences at week 0 and week 4 were of small-to-medium effect size (d = 0.33, and 0.43, respectively). At week 8, the mean plasma levels of adiponectin were significantly higher in the Control group (M = 32.92, SE = 5.23) compared to the EPA+DHA group (M = 10.73, SE = 8.76; p = .03) and a large effect size was identified (d = 0.80).
Due to differences in mean adiponectin levels at baseline, the changes in mean levels of adiponectin were calculated and between-group analyses were conducted. A small non-significant difference in change of mean adiponectin levels from week 0 to week 4 was identified between the EPA+DHA group (M = −0.7, SE = 5.8) and the Control group (M = 2.1, SE = 5.1), t(29) = −0.366, p = .72, d = 0.13. There was a medium non-significant difference in change of mean adiponectin levels from week 0 to week 8 between the EPA+DHA group (M = −6.5, SE = 5.5) and the Control group (M = 5.8, SE = 4.9), t(29) −1.66, p = .11, d = 0.60.
Concerning LARs, the within-group analyses of the Control group data detected no significant changes in mean plasma LARs over the study interval (week 0: M = 5.46, SE = 1.13; week 4: M = 5.88, SE = 1.62; week 8: M = 5.29, SE = 1.33). Likewise, there were no significant changes in the mean plasma LARs in the EPA+DHA group when comparing week 0 to week 4 or week 0 to week 8 (week 0: M = 6.64, SE = 1.53; week 4: M = 7.3, SE = 1.2; week 8: M = 5.6, SE = 1.5). However, the mean LAR was lower in the EPA+DHA group at week 8 compared to week 4, t(13) = −2.02, p = .065.
The between-group analyses showed that the mean plasma LARs for the EPA+DHA group (week 0: M = 6.64, SE = 1.53; week 4: M = 7.25, SE = 1.21; week 8: M = 5.64, SE = 1.45) compared to the Control group (week 0: M = 5.46, SE = 1.13; week 4: M = 5.88, SE = 1.62; week 8: M = 5.29, SE = 1.33) were not significantly different at any time point and the differences were of small effects sizes, t(29) = 0.63, p = .53, d = 0.23, t(29) = 0.66, p = .52, d = 0.24, t(29) = 0.18, p = .86, d = 0.08, respectively.
3.3. Correlations
Table 5 displays the Pearson Correlation coefficients of relationships between leptin, adiponectin, LAR, and the proinflammatory cytokines IL-6, IL-1β, and TNF-α. In the EPA+DHA group, there was a significant negative correlation between mean plasma levels of adiponectin and mean plasma levels of IL-1β at week 4 and mean plasma levels of TNF-α at week 8, r(N = 14) = −0.63, p = .02, r(N = 14) = −0.60, p = .03, respectively. In the Control group, mean plasma levels of leptin showed a strong negative relationship to both mean plasma levels of adiponectin and TNF-α at week 8, r(N = 17) = −0.57, p = .02, r(N = 17) = −0.50, p = .04, respectively. Mean plasma levels of leptin were also shown to have a moderate negative relationship to mean plasma levels of adiponectin at week 4, r(N = 17) = −0.49, p = .05.
Table 5.
Pearson Correlation coefficients (r) to determine relationships between leptin, adiponectin, leptin-to-adiponectin ratio (LAR) and cytokines.
| Measure | W | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1. Leptin | |||||||
| 0 | – | 0.08 | 0.81** | −0.10 | 0.40 | 0.08 | |
| 4 | – | 0.05 | 0.7** | 0.11 | 0.05 | −0.08 | |
| 8 | – | −0.19 | 0.25 | −0.03 | −0.28 | −0.27 | |
| 2. ADN | |||||||
| 0 | −0.03 | – | −0.27 | −0.14 | 0.14 | −0.31 | |
| 4 | −0.49* | – | −0.45 | −0.09 | −0.63* | −0.05 | |
| 8 | −0.57* | – | −0.09 | −0.48 | −0.47 | −0.60* | |
| 3. LAR | |||||||
| 0 | 0.84* | −0.52* | – | −0.14 | 0.46 | −0.06 | |
| 4 | 0.93** | −0.68** | – | −0.05 | 0.12 | −0.31 | |
| 8 | 0.94** | −0.73** | – | −0.19 | −0.04 | −0.42 | |
| 4. IL-6 | |||||||
| 0 | −0.13 | −0.19 | −0.03 | – | −0.32 | −0.1 | |
| 4 | −0.30 | 0.25 | −0.23 | – | −0.02 | −0.37 | |
| 8 | −0.32 | 0.20 | −0.30 | – | −0.01 | 0.15 | |
| 5. IL-1β | |||||||
| 0 | −0.25 | 0.26 | −0.25 | −0.23 | – | −0.01 | |
| 4 | −0.10 | −0.11 | −0.24 | −0.37 | – | 0.27 | |
| 8 | −0.39 | 0.09 | −0.22 | −0.17 | – | 0.44 | |
| 6. TNF-α | |||||||
| 0 | −0.41 | −0.19 | −0.33 | 0.23 | 0.11 | – | |
| 4 | −0.32 | 0.12 | −0.35 | 0.35 | 0.20 | – | |
| 8 | −0.50* | 0.03 | −0.33 | 0.33 | −0.01 | – | |
The numbers 1–6 across the top row represent the same variables that are listed with the corresponding number in the first column. EPA+DHA Group correlations are shown ABOVE the diagonal dashes and the Control Group correlations are shown BELOW the dash line. W = week; ADN = adiponectin; LAR = leptin-to-adiponectin ratio; IL-6 = interleukin-6; IL-1β = interleukin-1 beta; TNF-α = tumor necrosis factor-alpha;
p < .05,
p < .01.
