Abstract
Diabetic kidney disease (DKD) and its major clinical manifestation, progressive renal decline that leads to end-stage renal disease (ESRD), are a major health burden for individuals with diabetes. The disease process that underlies progressive renal decline comprises factors and pathways that increase risk of this outcome as well as factors and pathways that protect against progressive renal decline. Using an untargeted proteomic profiling of circulating proteins from patients in two independent cohorts with Type 1 and Type 2 diabetes and varying stages of DKD followed for 7–15 years, we identified 3 elevated plasma proteins, fibroblast growth factor 20 (OR=0.69; 95% CI: 0.54–0.88), angiopoietin-1 (OR=0.72; 95% CI: 0.57–0.91) and tumor necrosis factor ligand superfamily member 12 (OR=0.75; 95% CI: 0.59–0.95), that were combined effect of these 3 protective proteins was demonstrated by very low cumulative risk of ESRD in those who had baseline concentrations above median for all 3 proteins, whereas the cumulative risk of ESRD was high in those with concentrations below median for these proteins at the beginning of follow-up. This protective effect was shown to be independent from circulating inflammatory proteins and clinical covariates and was confirmed in a third cohort of diabetic individuals with normal renal function. These three protective proteins may serve as biomarkers to stratify diabetic individuals according to risk of progression to ESRD, and might also be investigated as potential therapeutics to delay or prevent the onset of ESRD.
One Sentence Summary:
Global proteomics profiling identified FGF20, TNFSF12 and ANGPT1 as protective against progression of renal function decline in those with diabetes
Introduction
Over the last several decades, considerable research efforts have been directed toward understanding the mechanisms of diabetic kidney disease (DKD) in humans with type 1 diabetes (T1D) as well as in type 2 diabetes (T2D). In that research, the major focus was on factors and markers that were associated with high risk of the development of various manifestations of DKD (1–6). Recent attention has focused on the search for factors and biomarkers associated with protection against DKD. It has been postulated that individuals who remained without late complications despite long duration of diabetes, so-called survivors with long diabetes duration, could be enriched for such protective factors/biomarkers. This approach has already provided findings that resulted not only in the development of a new hypothesis about DKD, but also in the identification of pyruvate kinase M2 (PKM2) as a new therapeutic target to prevent DKD (7).
For individuals with diabetes, the risk of end-stage renal disease (ESRD) remains relatively high despite improvements in glycemic control and advances in reno-protective therapies over the last 20 years for the prevention and treatment of DKD (8, 9). Findings from our Joslin Kidney Study, a longitudinal study of more than 3,500 individuals with diabetes, demonstrated that progressive renal decline is the major clinical manifestation of DKD that underlies progression to ESRD (10–13). The onset of progressive renal decline begins when patients have normal renal function and it progresses almost linearly to ESRD, although the rate of decline expressed as the slope of the estimated glomerular filtration rate (eGFR) varies tremendously among those individuals ranging from −72 to 3.0 ml/min/year (11, 13). By analogy to studies of long diabetes duration survivors, it is reasonable to postulate those individuals with impaired renal function but slow or minimal renal decline might have been enriched for protective factors/biomarkers against progressive renal decline and the development of ESRD.
In this study, we searched for such protective factors/biomarkers in two 7–15-year follow-up studies of individuals with T1D and T2D and moderately impaired renal function. We measured concentrations of 1,129 plasma proteins at baseline using an aptamer-based SOMAscan proteomic platform that uses single-stranded DNA aptamers (14, 15). We studied baseline plasma proteins that were elevated in those with slow or minimal renal decline and considered them as candidate protective factors/markers against progressive renal decline and progression to ESRD. The findings were validated in a cohort of T1D patients with normal renal function who were at high risk of progressive renal decline and progression to ESRD.
Results
Characteristics of the exploratory and replication cohorts
Our study included individuals participating in the ongoing Joslin Kidney Study. Two independent cohorts of individuals with diabetes and impaired renal function (chronic kidney disease (CKD) Stage 3) were assembled; an exploratory Joslin cohort of 214 individuals with T1D and a replication Joslin cohort of 144 individuals with T2D. These cohorts were followed for 7–15 years to determine eGFR slope and ascertain time of onset of ESRD. The clinical characteristics of these cohorts are shown in Table 1. All study participants included in the Joslin T1D cohort and 92% of study participants in the T2D cohort were Caucasian. At baseline, in comparison with individuals with T1D, those with T2D were older, had shorter duration of diabetes, higher body mass index (BMI), lower hemoglobin A1c (HbA1c) and lower urinary albumin to creatinine ratio (ACR) but similarly impaired eGFR.
Table 1.
Demographics and clinical characteristics of the Joslin Kidney Study cohorts with T1D and T2D.
|
|
|||
|---|---|---|---|
| EXPLORATORY | REPLICATION | ||
|
| |||
| Characteristics | Joslin T1D Cohort (N = 214) | Joslin T2D Cohort (N = 144) | P-value |
|
| |||
| At baseline | |||
| Male, n (%) | 104 (49%) | 94 (65%) | 0.002 |
| Ethnicity | <0.0001 | ||
| Caucasian, n (%) | 214 (100%) | 132 (92%) | |
| Non-Caucasian, n (%) | 0 (0%) | 12 (8%) | |
| Age at DM onset (years) | 13 (8, 20) | 44 (38, 50) | <0.0001 |
| Age at study entry (years) | 44 (38, 51) | 61 (56, 64) | <0.0001 |
| Duration of diabetes (years) | 28 (23, 36) | 15 (11, 21) | <0.0001 |
| BMI (kg/m2) | 26.4 (23, 28) | 33.4 (29, 37) | <0.0001 |
| Systolic BP (mm Hg) | 133 (124, 147) | 139 (128, 150) | 0.02 |
| Diastolic BP (mm Hg) | 78 (70, 84) | 74 (69, 81) | 0.04 |
| Insulin Rx, % | 100% | 65% | <0.0001 |
| Renoprotection Rx, % | 81% | 86% | 0.19 |
| HbA1c (%) | 8.6 (7.7, 9.6) | 7.3 (6.7, 8.3) | <0.0001 |
| ACR (mg/g creatinine) | 795 (274, 1803) | 255 (57, 1096) | <0.0001 |
| eGFR (ml/min/1.73m2) | 43.2 (35, 51) | 48.7 (40, 57) | <0.0001 |
| During follow-up | |||
| eGFR slope (ml/min/1.73m2/year) | −4.0 (−7.8, −2.1) | −3.1 (−6.4, −0.9) | 0.007 |
| Non-progressorsa, n (%) | 71 (33%) | 69 (48%) | |
| Progressorsa, n (%) | 143 (67%) | 75 (52%) | |
| New incidence of ESRD during | 108 (50%) | 35 (24%) | <0.0001 |
| 10-year follow-up, n (%) | |||
| Deaths unrelated to ESRD, n (%) | 15 (7%) | 8 (6%) | 0.58 |
T1D, Type 1 diabetes; T2D, Type 2 diabetes; DM, Diabetes mellitus; BMI, Body mass index; BP, Blood pressure; Rx, treatment; Renoprotection, Prescription of angiotensin-converting enzyme
inhibitor (ACE-I) or angiotensin II receptor blocker (ARB); HbAlc, Hemoglobin A1c; ACR, Albumin-to-creatinine ratio; eGFR, Estimated glomerular filtration rate; ESRD, End-stage renal disease.
Non-progressors were defined as eGFR loss < 3.0 ml/min/1.73m2/year and Progressors as eGFR loss ≥ 3.0 ml/min/1.73m2/year.
Data presented as median (25th, 75th percentile) or count (proportion) measures.
Differences between the two cohorts were tested using the Wilcoxon-rank-sum test for continuous variables, and the χ2 test for categorical variables.