4. Discussion and conclusions
4.1. Relevance
The daily dietary intake of the marine-derived omega-3 fatty acids EPA+DHA by the majority of people in the United States are below what many groups worldwide are currently recommending [35,44]. The World Health Organization advises adults (>18 years of age) to consume 200–500 mg/d of EPA+DHA and the United States’ National Institute of Medicine recommends women in this same age group consume at least 110 mg/d of EPA+DHA and men to consume at least 160 mg/d of EPA+DHA [45]. Nonetheless, findings from two recent studies suggest that between 60% [46] and 95% [45] of people do not consume the recommended daily amounts of EPA+DHA either by dietary intake or by supplementation.
Supplementing diets with EPA+DHA, long known for their anti-inflammatory effects, has been shown to improve symptoms and outcomes associated with some diseases common in aging that have an inflammatory component to their pathobiology (e.g., CVD, DM, arthritis) [47–49]. However, the mechanisms of action of EPA+DHA leading to inflammation reduction are not entirely known. Given that chronic low-grade systemic inflammation in aging is linked to multiple chronic diseases [5], that the adipokines leptin and adiponectin are involved in regulating inflammatory responses, and that some RCT findings suggest EPA+DHA supplementation may have beneficial effects on leptin and adiponectin, it is important to clarify the extent of the effects. It is also important to clarify the dose and duration of EPA+DHA supplementation required to affect a change, particularly in the LAR, a novel indicator of overall inflammatory status.
4.2. Summary and comparison with other studies
The primary purpose of this secondary data analysis was to determine the effects of supplementation with marine omega-3 fatty acids, EPA+DHA, on plasma levels of leptin and adiponectin in a sample of overweight and obese older adults with chronic inflammatory conditions. While supplementing diets with 2.5 g/d of EPA+DHA did not have a significant effect on plasma levels of leptin or adiponectin individually over the 8-week study interval, the mean LAR in the EPA+DHA group was reduced by 23% from week 4 to week 8. This is an important finding considering that emerging evidence suggests the LAR is an accurate indicator of systemic inflammation and risk for some inflammatory diseases in persons of all age groups [22,50,51]. Although normal reference values for LARs are not available to date, lower LARs have been shown to indicate less inflammation [52–56]. The current study finding of a 23% reduction in the LAR after EPA+DHA supplementation is aligned with a 2017 study by Jacobo-Cejudo et al. that assessed the effects of EPA+DHA supplementation (520 mg/d) on leptin and adiponectin over 24 weeks in 54 patients with DM [57]. In that study, individual levels of leptin and adiponectin did not change significantly over time, however, the LAR was reduced by 81.5% in the EPA+DHA treated group by the study end point compared to baseline. Future studies determining more definitive reference values for leptin, adiponectin, and the LAR in terms of inflammation status would significantly move this field of inquiry forward.
The finding that no significant changes in plasma levels of leptin or adiponectin were detected in the EPA+DHA group or between the two groups in the current study is similar to findings reported by several previous RCTs in adult populations [28,58,59]. However, the EPA+DHA dose per day, study duration, and populations of interest varied across these studies. Previous studies that have reported significantly lower levels of leptin or higher levels of adiponectin after EPA+DHA supplementation used doses ranging from 1.244 – 4.2 g/d for longer periods of time (12 – 24 weeks) than the current study [60,61]. For example, in 2006 Krebs and colleagues tested EPA+DHA supplementation (4.2 g/d) for 24 weeks in a sample of 67 overweight women (BMI > 27) with hyperinsulinemia and found significantly lower leptin levels and significantly higher adiponectin levels in the treatment group compared to the control group by the end of the study [60]. Taken together, the findings of these studies suggest that an EPA+DHA daily dose between ~1.2 and 4.2 g/d for at least 12 weeks may be necessary before significant beneficial changes to individual levels of leptin or adiponectin occur in overweight or obese older adults with inflammatory conditions.
There were no differences in BMI between groups across the current study interval. However, both groups exhibited higher levels of leptin at the three study time points than the current reference values that are categorized by sex, age, and BMI (Table 6). Additionally, while the mean adiponectin levels at the three study time points in the EPA+DHA group were normal according to the reference values (for people with BMIs > 30, (Table 6), the adiponectin levels in the control group were unusually high throughout the study compared to reference values (Table 7). Although the reasons for these variations are not clear, there are several factors known to influence leptin and adiponectin levels in addition to BMI [7,9,62,63]. For example, the antidiabetic drug metformin has been shown to activate the AMPK pathway, leading to increased expression of adiponectin by adipocytes [64]. Metformin has also been shown to improve leptin sensitivity [65]. Further, a 2011 review of clinical studies evaluating the effects of antidiabetic drugs on leptin and adiponectin in patients with DM reported that insulin increased both leptin and adiponectin, orlistat decreased leptin and increased adiponectin, glimepiride increased adiponectin but had no effect on leptin, and fenofibrate decreased leptin but had no effect on adiponectin [66]. In the current study, there was no difference in frequency of use of metformin or insulin between groups. Data on the use of other antidiabetic drugs were not collected.
Table 6.
Leptin reference values (ng/ml).
| Source | BMI | Sex | Age | Low Normal | High Normal |
|---|---|---|---|---|---|
| Mayo Clinic Labs [89] | 22 | M | NS | 0.7 | 5.3 |
| 22 | F | NS | 3.3 | 18.3 | |
| Cleveland Clinic Labs [90] | NS | M | 18 – 99 | 0.5 | 12.5 |
| NS | F | 18 – 99 | 0.5 | 15.2 | |
| Johns Hopkins Hospital Labs [91] & Quest Diagnostics [92] | 18 – 25 | M | 18 – 71 | 0.3 | 13.4 |
| 18 – 25 | F | 18 – 71 | 4.7 | 23.7 | |
| 25 – 30 | M | 19 – 60 | 1.8 | 19.9 | |
| 25 – 30 | F | 19 – 60 | 8.0 | 38.9 |
BMI = body mass index; NS = not specified.
Table 7.
Adiponectin reference values (mcg/ml).
| Source | BMI | Sex | Age | Low Normal | High Normal |
|---|---|---|---|---|---|
| Mayo Clinic Labs [93]; Cleveland Clinic Labs [94]; Johns Hopkins Hospital Labs [95] | < 25 | M | NS | 4 | 26 |
| < 25 | F | NS | 5 | 37 | |
| 25 – 30 | M | NS | 4 | 20 | |
| 25 – 30 | F | NS | 5 | 28 | |
| > 30 | M | NS | 2 | 20 | |
| > 30 | F | NS | 4 | 22 |
BMI = body mass index; NS = not specified.
The hormones testosterone, estrogen, and progesterone have also been reported to differentially affect leptin and adiponectin levels. Estrogen has been shown to upregulate pathways (PPAR-γ and AMPK) known to increase adipocyte production of leptin and adiponectin [7,9] while testosterone has been shown to down-regulate leptin and adiponectin by inhibiting mRNA and protein expression in adipocytes [7,9, 67]. As a result, men tend to have lower levels of leptin and adiponectin than women. Nevertheless, we did not find significant differences in leptin or adiponectin levels between men and women in the current study. Further investigation is needed to clarify the extent sex hormones impact leptin and adiponectin.
The strong negative correlations between adiponectin and IL-1β at week 4 and between adiponectin and TNF-α at week 8 in the EPA+DHA Group detected in the current study are congruent with reports from previous studies showing that adiponectin inhibits secretion of pro-inflammatory mediators by innate immune cells [68,69] and suggesting that adiponectin has anti-inflammatory actions [61,70–72]. Conversely, the significant negative relationship that emerged between leptin and TNF-α in the Control Group at week 8 differs from recent literature reporting that leptin upregulates TNF-α in innate immune cells [71,73]. The reasons for this unexpected relationship seen in the current study are unclear.
The combined EPA+DHA dose used in our parent study was 2.5 g/d for a period of eight weeks. This dose and length of treatment aligned with recommendations that a dose of greater than 2 g/d is needed to elicit anti-inflammatory effects and that up to 3 g/d is safe for the general public to consume [31,74,75]. However, this dose and duration of supplementation had no significant effect on plasma levels of leptin and adiponectin individually in the current study. Some human studies using comparable doses have reported similar null findings in terms of the effect of EPA+DHA supplementation on leptin and adiponectin levels [58,76–80]. Thus, a larger dose of EPA+DHA or a similar dose for a longer period of time may be necessary to affect a significant change in these individual adipokine levels, a hypothesis supported by previous RCTs reporting that EPA+DHA supplementation for 10 to 24 weeks leads to beneficial changes in leptin, adiponectin, or LARs in some populations [57,60,61,81–84].
No side effects or adverse events associated with EPA+DHA supplementation were reported in the parent study, which is in line with a recent systematic review of RCTs evaluating the incidence of side effects in 27 studies testing EPA+DHA supplementation [85]. The review concluded that the most commonly reported side effect was mild gastrointestinal disturbances, occurring in only 3.5% of all people randomized to the EPA+DHA groups in the included studies [85]. Additionally, there were no side effects or adverse events associated with the mineral oil placebo in the parent study. The potential for unintended effects of the comparator oil (e.g. mineral oil) on outcomes is a legitimate concern in lipid supplementation studies. In a recent review of 80 studies that used mineral oil as a placebo, researchers found lack of evidence that mineral oil significantly affected inflammatory markers [86]. While there has been concern that some of the positive results associated with icosapent ethyl (a type of n-3 fatty acid) in the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) were due to the potentially negative effects of the mineral oil placebo, after a review of the literature, authors concluded that mineral oil, in quantities typically used for placebos in clinical trials, does not notably impact study outcomes [86]. This increases confidence that results seen in the parent study and our analysis are related to EPA+DHA supplementation.