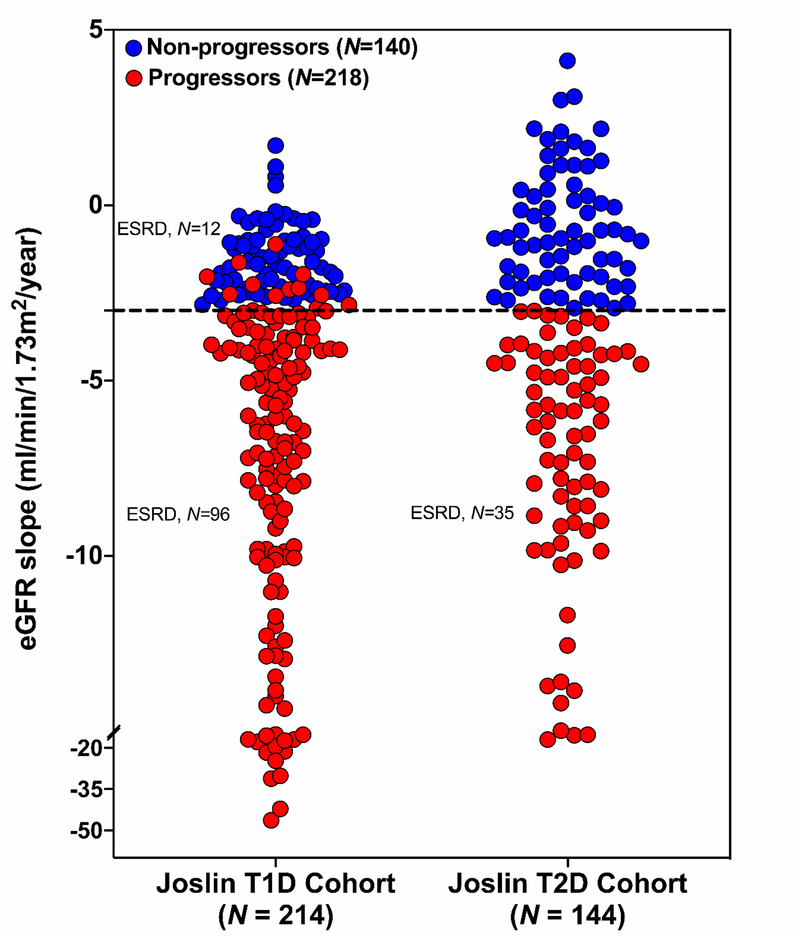
During 7–15 years of follow-up, the majority of participants in both cohorts had progressive renal decline. However, eGFR slopes varied greatly among individuals, with slopes being slightly steeper in individuals with T1D than in those with T2D. Figure 1 shows the distribution of eGFR slopes in the Joslin cohorts with T1D and T2D. The number of slow decliners (referred to as non-progressors) defined as eGFR loss < 3.0 ml/min/year was 71 (33%) and 69 (48%) in the T1D exploratory and T2D replication cohorts, respectively (Table 1). These non-progressors had very shallow eGFR slopes, with the median (25th, 75th percentile) being −1.6 ml/min/year (−2.3, −1.0) and −0.9 ml/min/year (−2.0, 0.4) in T1D and T2D cohorts, respectively. None of these individuals progressed to ESRD during the 7–15 years of follow-up. In contrast, a large proportion (61% of combined cohorts) of fast decliners defined as eGFR loss ≥ 3.0 ml/min/year or progression to ESRD within 10 years of follow-up (referred to as progressors) (Table 1).
Fig. 1. Distribution of eGFR slopes (ml/min/1.73m2/year) in the Joslin Kidney Study cohorts with T1D and T2D.
Non-progressors were defined as eGFR loss < 3.0 ml/min/1.73m2/year and progressors as eGFR loss ≥ 3.0 ml/min/1.73m2/year or progression to ESRD. In each cohort, only ESRD cases that developed during the first 10 years after study entry were considered in the present study. Dash line indicates eGFR loss equal to 3.0 ml/min/1.73m2/year.
Profiling plasma proteins that protect against progressive renal decline
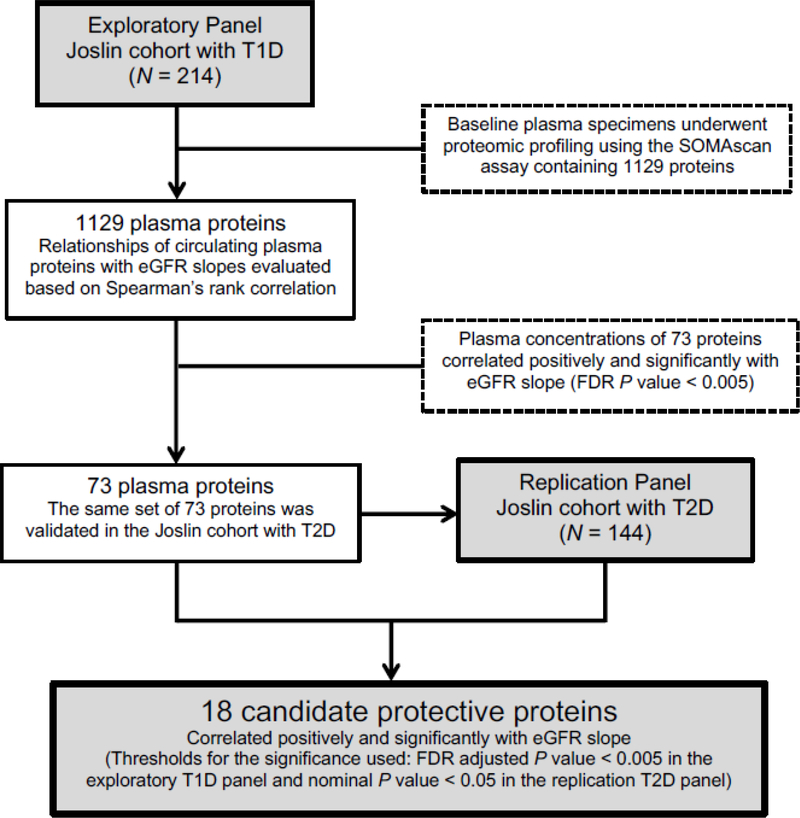
The SOMAscan proteomic platform was used to measure 1,129 plasma proteins (table S1). These plasma proteins were examined for elevated concentrations in non-progressors at baseline. The schematic representation of this study is outlined in Figure 2. In the Joslin exploratory T1D cohort, baseline plasma concentrations of 73 proteins were positively correlated with eGFR slope at a false discovery rate (FDR) adjusted P<0.005 (table S2), therefore, elevated baseline concentrations of these proteins were associated with slow or minimal renal decline during follow-up. These proteins were considered candidate protective factors/biomarkers against progressive renal decline. Proteins that were negatively correlated with eGFR slope might be considered candidate factors/biomarkers increasing the risk of progressive renal decline and progression to ESRD, however, they are the subject of other studies. We have recently published in a separate study the association of 194 inflammatory circulating proteins with the risk of progression to ESRD in these two Joslin cohorts using the same SOMAscan proteomic platform (5).
Fig. 2. Overview of the study design.
Schematic representation of study design showing the study participants in the exploratory and replication panels and how the candidate protective proteins were selected.
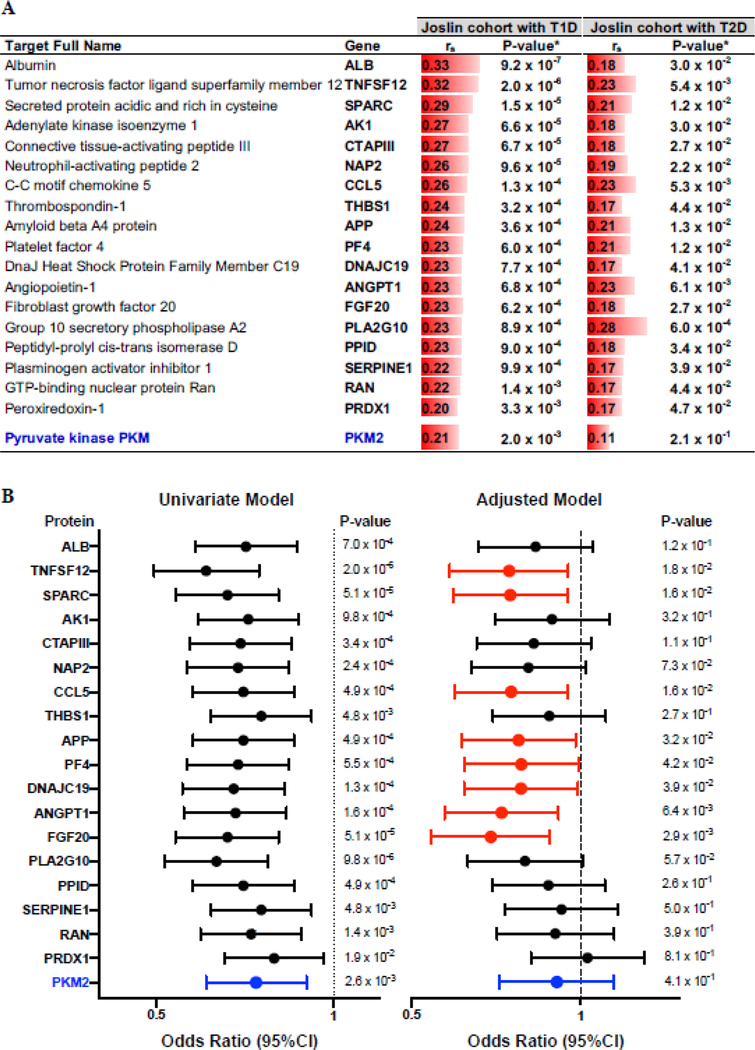
The 73 plasma proteins positively correlated with eGFR slope in patients with T1D were analyzed further in the replication cohort of patients with T2D. Eighteen proteins were found positively correlated with eGFR slope at a nominal P<0.05 (table S2). Elevated concentrations of PKM2 in kidney tissue and in plasma were recently demonstrated as a new biomarker and potential therapeutic target protecting against DKD in individuals with long duration of T1D (7). To determine whether this protein may be also involved in protection against progressive renal decline in individuals with impaired renal function, we included PKM2, along the 18 candidate proteins, in further analyses despite a weak correlation with eGFR slope in those with T2D. The names of the 19 plasma proteins, correlation coefficients and P-values for each positively correlated protein with eGFR slope in the T1D and T2D cohorts, respectively, are presented in Figure 3A. Correlations were generally slightly weaker in those with T2D, but all proteins were correlated positively with eGFR slope.
Fig. 3. Candidate circulating proteins associated with protection against progressive renal decline.
(A) Spearman’s rank correlation coefficients (rS) between baseline concentration of 19 plasma proteins and eGFR slope in the Joslin cohorts with T1D (N = 214) and T2D (N = 144). Red bars are a graphic representation of the effect size. Corresponding two-sided P-values have been provided. *Thresholds for the significance used: FDR adjusted P < 0.005 in the T1D exploratory cohort and a nominal P < 0.05 in the T2D replication cohort. (B) Odds ratios (95% CI) for the 19 candidate protective proteins and progressive renal decline (eGFR loss ≥ 3.0 ml/min/year) in the combined cohorts with T1D and T2D in univariate and adjusted logistic regression models. The effect is shown as an odds ratio (95% CI) per one quartile increase in circulating baseline concentration of the specific protein. The final model was adjusted for baseline eGFR, HbA1c and ACR with stratification by type of diabetes. The 8 selected markers are in red. PKM2, included in the analysis based on previous publication, is in blue.
Multivariable logistic analysis of plasma proteins protecting against progressive renal decline
As both Joslin cohorts had impaired renal function (CKD Stage 3) at baseline and had homogenous strength of association with eGFR slope, the SOMAscan results from both cohorts were combined. The association of baseline plasma concentrations of each of the 19 proteins and the rate of progressive renal decline were analyzed using logistic regression analysis. Participants from the combined Joslin cohorts were grouped into those with (i) fast renal decline (eGFR loss ≥ 3.0 ml/min/year) or progression to ESRD; or (ii) slow or minimal renal decline (eGFR loss < 3.0 ml/min/year). To assess statistical independence of protective effect from clinical characteristics and risk factors associated with progressive renal decline, we performed first univariate and then multivariable logistic models adjusted for baseline clinical covariates. The list of potential confounders included age, gender, ethnicity/race, duration of diabetes, insulin treatment, renoprotection treatment, BMI, systolic and diastolic blood pressures, HbA1c, eGFR and ACR. The key covariates, consisting of HbA1c, eGFR and ACR were included in the final logistic model. Information about selection of covariates into the logistic models is provided in table S3. The results of univariable and multivariable analyses are shown in Figure 3B. All models were adjusted for type of diabetes. The effects are shown as odds ratios (OR) with 95% confidence interval (95% CI) per one quartile increase in baseline plasma concentration of the specific protein. In the univariate model, all 19 proteins including PKM2 (Fig. 3B – marked in blue) protected (had OR<1.0) against progressive renal decline. Elevated plasma concentrations of 8 proteins remained associated with protection against progressive renal decline in the final model adjusted for baseline clinical covariates including eGFR, HbA1c, ACR and type of diabetes (Fig. 3B and table S4). These 8 plasma proteins, referred to as “confirmed” protective proteins, included TNFSF12, SPARC, CCL5, APP, PF4, DNAJC19, ANGPT1 and FGF20 (Fig. 3B – marked in red). Baseline concentrations of PKM2 were not associated with protection against progressive renal decline after further adjustment by clinical covariates.
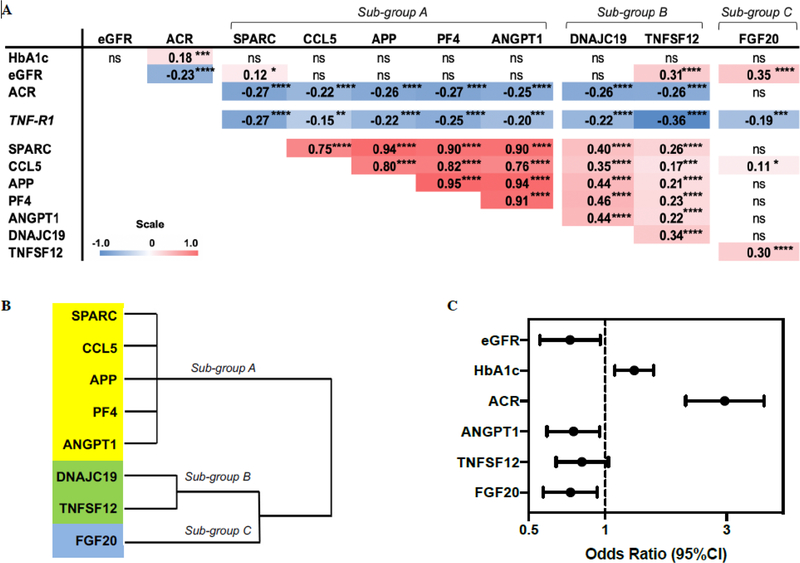
To examine which of the confirmed protective proteins contributed independently to protection against progressive renal decline, first, relationships at baseline were analyzed among the 8 proteins and important clinical covariates using a Spearman’s rank correlation. The correlation matrix shown in Figure 4A indicates that variation in baseline HbA1c had no impact on variation of the 8 protective proteins, whereas variation in baseline eGFR correlated weakly with TNFSF12 and FGF20. In contrast, baseline ACR correlated weakly with all of the proteins (Fig. 4A and fig. S1) except for FGF20. In addition, all of the protective proteins correlated negatively with plasma tumor necrosis factor receptor 1 (TNF-R1) concentrations, reported by us previously as one of the circulating inflammatory proteins associated with increased risk of progression to ESRD (5), indicating decreased plasma TNF-R1 concentrations with increasing concentrations of the protective proteins. The confirmed protective proteins were grouped into three sub-groups according to their correlation coefficients with each other (Fig. 4A). Sub-group (A) contained 5 extremely highly inter-correlated proteins; SPARC, CCL5, APP, PF4 and ANGPT1. Sub-group (B) contained 2 proteins; DNAJC19 and TNFSF12, that were moderately correlated between themselves and with proteins in sub-group (A). Sub-group (C) contained FGF20, a protein not correlated with any of the other proteins except for moderate correlation with TNFSF12. This pattern of grouping of proteins was preserved and confirmed in the hierarchical cluster analysis (Fig. 4B). This finding suggests that plasma concentrations of these three sub-groups of proteins are regulated by different mechanisms. In contrast, the 5 proteins in sub-group (A) showed such strong inter-correlation that one can hypothesize that they are regulated by the same mechanisms.
Fig. 4. Association of 8 confirmed protective proteins with clinical covariates and with risk of progressive renal decline.