4.3. Strengths and limitations
The current study had several strengths. First, the parent study was a RCT, the gold standard for determining cause and effect relationships. Additionally, the combined EPA+DHA dose of 2.5 g/d was aligned with the literature reporting that at least 2 g/d is needed to elicit anti-inflammatory effects [31,33,87]. Finally, this study evaluated the effects of the intervention on the LAR, which, to our knowledge, has been done in only one previous study according to a recent systematic review of RCTs testing the effects of EPA+DHA supplementation on leptin and adiponectin [88].
A limitation of this study is that because it was a secondary analysis of an existing data set, potential covariates such as estrogen or testosterone were not considered in the statistical analysis because they were not measured in the parent study. A second limitation is the relatively small sample size that may have reduced our ability to find a statistical effect in the sample if the effect exists in the population. Testing the intervention in a larger sample for a longer period of time may lead to different findings.
4.4. Conclusion
The current study evaluated the effects of EPA+DHA oral supplementation on plasma levels of leptin, adiponectin, and the LAR in a sample of overweight and obese older adults with chronic inflammatory conditions. We hypothesized that EPA+DHA oral therapy would have beneficial effects on plasma levels of leptin and adiponectin, adipokines involved in modulating inflammatory responses. While we report no significant effects of EPA+DHA supplementation on levels of leptin or adiponectin individually, there was a trend toward a reduction in the LAR (by 23%) between weeks 4 and 8 among the EPA+DHA group. Future studies using larger samples, high versus low dose EPA+DHA, and perhaps variable ratios of EPA to DHA for longer treatment periods are needed to make clear the extent that EPA+DHA supplementation alters leptin, adiponectin, and the LAR.
Funding
This paper presents independent research funded by The Robert Wood Johnson Foundation (RWJF), Future of Nursing Scholars (FNS) program in collaboration with The Ohio State University (OSU). The parent study was funded by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) (R21NR012803); and the Clinical and Translational Science Award (UL1TR001070). This paper was completed without input from funders. Views expressed are of the authors and not necessarily those of RWJF FNS, OSU, NINR, or NIH.
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- DM
diabetes mellitus
- EPA
eicosapentaenoic acid
- IL
interleukin
- LAR
leptin-to-adiponectin ratio
- mRNA
messenger ribonucleic acid
- TNF
tumor necrosis factor
Footnotes
Data
The data used in this work are not available for public use. For information on data sharing contact the corresponding author.
Declaration of Competing Interest
Authors declare there are no conflicts of interest concerning this manuscript.
References
- [1].Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T, Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments, Int J Mol. Sci 20 (2019), 10.3390/ijms20184472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bektas Schurman, Sen Ferrucci, Aging, inflammation and the environment, Exp. Gerontol (2017). 10.1016/j.exger.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calçada D, Vianello D, Giampieri E, Sala C, Castellani G, de Graaf A, Kremer B, van Ommen B, Feskens E, Santoro A, Franceschi C, Bouwman J, The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach, Mech. Agei. Dev 136–137 (2014) 138–147, 10.1016/j.mad.2014.01.004. [DOI] [PubMed] [Google Scholar]
- [4].Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F, Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition, Agei. Res. Rev 40 (2017) 95–119, 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [5].Ferrucci L, Fabbri E, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nat. Rev Cardiol 15 (2018) 505–522, 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, Inflamm-aging. an evolutionary perspective on immunosenescence, Ann. N Y Acad. Sci 908 (2000) 244–254, 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- [7].Dagogo-Jack, Leptin Regulation and Clinical Applications, Springer International Publishing, Cham, 2015. [Google Scholar]
- [8].Ghantous Azrak, Hanache Abou-Kheir, Zeidan, Differential role of leptin and adiponectin in cardiovascular system, Int. J. Endocrinol (2015), 534320, 10.1155/2015/534320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].ed. Litwack, Adiponectin, Elsevier/Academic Press, London; Waltham, MA, 2012. [Google Scholar]
- [10].Martyniak K, Masternak MM, Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation, Exp. Gerontol 94 (2017) 59–63, 10.1016/j.exger.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murray ET, Hardy R, Hughes A, Wills A, Sattar N, Deanfield J, Kuh D, Whincup P, Overweight across the life course and adipokines, inflammatory and endothelial markers at age 60–64 years: evidence from the 1946 birth cohort, Int. J Obes. (Lond) 39 (2015) 1010–1018, 10.1038/ijo.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gairolla Kler, Modi Khurana, Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke, Rev. Neurosci 28 (2017) 295–306, 10.1515/revneuro-2016-0055. [DOI] [PubMed] [Google Scholar]