(A) Spearman’s rank correlation matrix among 8 candidate protective proteins with TNF-R1 and important clinical covariates in the two cohorts adjusted for type of diabetes. Correlation coefficients (rs) are presented as shades of red (positive) and blue (negative) which correspond to the magnitude of the effect size. (B) Hierarchical cluster analysis in the combined Joslin cohorts. (C) Odds ratios (95% CI) of covariates selected from a backward selection of covariates using the significance criterion α = 0.1. The effects of eGFR and HbA1c on progressive renal decline are estimated per 10 ml/min/1.73m2 increase and per 1% increase, respectively. The effect of ACR on progressive renal decline is estimated as one unit increase of log10 ACR. The effect of each protein is shown as an odds ratio (95% CI) per one quartile increase in circulating baseline concentration of the relevant protein. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; ns, not significant.
To further test which of these 8 proteins (three sub-groups) independently contributed to protection against progressive renal decline, a multivariable logistic regression analysis was performed with backward elimination of proteins and clinical covariates that had no or weak effects (α>0.1) (table S5). All relevant clinical characteristics and the 8 confirmed protective proteins were included in the analysis. In the final model, baseline clinical variables, HbA1c and ACR increased the risk of progressive renal decline, whereas eGFR and three baseline plasma proteins, ANGPT1 (exemplar of sub-group A), TNFSF12 (exemplar of sub-group B) and FGF20 (sub-group C) protected against progressive renal decline. The odds ratios (95% CI) obtained from the multivariable logistic regression analysis for the clinical covariates and the exemplar protective proteins are shown in Figure 4C.
Combined effect of the three exemplar protective proteins
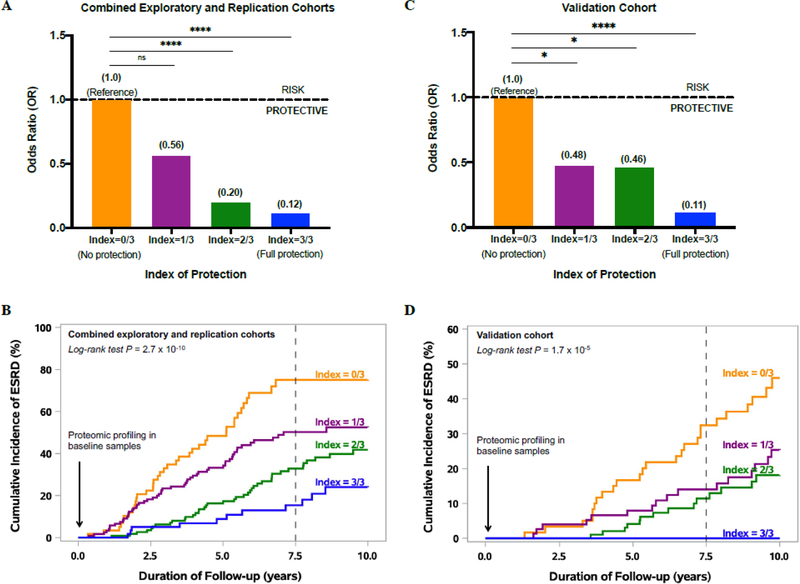
To estimate the combined effect of the three exemplar protective proteins on risk of progressive renal decline and progression to ESRD, an “index of protection” was developed. The plasma concentrations of the three exemplar protective proteins (ANGPT1, TNFSF12 and FGF20) were evaluated in each individual. Value above median for each protein was scored as 1 and below as 0; by summing up the scores, an individual could have a total protection index varying between 0 (all proteins below median) and 3 (all proteins above median). The association between the index of protection and progressive renal decline is shown in Figure 5A. The odds ratio (95% CI) for progressive renal decline was 0.56 (0.26, 1.25), 0.20 (0.1, 0.45) and 0.12 (0.05, 0.28) for individuals with the total index of protection 1, 2 and 3, respectively, when compared with those with the protection index value 0. To visualize the combined effect of the three protective proteins, the cumulative risk of progression to ESRD was analyzed in the combined study cohorts according to the index of protection. Figure 5B shows the cumulative incidence of ESRD during 7.5 years of follow-up according to values of the protection index. Individuals with all 3 protective protein values above median had very low risk of developing ESRD, with a cumulative incidence of 16% during 7.5 years of follow-up. In contrast, those with the protective index value 0 (all three protective protein values below median), had a very high cumulative incidence of ESRD of 80%. The difference in cumulative incidence of ESRD among the 4 subgroups was highly statistically significant (P = 2.7×10−10).
Fig. 5. The combined effect of protective proteins FGF20, TNFSF12 and ANGPT1 on risk of progressive renal decline and progression to ESRD in the combined exploratory and replication cohorts (A and B), and in the validation cohort (C and D).
Odds ratios for progressive renal decline according to index of protection considered as a discrete covariate in (A) the combined exploratory and replication cohorts (N = 358) with both types of diabetes and impaired renal function, and (C) the validation cohort (N =294) of T1D individuals with normal renal function.
Cumulative incidence of ESRD (%) according to discrete values of index of protection in (B) the combined exploratory and replication cohorts and (D) the validation cohort. Index of protection: Value above median for each protein was scored as 1 and below as 0; by summing up these scores, an individual could have a total protection index varying between 0 (all proteins below median) and 3 (all proteins above median). *P<0.05; ****P<0.0001; ns, not significant.
To examine whether the results shown in Figure 5A could have been confounded by inflammatory circulating proteins (such as high TNF-R1 plasma concentrations) or clinical covariates, the logistic regression analysis was performed in the combined Joslin cohorts (T1D and T2D). In this analysis, the protection index was considered as a continuous variable as opposed to discrete variable as in Figure 5A. As shown in Table 2, the effect of index of protection was statistically significant (P<0.0001), the odds ratio was 0.47 (95% CI: 0.32–0.60). By including into the model one inflammatory protein, TNF-R1, reported by us previously (5), the protective effect of the index was attenuated, the odds ratio increased to 0.60 (95% CI: 0.45–0.78) but remained statistically significant (P<0.0002). Adding into the model many clinical covariates did not substantially change the odds ratio for the protective index.
Table 2.
Effect estimates measured as odds ratios (95% CI) of index of protection on risk of progressive renal decline in univariable and multivariable logistic regression models in both Joslin cohorts combined.
|
|
||||||
|---|---|---|---|---|---|---|
| Model | Model comparisons (P-value) | |||||
|
| ||||||
| Predictive metrics | 1 | 2 | 3 | 2 vs 1 | 3 vs 2 | 3 vs 1 |
|
| ||||||
| C-statistics ± SE | 0.687 ± 0.03 | 0.765 ± 0.03 | 0.833 ± 0.02 | 0.0005 | <0.0001 | <0.0001 |
| −2 Log Likelihood | 439 | 401 | 352 | |||
| AIC | 443 | 407 | 364 | |||
| Covariates | Odds Ratio (95% CI) | Significance (P-value) | ||||
| Protection Index | 0.47 (0.36, 0.60) | 0.60 (0.45, 0.78) | 0.61 (0.45, 0.82) | <0.0001 | 0.0002 | 0.001 |
| TNF-R1 | 2.04 (1.61, 2.58) | 1.63 (1.23, 2.15) | <0.0001 | 0.0007 | ||
| HbAlc | 1.32 (1.12, 1.56) | 0.001 | ||||
| ACR | 2.54 (1.77, 3.63) | <0.0001 | ||||
| eGFR | 0.99 (0.96, 1.02) | 0.49 | ||||
SE, Standard error; AIC, Akaike information criterion; CI, Confidence intervals; TNF-R1, Tumor necrosis factor receptor 1; HbAlc, Hemoglobin A1c; ACR, Albumin-to-creatinine ratio; eGFR, Estimated glomerular filtration rate.
Model 1 has been compared to the model with the same protection index in the presence of TNF-R1 (Model 2) and to the model with same protection index and TNF-R1, in the presence of important clinical covariates (Model 3).
Validation of the three exemplar protective proteins in early DKD
To demonstrate the robustness of these findings, a validation study was conducted in an independent Joslin cohort of 294 individuals with T1D who had had albuminuria but normal renal function at baseline. This cohort was followed for 7–15 years to determine eGFR slope and ascertain time of onset of ESRD. Plasma samples from the validation study of 294 T1D individuals underwent profiling of the proteins of interest using the same SOMAscan platform. In contrast to the exploratory and replication cohorts, which had impaired renal function (CKD Stage 3) at baseline, the validation cohort had normal renal function (CKD Stages 1 and 2; median eGFR (25th, 75th percentile): 100 (82, 114) ml/min/1.73m2) at baseline. The clinical characteristics of the validation cohort are shown in table S6.