- [13].Pérez-Pérez A, Sánchez-Jiménez F, Vilariño-García T, Sánchez-Margalet V, Role of leptin in inflammation and vice versa, IJMS 21 (2020) 5887, 10.3390/ijms21165887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Franceschi Campisi, Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases, J. Gerontol. A Biol. Sci. Med. Sci 69 (Suppl 1) (2014) S4–S9, 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- [15].Ouchi Walsh, Adiponectin as an anti-inflammatory factor, Clinic. Chimic. Acta 380 (2007) 24–30, 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ajuwon KM, Spurlock ME, Adiponectin inhibits lps-induced nf-κb activation and il-6 production and increases pparγ2 expression in adipocytes, Am. J. Physiology-Regulatory, Integra. Comparative Physiol 288 (2005) R1220–R1225, 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- [17].Manigrasso MR, Ferroni P, Santilli F, Taraborelli T, Guagnano MT, Michetti N, Davì G, Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss, J.Clin. Endocrinol. Metabol 90 (2005) 5876–5879, 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]
- [18].Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J, Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. relation with obesity-associated cardiometabolic risk, Adipoc 7 (2018) 57–62, 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Golbahar Das, Al-Ayadhi Gumaa, Leptin-to-adiponectin, adiponectin-to-leptin ratios, and insulin are specific and sensitive markers associated with polycystic ovary syndrome: a case-control study from bahrain, Metab. Syndr. Relat. Disord 10 (2012) 98–102, 10.1089/met.2011.0075. [DOI] [PubMed] [Google Scholar]
- [20].Lekva Michelsen, Aukrust Henriksen, Bollerslev Ueland, Leptin and adiponectin as predictors of cardiovascular risk after gestational diabetes mellitus, Cardiovasc. Diabetol 16 (2017) 5, 10.1186/s12933-016-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sarray Madan, Saleh Mahmoud, Almawi, Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome, Fertil. Steril 104 (2015) 460–466, 10.1016/j.fertnstert.2015.05.007. [DOI] [PubMed] [Google Scholar]
- [22].Chen Chen, Chiu Lin, Li Lu, Leptin/adiponectin ratio as a potential biomarker for metabolic syndrome in patients with schizophrenia, Psychoneuroendocrinol 92 (2018) 34–40, 10.1016/j.psyneuen.2018.03.021. [DOI] [PubMed] [Google Scholar]
- [23].Drevon, Fatty acids and expression of adipokines, Biochim. Biophys. Acta 1740 (2005) 287–292, 10.1016/j.bbadis.2004.11.019. [DOI] [PubMed] [Google Scholar]
- [24].Flachs Mohamed-Ali, Horakova Rossmeisl, Hosseinzadeh-Attar Hensler, Kopecky Ruzickova, Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet, Diabetolog 49 (2006) 394–397, 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- [25].Lombardo Hein, Chicco, metabolic syndrome: effects of n-3 pufas on a model of dyslipidemia, insulin resistance and adiposity, Lip 42 (2007) 427–437, 10.1007/s11745-007-3039-3. [DOI] [PubMed] [Google Scholar]
- [26].Selenscig Rossi, Chicco Lombardo, Increased leptin storage with altered leptin secretion from adipocytes of rats with sucrose-induced dyslipidemia and insulin resistance: effect of dietary fish oil, Metab. Clin. Exp 59 (2010) 787–795, 10.1016/j.metabol.2009.09.025. [DOI] [PubMed] [Google Scholar]
- [27].Itariu Zeyda, Hochbrugger Neuhofer, Prager Schindler, Bohdjalian Mascher, Vangala Schranz, Krebs Bischof, Stulnig, long-chain n-3 pufas reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial, Am. J. Clin. Nutr 96 (2012) 1137–1149, 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- [28].Poreba Mostowik, Siniarski Golebiowska-Wiatrak, Malinowski Haberka, Konduracka Nessler, Undas Gajos, Treatment with high-dose n-3 pufas has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes, Cardiovasc. Diabetol 16 (2017), 10.1186/s12933-017-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huerta Prieto-Hontoria, Sainz Martinez, Moreno-Aliaga, Supplementation with alpha-lipoic acid alone or in combination with eicosapentaenoic acid modulates the inflammatory status of healthy overweight or obese women consuming an energy-restricted diet, J. Nutr 146 (2016) 889S–896S, 10.3945/jn.115.224105. [DOI] [PubMed] [Google Scholar]
- [30].Banga Unal, Tripathi Pokrovskaya, Owens Kern, Ranganathan, Adiponectin translation is increased by the ppargamma agonists pioglitazone and omega-3 fatty acids, Am. J. Physiol. Endocrinol. Metab 296 (2009) E480–E489, 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Calder, Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance, Biochim. Biophys. Acta 1851 (2015) 469–484, 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- [32].Harris, The omega-3 index as a risk factor for coronary heart disease, Am. J. Clin. Nutr 87 (2008) 1997S–2002S, 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- [33].Tan A, Sullenbarger B, Prakash R, McDaniel JC, Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study, Prostagl, Leukot. Essent. Fat, Acids 132 (2018) 23–29, 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McDaniel Belury, Ahijevych Blakely, Omega-3 fatty acids effect on wound healing, Wou. Repa. Regen 16 (2008) 337–345, 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Calder, Fatty acids and inflammation: the cutting edge between food and pharma, Eur. J. Pharmacol 668 (Suppl 1) (2011) S50–S58, 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- [36].Grenon Owens, Nosova Hughes-Fulford, Alley Chong, Perez Yen, Boscardin Hellmann, Spite Conte, Short-Term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the omega-pad i trial), J. Am. Heart Assoc 4 (2015), e002034, 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products Labeling and Dietary Supplements (FDA/CSFAN), FDA Announces New Qualified Health Claims for EPA and DHA Omega-3 Consumption and the Risk of Hypertension and Coronary Heart Disease, FDA, 2019. [Google Scholar]
- [38].McDaniel Massey, Nicolaou, Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds, Wou. Repa. Regen 19 (2011) 189–200, 10.1111/j.1524-475X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harris Taylor, Thielke Payne, Gonzalez Conde, Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2009) 377–381, 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meso Scale Discovery, V-PLEX Human Leptin Kit, (n.d.). https://www.mesoscale.com/en/products/v-plex-human-leptin-kit-k151v5d/ (accessed March 4, 2021).