The plasma concentrations of the three exemplar protective proteins ANGPT1, TNFSF12 and FGF20 were evaluated and the index of protection was calculated. The association between the index of protection and progressive renal decline is shown in Figure 5C. The odds ratio (95% CI) for progressive renal decline was 0.48 (0.24, 0.95), 0.46 (0.24, 0.89) and 0.11 (0.05, 0.27) for individuals with the index of protection value of 1, 2 and 3, respectively, when compared with individuals with the index value of 0. The cumulative risk of progression to ESRD was also analyzed in the validation cohort according to the index of protection. Figure 5D shows the cumulative incidence of ESRD during 7.5 years of follow-up according to values of the index of protection. None of the individuals with all 3 protective protein values above median progressed to ESRD during 7.5 years of follow-up. The low cumulative incidence of ESRD was observed for those with the protection index values 1 and 2; 14% and 11%, respectively, when compared with individuals with the protection index value 0 with the cumulative incidence of 33% during 7.5 years of follow-up. These differences were highly statistically significant (P = 1.7×10−5).
Protective effect of ANGPT1 versus the risk effect of ANGPT2
ANGPT1 and angiopoietin-2 (ANGPT2) are both ligands for the Tie-2 receptor. Since ANGPT1 and ANGPT2 have opposite actions and compete with each other for the receptor, it is not clear whether the protective effect of ANGPT1 was independent from the variation of ANGPT2. Since ANGPT2 was measured on the SOMAscan platform and the results were available for this study, we compared the protective effect of ANGPT1 versus the risk effect of ANGPT2 as well as the effect of ratio of ANGPT1/ANGPT2 (in favor of ANGPT1) on the risk of progressive renal decline. The findings of these analyses did not show a stronger protective effect of the ratio of the two angiopoietins in comparison with the protective effect of ANGPT1 alone (table S7), supporting the protective role of ANGPT1 alone against progressive renal decline rather than the ratio of the two angiopoietins.
Plasma concentrations of protective proteins in non-diabetic and diabetic individuals
Two possibilities may explain the elevated concentrations of protective proteins in non-progressors vs. those at risk of progression. The first possibility is that non-progressors had high concentrations of these proteins, whereas those at risk of progression had low concentrations of these proteins before they developed diabetes, a pattern consistent with true protection. The second possibility is that diabetes-related abnormalities resulted in lowering concentration of the protective proteins in individuals at risk of progression but not in non-progressors, a pattern consistent with protective proteins being just markers of risk of progressive renal decline. To distinguish between the two possibilities, plasma concentrations of the protective proteins were compared among healthy non-diabetic parents of T1D individuals, non-progressors and progressors with T1D and T2D, using the same SOMAscan platform. Baseline clinical characteristics and baseline values of the protective proteins among the three study sub-groups are shown in Table 3. The healthy individuals were older, had normal HbA1c, normal ACR and almost normal eGFR in comparison with individuals with diabetes. By design, non-progressors and progressors had similarly impaired renal function at baseline but dramatically different eGFR slopes during 7–15 years of follow-up. With regard to the 8 confirmed protective proteins, the lowest baseline concentrations were observed in healthy individuals and the highest values were observed in non-progressors, while progressors’ concentrations fell between the two other sub-groups. A graphical comparison of plasma concentration of the 3 exemplar protective proteins among the 3 sub-groups are shown in figure S2.
Table 3.
Clinical characteristics and plasma concentrations of 8 confirmed protective proteins in non-diabetic parents of T1D individuals and in the combined Joslin cohorts, for non-progressors and progressors.
|
|
|||
|---|---|---|---|
| Combined Joslin cohorts (N = 358) | |||
|
| |||
| Characteristics | Non-diabetics (N = 79) | Non-progressors (N = 140) | Progressors (N = 218) |
|
| |||
| At baseline | |||
| Male, n | 40 (51%) | 78 (56%) | 120 (55%) |
| Age at study entry (years) | 61 (57, 66) | 56 (48, 61) | 47 (40, 60) |
| Duration of diabetes (years) | - | 24 (14,34) | 24 (18, 31) |
| BMI (kg/m2) | - | 29 (25, 34) | 27 (24,33) |
| Systolic BP (mm Hg) | - | 133 (122, 148) | 136 (126, 149) |
| Diastolic BP (mm Hg) | - | 72 (67, 81) | 78(70,83) |
| Insulin Rx, % | - | 81% | 89% |
| Renoprotection Rx, % | - | 82% | 83% |
| HbAlc (%) | 5.4 (5.2, 5.6) | 7.4 (6.9, 8.6) | 8.4 (7.4, 9.6) |
| eGFR (ml/min/1.73m2) | 71.2 (62, 82) | 49 (42,55) | 42 (34, 51) |
| ACR (mg/g creatinine) | 5.8 (3.9, 7.8) | 175 (40, 502) | 1106 (402, 2232) |
| During follow-up | |||
| eGFR slope (ml/min/1.73m2/year) | - | −1.2 (−2.2, −0.31) | −6.2 (−9.8, −4.1) |
| Deaths unrelated to ESRD, n (%) | - | 10 (7%) | 13 (6%) |
| Baseline plasma concentrations (RFU) | |||
| Sub-group A | |||
| SPARC | 17775 (13587, 28777) | 43192 (30001, 62701) | 33266 (21572, 49352) |
| CCL5 | 14900 (7180, 24835) | 25351 (13498, 46022) | 18973 (10635, 32056) |
| APP | 23162 (17824, 36917) | 45776 (29392, 72317) | 35561 (23106, 52307) |
| PF4 | 20031 (9581, 46044) | 52730 (21260, 100893) | 31230 (13449, 70052) |
| ANGPT1 | 757 (640,1189) | 1564 (1093, 2522) | 1248 (934,1916) |
| Sub-group B | |||
| DNAJC19 | 540 (484,585) | 587 (540, 675) | 555 (507, 604) |
| TNFSF12 | 270 (240, 296) | 291 (269, 316) | 267 (244, 288) |
| Sub-group C | |||
| FGF20 | 371 (311, 417) | 491 (449, 550) | 460 (421, 507) |
BMI, Body mass index; BP, Blood pressure; Rx, treatment; Renoprotection, Prescription of angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB); HbA1c, Hemoglobin A1c; eGFR, Estimated glomerular filtration rate; ACR, Albumin-to-creatinine ratio; RFU, Relative fluorescence unit. Data presented as median (25th, 75th percentile) or count (proportion) measures.
To examine whether plasma concentration pattern of protective proteins preceded the diabetic state and the development of early renal decline, a comparative analysis was performed on plasma concentrations of ANGPT1, TNFSF12 and FGF20 in non-diabetic parents of two categories of T1D probands, normo-albuminuria or ESRD (or proteinuria). Baseline characteristics and baseline values of the 3 protective proteins among non-diabetic parents of the two categories of T1D probands are shown in table S8. Interestingly, parents of children who remained without kidney complications despite long diabetes duration had higher concentration of circulating FGF20 in comparison with parents who had children with kidney complications (ESRD or Proteinuria) (table S8). We did not observe any differences among the parents regarding the concentrations of ANGPT1 and TNSF12 most likely due to small study groups.
Discussion
Through unbiased proteomic profiling, the present study searched for circulating plasma proteins that were specifically associated with protection against progressive renal decline and progression to ESRD. We identified 8 circulating proteins. When these 8 proteins were considered together, only three ANGPT1, TNFSF12 and FGF20, showed a strong independent protective effect against progressive renal decline. The combined effect of these 3 protective proteins was additive and was nicely demonstrated by very low risk of ESRD in individuals who had had values for all 3 proteins above median at the beginning of follow-up. Furthermore, the fact that the concentrations of these protective proteins were much higher in non-progressors than in non-diabetic parents provides strong evidence that the proteins or the pathways that they represent, are causally involved in protection against progressive renal decline. Our study findings are highly generalizable as the importance of these 3 protective proteins is confirmed in independent cohorts of individuals with different types of diabetes and those with early and late stages of DKD. Following, we will discuss the biology of each protective protein and possible mechanisms through which they may contribute to protection against progressive renal decline.
Angiopoietins (ANGPT) are growth factors involved in angiogenesis and vascular inflammation. Among the members of the ANGPT family, Angiopoietin-1 (ANGPT1) and Angiopoietin-2 (ANGPT2) are both ligands for the tyrosine kinase receptor (TIE-2) (16, 17). ANGPT1 is a major ligand and activator of the TIE-2 receptor, maintaining vessel integrity (18), therefore protecting the endothelium from excessive activation by growth factors and cytokines (19). ANGPT2, on the other hand, is considered a natural antagonist of ANGPT1 by preventing the binding of ANGPT1 to the TIE-2 receptor, consequently reducing ANGPT1/ TIE-2 pathway activation and promoting blood vessel wall destabilization and vascular leakage (17, 19). Since ANGPT1 and ANGPT2 are competing with each other for the TIE-2 receptor and have opposite actions, some authors postulated that it would be beneficial to measure both angiopoietins to assess the equilibrium of the ongoing angiogenesis process (20). Disruption of the equilibrium might lead to diabetes-mediated destabilization of blood vessel walls, promotes inflammation and fibrosis (21). This study showed that the measurement of circulating ANGPT1 alone is a sufficient predictor of rate of progressive renal decline and risk of ESRD.
Tumor Necrosis Factor (TNF) Ligand Superfamily Member 12 (TNFSF12), also known as TWEAK, is a member of a large TNF superfamily of ligands and receptors (22). Findings from in vitro and in vivo models have shown that the administration of TNFSF12 increases inflammatory cytokine production in renal tubular cells, such as increased mRNA and protein expression of monocyte chemoattractant protein-1 and interleukin-6 (IL-6), whereas the blockage of TNFSF12 prevented tubular chemokine and IL-6 expression, interstitial inflammation and macrophage infiltration in mice (23). The role of TNFSF12 in the development/progression of DKD remains unclear. So far there has been sparse literature devoted to this topic; a few cross-sectional studies have investigated a relationship between circulating TNFSF12 concentrations and DKD. One study reported decreased circulating TNFSF12 concentrations in T2D and ESRD individuals (24). The actions of TNFSF12 in other kidney diseases and other forms of diabetes have also been reported (25–27). In experimental folic acid-induced acute kidney injury, TNFSF12 deficiency reduced kidney apoptosis and inflammation and improved renal function (25). A case-control study involving women with and without gestational diabetes mellitus (GBM) reported decreased TNFSF12 concentrations in women with GBM compared to pregnant volunteers without GBM (26). The present study is the only follow-up observation in which very robust findings point to TNFSF12 as a protective protein against progressive renal decline, contrary to findings in the aforementioned studies. This finding needs to be explored further in humans and in animal studies.
Fibroblast growth factor 20 (FGF20) is a member of a large family of 22 fibroblast growth factors (FGFs), comprising 7 sub-families consisted of secreted signaling proteins and intracellular non-signaling proteins (28). Seventeen out of 22 FGFs were measured on the SOMAscan proteomic platform and only FGF20 was robustly associated with protection against progressive renal decline. FGF20 is a neurotrophic factor that was originally identified in the rat brain (29) and has been suggested to play vital roles in the development of dopaminergic neurons (30, 31). In addition, numerous studies have reported correlations between Parkinson’s disease susceptibility with FGF20 genetic polymorphisms in different ethnicities (32–34) although some studies reported no evidence of association between FGF20 and Parkinson’s disease (35, 36). Interestingly, a previous study demonstrated the essential role of FGF20/Fgf20 in the development of kidney by maintaining the stemness of nephron progenitors both in humans and in mice (37). FGF20 was expressed exclusively in nephron progenitors in the kidney. Loss of FGF20/Fgf20 in humans and in mice resulted in kidney agenesis (37), a condition in which one or both fetal kidneys fail to develop and hence a newborn was missing one or both kidneys.
The present study demonstrates FGF20 as one of the confirmed protective proteins that is most strongly associated with protection against progressive renal decline and progression to ESRD in the combined cohorts with T1D and T2D. The association is independent from circulating inflammatory proteins and relevant clinical covariates. High plasma concentrations of FGF20 at baseline predicted less renal decline during 7–15 years of follow-up. This association points to the involvement of FGF20 and its independent role to retard or decrease the risk of progressive renal decline and development of ESRD. As such, FGF20 may be a useful target for preventing or delaying the onset of progressive renal decline and ESRD in diabetes. Another interesting finding from our study was observed in plasma profiles of non-diabetic parents of two categories of T1D probands, either normo-albuminuria or ESRD/Proteinuria. Surprisingly, non-diabetic parents of T1D offspring with ESRD/Proteinuria had lower plasma concentrations of FGF20 than those parents with T1D offspring without kidney complications. These findings prompt a question and/or speculation whether a genetic predisposition or component inherited from a parent may modulate corresponding protein concentrations in their offspring, and if confirmed in larger studies, could have a profound implication in future research on determinants of progressive renal decline in T1D (and also in T2D).
Recent interest in studies on protective factors against late diabetic complications, including DKD, has been initiated by the Joslin Medalist Study (7). This cross-sectional study enrolled nationwide individuals who survived with T1D for at least 50 years. Those who remained without late diabetic complications have been compared with regard to a large number of characteristics including various -omics profiles of biospecimens with non-diabetic spouses and with those who developed complications very late in the diabetes course. Comparing proteomic profiles of kidney tissues obtained from individuals in the three sub-groups, several glucose metabolic enzymes/proteins were identified in the glomeruli, including PKM2, which were highly elevated among those who remained without DKD despite extremely long duration of diabetes. By following this finding with a series of functional studies, the authors concluded that the upregulation of PKM2 may be a way of preventing the development of DKD (7).
The present study also searched for protective factors but was very different from the Medalist study. Where the latter was cross-sectional and searched for candidate protective proteins to be investigated in cellular and animal studies, this study was a Joslin clinic population-based prospective observational study that investigated the association between baseline circulating plasma proteins that protected against progressive renal decline and fast progression to ESRD during 7–15 years of follow-up. Furthermore, the two studies were based on two different premises. The Medalist study aimed to find protective proteins against onset/development of late diabetic complications whereas this study aimed to identify protective proteins against progressive renal decline in individuals with already existing mild renal impairment. This is most likely the reason we could not confirm the PKM2 finding obtained in the Joslin Medalist study (7).
The strengths of this study include its prospective design, long-term follow-up observations of three independent study cohorts, the consistency of data in T1D and T2D, and the use of SOMAscan proteomic platform to measure protein concentrations in all Joslin cohorts. Furthermore, in this study, we adjusted our findings for key potential confounders and type of diabetes. However, the present study must be also considered in light of potential limitations. First, this is an observational study and while these proteins might directly protect against progression of renal decline, they could alternatively be indirect reporters of protective processes. Causal explanations of our findings will need to be established through animal models and clinical trials for confirmation that they are directly protective. Second, our findings are restricted to Caucasian individuals with diabetes who have chronic kidney disease and impaired renal function, therefore, the results may not be generalizable to individuals in other populations and with other kidney diseases. Third, the baseline plasma samples were not taken at the onset of diabetes or at the onset of renal function decline, hence, slow or fast progressive renal decline is relative to the time of blood sampling (13).
Materials and Methods
Study design
The primary goal of this observational study was to search for protective proteins against progressive renal decline and progression to ESRD, not only in T1D patients with impaired renal function but also in any diabetic patients at any stages of DKD. Therefore, to demonstrate the robustness of our findings, we selected three very different cohorts with different baseline characteristics; the T1D exploratory (T1D patients with late stage of DKD), the T2D replication (T2D patients with late stage of DKD) and the T1D validation (T1D patients with early stage of DKD) cohorts. The schematic representation of this study is outlined in Figure 2.
The study participants were selected among individuals participating in the Joslin Kidney Study (JKS). The Joslin Diabetes Center Committee on Human Studies approved the informed consent, recruitment and examination protocols for the JKS, a longitudinal observational study that investigates the determinants and natural history of renal function decline in both types of diabetes. A detailed description of the JKS is provided in the Supplementary Materials. Sample size calculations were not performed in this study. Randomization and blinding were not applicable to this study.