- [41].Meso Scale Discovery, R-PLEX Human Adiponectin Antibody Set, (n.d.). https://www.mesoscale.com/en/products/r-plex-human-adiponectin-antibody-set-f21yt/ (accessed March 4, 2021).
- [42].Quehenberger O, Armando AM, Dennis EA, High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry, Biochim. Biophys. Acta 1811 (2011) 648–656, 10.1016/j.bbalip.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Templeton A two-step approach for transforming continuous variables to normal: implications and recommendations for is research, Commu. Assoc. Info. Systems 28 (2011), 10.17705/1CAIS.02804. [DOI] [Google Scholar]
- [44].Bibus Raatz (Ed.), Fish and Fish Oil in Health and Disease Prevention, Elsevier/Academic Press, Amsterdam, 2016. [Google Scholar]
- [45].Thompson Hein, Hanson Smith, Anderson-Berry Richter, Bisselou Stessy, Appiah Kusi, Kris-Etherton, skulas-ray, nordgren, omega-3 fatty acid intake by age, gender, and pregnancy status in the united states: national health and nutrition examination survey 2003–2014, Nutrie 11 (2019), 10.3390/nu11010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang Z, Fulgoni VL, Kris-Etherton PM, Mitmesser SH, Dietary intakes of epa and dha omega-3 fatty acids among us childbearing-age and pregnant women: an analysis of nhanes 2001–2014, Nutrie 10 (2018), 10.3390/nu10040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Centers for Disease Control, FastStats Heart Disease, (2020). https://www.cdc.gov/nchs/fastats/heart-disease.htm (accessed January 25, 2021).
- [48].Centers for Disease Control, FastStats Diabetes, (2020). https://www.cdc.gov/nchs/fastats/diabetes.htm (accessed January 25, 2021).
- [49].Centers for Disease Control, FastStats Arthritis, (2020). https://www.cdc.gov/nchs/fastats/arthritis.htm (accessed January 25, 2021).
- [50].Ayina Endomba, Mandengue Noubiap, Ngoa Boudou, Gautier Mbanya, Sobngwi, association of the leptin-to-adiponectin ratio with metabolic syndrome in a sub-saharan african population, Diabetol. Metab. Syndr 9 (2017) 66, 10.1186/s13098-017-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nixon, The inverse relationship between cancer and alzheimer’s disease: a possible mechanism, Curr. Alzhei. Res 14 (2017) 883–893, 10.2174/1567205014666170216152905. [DOI] [PubMed] [Google Scholar]
- [52].Chou Hsu, Wu Teng, Sun Ko, Leptin-to-adiponectin ratio is related to low grade inflammation and insulin resistance independent of obesity in non-diabetic taiwanese: a cross-sectional cohort study, Acta. Cardiol. Sin 30 (2014) 204–214. [PMC free article] [PubMed] [Google Scholar]
- [53].Fruhbeck Catalán, Rodríguez Ramírez, Becerril Salvador, Portincasa Colina, Gómez-Ambrosi, Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome, Sci. Rep 7 (2017) 6619, 10.1038/s41598-017-06997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jialal Adams-Huet, Duong Smith, Relationship between retinol-binding protein-4/adiponectin and leptin/adiponectin ratios with insulin resistance and inflammation, Metab. Syndr. Relat. Disord 12 (2014) 227–230, 10.1089/met.2014.0013. [DOI] [PubMed] [Google Scholar]
- [55].Liang Hur, Kang Rhee, Kim Lee, Effect of the anti-il-17 antibody on allergic inflammation in an obesity-related asthma model, Kore. J. Intern. Med 33 (2018) 1210–1223, 10.3904/kjim.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Panahi Khalili, Sahebi Namazi, Atkin Majeed, Sahebkar, Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus, Curr. Clin. Pharmacol 12 (2017) 253–258, 10.2174/1574884713666180104095641. [DOI] [PubMed] [Google Scholar]
- [57].Jacobo-Cejudo Valdés-Ramos, Guadarrama-López Pardo-Morales, Harbige Martínez-Carrillo, Effect of n-3 polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers in type 2 diabetes mellitus patients, Nutrie 9 (2017), 10.3390/nu9060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vargas Almario, Buchan Kim, Karakas, metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome, Metab.-Clin. Exp 60 (2011) 1711–1718, 10.1016/j.metabol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yamamoto Kajikawa, Otani Yamada, Takemoto Hirota, Ikeda Iwagaki, Saito Fujiwara, Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: the possible involvement of adiponectin, Acta Med. Okayama 68 (2014) 349–361, 10.18926/AMO/53024. [DOI] [PubMed] [Google Scholar]