Joslin Kidney Study
Briefly, the JKS comprises two cohorts, T1D and T2D. Individuals in the T1D cohort (N=2,000) were recruited consecutively from among adults 18–64 years old who attended the Joslin Clinic between 1991 and 2009. Individuals in the T2D cohort (N=1,500) were recruited consecutively from among adults 35–64 years old who attended the Joslin Clinic between 2003 and 2009. All participants enrolled into the JKS had biannual examinations either during routine clinic visits or were invited for a special visit or were examined at their homes. These examinations were conducted until they developed ESRD, died, were lost to follow-up or until the end of follow-up in 2015. Biospecimens obtained at examinations were stored in −85°C. Serum creatinine was used to determine renal function at baseline and its changes during follow-up visits. Protocols to calibrate serum creatinine measurements over time were described previously (38). Estimates of GFR were obtained using the Chronic Kidney Disease Epidemiology Collaboration formula (39). To estimate the eGFR slope, we applied an approach described by Jones and Molitoris (40) and used by Shah and Levey (41), to examine an individual’s serial renal function changes during follow-up. Details of this approach are provided in the Supplementary Materials and were also described in our earlier publication (38).
The exploratory, replication and validation cohorts used in the current study
The current study comprises three JKS cohorts; the exploratory cohort of 214 individuals with T1D and the replication cohort of 144 individuals with T2D, who previously participated in our study to determine cut-point values of serum TNF-R1 concentrations for the prediction of development of ESRD in T1D and T2D (42). In contrast to the previous study which included individuals with CKD Stages 3 and 4, the present study included individuals in the JKS who had CKD Stage 3 at baseline examination. The validation cohort consists of 294 individuals with T1D who had CKD Stages 1 and 2 at baseline and was used to examine the importance of three protective proteins observed in late DKD cohorts in individuals with an early stage of DKD.
Those with T1D and T2D had macro- (ACR ≥ 300 μg/mg) and micro-albuminuria (ACR ≥ 30 μg/mg). These individuals were followed for 7–15 years to determine the rate of eGFR decline (eGFR slopes) and to ascertain onset of ESRD. All clinical data and plasma specimens from these individuals were available for the current study. Detailed descriptions of these cohorts, measurements of clinical characteristics, determinations of eGFR slopes from serial measurements of serum creatinine, and ascertainment of onset of ESRD were described in our recent publications (5, 42). In all 3 cohorts, we selected eGFR loss < 3.0 ml/min/year as the threshold to define those with slow (non-progressors) or fast (progressors) progressive renal decline. The rationale for such a threshold was well documented and used in our previous publications (11, 43) and corresponds to the 2.5th percentile of the distribution of annual renal function loss in a general population (44).
Healthy non-diabetic parents of T1D individuals
During the Joslin Kidney Study, we also examined living parents of individuals with T1D. The group of non-diabetic parents of T1D individuals was derived from our genetic study on determinants of DKD in T1D. Parents had baseline examinations performed according to the same protocols as all participants of the JKS. Biospecimens obtained at examinations were stored at −85°C. For the purpose of this study, 79 white non-diabetic parents aged 50–69 years at baseline examination were selected to be used as non-diabetic controls. Forty parents had children who remained without kidney complications despite long duration of diabetes and 39 parents had children who had advanced DKD (impaired renal function or ESRD). The clinical phenotype of the T1D offspring of the non-diabetic parents is either normo-albuminuria (N=40), or ESRD or proteinuria (N=39). Plasma specimens obtained at baseline examination were subjected to the SOMAscan analysis.
The SOMAscan proteomic analysis
The SOMAscan proteomic platform uses single-stranded DNA aptamers that measure 1129 protein concentrations (a complete list of the proteins is available in Table S1) in only 50 μl plasma, serum or equally small amounts of a variety of other biological matrices. The SOMAscan platform is facilitated by a new generation of the Slow Off-rate Modified Aptamer (SOMAmer) reagents that benefit from the aptamer technology developed over the past 20 years (45, 46). The SOMAmer reagents are selected against proteins in their native folded conformations and bind to folded proteins and thus three-dimensional shape epitopes rather than linear peptide sequences. The SOMAscan platform offers a remarkably dynamic range, and this large dynamic range results from the detection range of each SOMAmer reagent in combination with three serial dilutions of the sample of interest. The dilutions are separated into three pools: the 40% (the most concentrated sample to detect the least abundant proteins – fM to pM in 100% sample), 1% (mid-range) and 0.005% (the least concentrated sample designs to detect the most abundant proteins – ~μM in 100% sample). The assay readout is reported in relative fluorescent units (RFU) and is directly proportional to the target protein amount in the original sample. The details of the SOMAscan proteomics platform are described elsewhere (14, 15).
Proteomic profiling was performed using the SOMAscan platform based at the SomaLogic laboratory (Boulder, CO). The Human Plasma SOMAscan 1.1k kit with a set of calibration and normalization samples was used following the manufacturer’s recommended protocol. Data standardization was performed according to the SOMAscan platform data quality-control protocols. To standardize SOMAscan assay results, raw SOMAscan assay data was first normalized to remove hybridization variation within a run (hybridization normalization) followed by median signal normalization across all samples to remove other assay biases within the run and finally calibrated to remove assay differences between runs. The acceptance criteria for hybridization and median signal normalization scale factors are expected to be in the range of 0.4–2.5. The median of the calibration scale factors is expected to be within ± 0.2 from 1.0 and a minimum of 95% of individual SOMAmer reagents in the total array must be within ± 0.4 from the median. SOMAscan data from all samples passed quality control criteria and were fit for analysis.
Validation of SOMAscan measurements of the protective proteins
We further validated ANGPT1 and FGF20 proteins using different platforms. ANGPT1 measurements were validated in a subset of samples (N=32) using the Human Ang-1 MSD R-Plex assay (F21YQ-3, Meso Scale Diagnostics). Assay protocols are provided in the Supplementary Materials. The Spearman’s rank correlation coefficient between the SOMAscan and MSD ANGPT1 measurements was rs = 0.76, P<0.0001. To analytically validate SOMAmer specificity, we developed protocols integrating DNA-based affinity pull-down of intact proteins with mass spectrometry. Fourteen FGF20 tryptic peptides spanning amino acids (a.a.) 50–211 of the FGF20 protein sequence were identified in the FGF20 SOMAmer plasma pull-downs spiked with recombinant FGF20, whereas no FGF20 peptides were identified in the FGF20 SOMAmer plasma pull-downs that were not spiked with recombinant FGF20. An example of an extracted ion chromatogram of FGF20 tryptic peptide GGPGAAQLAHLHGILR (a.a. 50–65) is shown in figure S3. This FGF20 peptide was identified in the plasma pull-down spiked with recombinant FGF20 but was not detected in the plasma pull-down not spiked with recombinant FGF20, thereby verifying the FGF20 SOMAmer specificity on the SOMAscan platform. A detailed pull-down protocol is provided in the Supplementary Materials.
Statistical analysis
All statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute). All data were presented as either mean and standard deviations, median (25th and 75th percentiles) or count (proportion) measures, where applicable. Correlations between circulating plasma concentrations with eGFR slopes, TNF-R1 and clinical covariates were assessed using a Spearman’s rank correlation (rs). Clusters of protective proteins were identified using a hierarchical cluster analysis (Ward’s method). Baseline protein RFU concentrations (N=1,129) were natural log transformed and then were categorized into quartiles of their distributions prior to association testing. The distributions of the top 3 protective proteins after natural log transformation in the combined discovery and replication cohorts, and in the validation cohort are shown in figure S4. Univariable and multivariable logistic regression models were used to test associations of relevant circulating plasma proteins measured at baseline with the outcome measure (being a progressor, if eGFR loss ≥ 3.0 ml/min/year or progression to ESRD) and expressed as odds ratios per one quartile increase in circulating plasma concentration of the relevant protein with corresponding 95% confidence intervals. The cumulative incidence rate of ESRD according to the index of protection – the combined effect of the three exemplar protective proteins, was analyzed using PROC LIFETEST in SAS software. Comparisons between plasma protein concentrations in non-diabetics, non-progressors and progressors were examined using one-way ANOVA with Dunn’s multiple comparisons test. Significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Material
Fig. S1 – S4. -Supplementary Figures.
Table S2. Global proteomic profiling data of the circulating plasma proteins in the exploratory cohort of 214 T1D individuals and in the replication cohort of 144 T2D individuals.
Table S3. Selection of potential covariates into the logistic regression model.