- [60].Krebs Browning, McLean Rothwell, Mishra Moore, Jebb, additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women, Int. J Obes. (Lond) 30 (2006) 1535–1544, 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- [61].Qin Zhou, Chen Zhao, Ran Zeng, Wu Chen, Kang Shu, Zhang Mi, Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin e2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial, PLoS ONE 10 (2015), e0133496, 10.1371/journal.pone.0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Everson-Rose Clark, Wang Guo, Mancuso Kravitz, Bromberger, depressive symptoms and adipokines in women: study of women’s health across the nation, Psychoneuroendocrinol 97 (2018) 20–27, 10.1016/j.psyneuen.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Katsiki Mikhailidis, Banach, leptin, cardiovascular diseases and type 2 diabetes mellitus, Acta. Pharmacol. Sin 39 (2018) 1176–1188, 10.1038/aps.2018.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Su Lu, Su Zhao, Dong Sun, Zhao Li, Relationship of serum adiponectin levels and metformin therapy in patients with type 2 diabetes, Horm. Metab. Res 48 (2016) 92–98, 10.1055/s-0035-1569287. [DOI] [PubMed] [Google Scholar]
- [65].Malin Kashyap, Effects of metformin on weight loss: potential mechanisms, Curr Opin Endocrinol Diab. Obes 21 (2014) 323–329, 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- [66].Katsiki Mikhailidis, Gotzamani-Psarrakou Yovos, Karamitsos, effect of various treatments on leptin, adiponectin, ghrelin and neuropeptide y in patients with type 2 diabetes mellitus, Expe. Opin. Ther. Targ 15 (2011) 401–420, 10.1517/14728222.2011.553609. [DOI] [PubMed] [Google Scholar]
- [67].Schooling Luo, Yeung Au, Thompson Karthikeyan, Bolton Mason, Ingelsson Burgess, Genetic predictors of testosterone and their associations with cardiovascular disease and risk factors: a mendelian randomization investigation, Int. J. Cardiol 267 (2018) 171–176, 10.1016/j.ijcard.2018.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tilija Pun N, Park P–H, Adiponectin inhibits inflammatory cytokines production by beclin-1 phosphorylation and b-cell lymphoma 2 mrna destabilization: role for autophagy induction, Br. J. Pharmacol 175 (2018) 1066–1084, 10.1111/bph.14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Elfeky M, Yoneshiro T, Okamatsu-Ogura Y, Kimura K, Adiponectin suppression of late inflammatory mediator, hmgb1-induced cytokine expression in raw264 macrophage cells, J. Biochem 163 (2018) 143–153, 10.1093/jb/mvx069. [DOI] [PubMed] [Google Scholar]
- [70].Liu Feng, Li Wang, Li Hua, Adiponectin, tnf-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis, Cytok 86 (2016) 100–109, 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- [71].Lopez-Jaramillo Gómez-Arbeláez, López-López López-López, Martínez-Ortega Gómez-Rodríguez, Triana-Cubillos, The role of leptin/adiponectin ratio in metabolic syndrome and diabetes, Horm. Mol. Biol. Clin. Investig 18 (2014) 37–45, 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- [72].Rapaport Nierenberg, Schettler Kinkead, Cardoos Walker, Mischoulon, inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study, Mol. Psychia 21 (2016) 71–79, 10.1038/mp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Borges Franca, Fujimori Silva, Deluque de Marchi, de Abreu Honorio-Franca, Relationship between proinflammatory cytokines/chemokines and adipokines in serum of young adults with obesity, Endocr. Metab. Immu. Disord. Drug Targ 18 (2018) 260–267, 10.2174/1871530318666180131094733. [DOI] [PubMed] [Google Scholar]
- [74].Leaf Jorgensen, Jacobs Cote, Scheer Schoenfeld J, Weiner Slack, Kellett Raizner, Do fish oils prevent restenosis after coronary angioplasty? Circul 90 (1994) 2248–2257, 10.1161/01.cir.90.5.2248. [DOI] [PubMed] [Google Scholar]
- [75].U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products Labeling and Dietary Supplements (FDA/CSFAN)., Letter Regarding Dietary Supplement Health Claim For omega-3 Fatty Acids and Coronary Heart disease. (Docket No. 91N-0103), (2000).
- [76].Krantz Havranek, Pereira Beaty, Mehler Long, Effects of omega-3 fatty acids on arterial stiffness in patients with hypertension: a randomized pilot study, J. Negat. Resul.Biomed 14 (2015) 21, 10.1186/s12952-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lee Ivester, Hester Sergeant, Case Morgan, Kouba Chilton, The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population, Lip. Heal. Dis (2014). 10.1186/1476-511X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Simao Lozovoy, Bahls Morimoto, Matsuo, Simão Dichi, Blood pressure decrease with ingestion of a soya product (kinako) or fish oil in women with the metabolic syndrome: role of adiponectin and nitric oxide, Br. J. Nutr 108 (2012) 1435–1442, 10.1017/S0007114511006921. [DOI] [PubMed] [Google Scholar]
- [79].Spencer Finlin, Unal Zhu, Morris Shipp, Lee Walton, Adu Erfani, Campbell McGehee, Peterson Kern, Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance, Diab 62 (2013) 1709–1717, 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Troseid Arnesen, Hjerkinn Seljeflot, Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men, Metab. Clin. Exp 58 (2009) 1543–1549, 10.1016/j.metabol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- [81].Allaire Couture, Leclerc Charest, Lépine Marin M-C, Talbot D, Tchernof A, Lamarche B, A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the comparing epa to dha (compared) study, Am. J. Clin. Nutr 104 (2016) 280–287, 10.3945/ajcn.116.131896. [DOI] [PubMed] [Google Scholar]
- [82].Haidari Tavakoli, Heybar Helli, Mohammadshahi, The effect of omega-3 on the level of serum lipid profile, adipocytokines, and indicator of vascular inflammation in patients diagnosed with myocardial infarction, J. Babol Uni. Medi. Sci 17 (2015) 7–17. [Google Scholar]
- [83].Satoh Shimatsu, Kotani Himeno, Majima Yamada, Suganami Ogawa, Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid a-ldl in metabolic syndrome, Hypertens. Res 32 (2009) 1004–1008, 10.1038/hr.2009.145. [DOI] [PubMed] [Google Scholar]
- [84].Satoh-Asahara Shimatsu, Sasaki Nakaoka, Himeno Tochiya, Kono Takaya, Ono Wada, Suganami Hasegawa, Ogawa, highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia, Diab. Care 35 (2012) 2631–2639, 10.2337/dc12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Villani Crotty, Cleland James, Fraser Cobiac, Miller, fish oil administration in older adults with cardiovascular disease or cardiovascular risk factors: is there potential for adverse events? a systematic review of the literature, Int. J. Cardiol 168 (2013) 4371–4375, 10.1016/j.ijcard.2013.05.054. [DOI] [PubMed] [Google Scholar]
- [86].Olshansky B, Chung MK, Budoff MJ, Philip S, Jiao L, Doyle RT Jr., Copland C, Giaquinto A, Juliano RA, Bhatt DL, Mineral oil: safety and use as placebo in reduce-it and other clinical studies, Euro. Hear. J. Suppl 22 (2020) J34–J48, 10.1093/eurheartj/suaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sakamoto A, Saotome M, Iguchi K, Maekawa Y, Marine-Derived omega-3 polyunsaturated fatty acids and heart failure: current understanding for basic to clinical relevance, Int J Mol Sci 20 (2019), 10.3390/ijms20164025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rausch J, Gillespie S, Orchard T, Tan A, McDaniel JC, Systematic review of marine-derived omega-3 fatty acid supplementation effects on leptin, adiponectin, and the leptin-to-adiponectin ratio, Nutr. Res. (2020). [DOI] [PubMed] [Google Scholar]
- [89].Mayo Clinic Labs, Leptin. (n.d.). https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/91339 (accessed March 3, 2021).
- [90].Cleveland Clinic Laboratories Test Directory, Leptin. (n.d.). https://ltd.clevelandcliniclabs.com/test/3201 (accessed March 4, 2021).
- [91].Johns Hopkins Hospital, Leptin (n.d.). https://pathology.jhu.edu/jhml-services/test-directory/ (accessed March 3, 2021).
- [92].Quest Diagnostics, Leptin. (n.d.). https://testdirectory.questdiagnostics.com/test/test-detail/90367/leptin?cc=MASTER (accessed March 3, 2021).
- [93].Mayo Clinic Labs, Adiponectin. (n.d.). https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/75607 (accessed March 3, 2021).
- [94].Cleveland Clinic Laboratories Test Directory, Adiponectin. (n.d.). https://ltd.clevelandcliniclabs.com/test/4199 (accessed March 4, 2021).
- [95].Johns Hopkins Hospital, Adiponectin. (n.d.). https://pathology.jhu.edu/jhml-services/test-directory/ (accessed March 3, 2021).