Table S4. Logistic regression models examining the association of 19 circulating plasma proteins and progressive renal decline in the combined Joslin cohorts with T1D and T2D.
Table S5. Ranking of proteins/clinical covariates for elimination from the multivariable logistic regression analysis using backward elimination procedure.
Table S6. Demographics and clinical characteristics of an independent validation cohort of T1D individuals with normal renal function.
Table S7: Circulating plasma concentrations of top 3 protective proteins in non-diabetic parents of two categories of T1D probands.
Table S8. Logistic regression models comparing the protective effect of ANGPT1, the risk effect of ANGPT2 and the effect of ANGPT1/ANGPT2 ratio on the risk of progressive renal decline in the combined Joslin cohorts.
Table S1. A complete list of all proteins (N=1,129) measured on the SOMAscan platform.
Funding:
We acknowledge grants support from the JDRF 17-2013-311 (to A.S.K.), the National Institutes of Health DK041526-27 (to A.S.K.), the Novo Nordisk Foundation 14OC0013659 (PROTON) (to A.S.K), the Sunstar Foundation (Hiroo Kaneda Scholarship) (to E.S.), the Foundation for Growth Science from Japan (to E.S.), the Uehara Memorial Foundation (Postdoctoral Fellowship) (to H.K.), the Japan Society for the Promotion of Science (Overseas Research Fellowship) (to H.K.) and the NIH Diabetes Research Center (DRC) grant to Joslin Diabetes Center (P30 DK36836).
Footnotes
Competing interests: A.S.K. is a co-inventor of the “TNF-R1 and TNF-R2 patent for predicting risk of ESRD”. This patent was licensed by the Joslin Diabetes Center to the Renalytix AI PLC. J.M.W is an employee of Eli Lilly and Company and holds equity in Eli Lilly and Company. K.L.D. is an employee of Eli Lilly and Company and has ownership interest in Eli Lilly and Company and Pfizer. The other authors of this report declare no competing conflicts of interest.
Data and materials availability: All data associated with this study are present in the paper or in the supplementary materials. Individual proteomics data may become available for collaborative research from Dr. A.S. Krolewski.
References and Notes:
- 1.Parving HH, Mauer M, Ritz E, Diabetic Nephropathy. In: Brenner BM, ed. Brenner and Rector’s The Kidney. 7th ed. Philadelphia. (Elsevier, 2004). [Google Scholar]
- 2.Diabetes, Control Complications Trial/Epidemiology of Diabetes, Interventions Complications Research (DCCT/EDIC) Group: Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290, 2159–2167 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998). [PubMed] [Google Scholar]
- 4.Nowak N, Skupien J, Smiles AM, Yamanouchi M, Niewczas MA, Galecki AT, Duffin KL, Breyer MD, Pullen N, Bonventre JV, Krolewski AS, Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney international 93, 1198–1206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ, Satake E, Simeone CA, Shah H, Qiu C, Looker HC, Fiorina P, Ware CF, Sun JK, Doria A, Kretzler M, Susztak K, Duffin KL, Nelson RG, Krolewski AS, A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25, 805–813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahluwalia TS, Kilpelainen TO, Singh S, Rossing P, Editorial: Novel Biomarkers for Type 2 Diabetes. Front Endocrinol (Lausanne) 10, 649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, Yorek MA, Wu IH, Lockhart S, Coppey LJ, Pfenninger A, Liew CW, Qiang G, Burkart AM, Hastings S, Pober D, Cahill C, Niewczas MA, Israelsen WJ, Tinsley L, Stillman IE, Amenta PS, Feener EP, Vander Heiden MG, Stanton RC, King GL, Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23, 753–762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS, Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22, 545–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J, Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305, 2532–2539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS, Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348, 2285–2293 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS, Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18, 1353–1361 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Krolewski AS, Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes care 38, 954–962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krolewski AS, Skupien J, Rossing P, Warram JH, Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney international 91, 1300–1311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, Flather D, Forbes A, Foreman T, Fowler C, Gawande B, Goss M, Gunn M, Gupta S, Halladay D, Heil J, Heilig J, Hicke B, Husar G, Janjic N, Jarvis T, Jennings S, Katilius E, Keeney TR, Kim N, Koch TH, Kraemer S, Kroiss L, Le N, Levine D, Lindsey W, Lollo B, Mayfield W, Mehan M, Mehler R, Nelson SK, Nelson M, Nieuwlandt D, Nikrad M, Ochsner U, Ostroff RM, Otis M, Parker T, Pietrasiewicz S, Resnicow DI, Rohloff J, Sanders G, Sattin S, Schneider D, Singer B, Stanton M, Sterkel A, Stewart A, Stratford S, Vaught JD, Vrkljan M, Walker JJ, Watrobka M, Waugh S, Weiss A, Wilcox SK, Wolfson A, Wolk SK, Zhang C, Zichi D, Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One 5, e15004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, Gordish-Dressman H, Hache L, Henricson E, Hoffman EP, Kobayashi YM, Lorts A, Mah JK, McDonald C, Mehler B, Nelson S, Nikrad M, Singer B, Steele F, Sterling D, Sweeney HL, Williams S, Gold L, Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 112, 7153–7158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD, Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD, Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Brindle NP, Saharinen P, Alitalo K, Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98, 1014–1023 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler U, Augustin HG, Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27, 552–558 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Khalaf N, Helmy H, Labib H, Fahmy I, El Hamid MA, Moemen L, Role of Angiopoietins and Tie-2 in Diabetic Retinopathy. Electron Physician 9, 5031–5035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnudi L, Angiopoietins and diabetic nephropathy. Diabetologia 59, 1616–1620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL, TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272, 32401–32410 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A, The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19, 695–703 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Bluher M, Stumvoll M, Fasshauer M, Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 199, 440–444 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A, Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J Cell Mol Med 13, 3329–3342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dereke J, Nilsson J, Nilsson C, Strevens H, Landin-Olsson M, Hillman M, Soluble CD163 and TWEAK in early pregnancy gestational diabetes and later glucose intolerance. PLoS One 14, e0216728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardi S, Voltan R, Rimondi E, Melloni E, Milani D, Cervellati C, Gemmati D, Celeghini C, Secchiero P, Zauli G, Tisato V, TRAIL OPG, and TWEAK in kidney disease: biomarkers or therapeutic targets? Clin Sci (Lond) 133, 1145–1166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh N, Ornitz DM, Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149, 121–130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmachi S, Watanabe Y, Mikami T, Kusu N, Ibi T, Akaike A, Itoh N, FGF-20, a novel neurotrophic factor, preferentially expressed in the substantia nigra pars compacta of rat brain. Biochem Biophys Res Commun 277, 355–360 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Correia AS, Anisimov SV, Roybon L, Li JY, Brundin P, Fibroblast growth factor-20 increases the yield of midbrain dopaminergic neurons derived from human embryonic stem cells. Front Neuroanat 1, 4 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada H, Yoshimura N, Tsuji A, Kunisada T, Differentiation of dopaminergic neurons from human embryonic stem cells: modulation of differentiation by FGF-20. J Biosci Bioeng 107, 447–454 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Pan J, Li H, Wang Y, Ma JF, Zhang J, Wang G, Liu J, Wang XJ, Xiao Q, Chen SD, Fibroblast growth factor 20 (FGF20) polymorphism is a risk factor for Parkinson’s disease in Chinese population. Parkinsonism Relat Disord 18, 629–631 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Sadhukhan D, Das G, Biswas A, Ghosh S, Das SK, Ray K, Ray J, Evaluation of FGF 20 variants for susceptibility to Parkinson’s disease in Eastern Indians. Neurosci Lett 675, 68–73 (2018). [DOI] [PubMed] [Google Scholar]
- 34.van der Walt JM, Noureddine MA, Kittappa R, Hauser MA, Scott WK, McKay R, Zhang F, Stajich JM, Fujiwara K, Scott BL, Pericak-Vance MA, Vance JM, Martin ER, Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease. Am J Hum Genet 74, 1121–1127 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarimon J, Xiromerisiou G, Eerola J, Gourbali V, Hellstrom O, Dardiotis E, Peuralinna T, Papadimitriou A, Hadjigeorgiou GM, Tienari PJ, Singleton AB, Lack of evidence for a genetic association between FGF20 and Parkinson’s disease in Finnish and Greek patients. BMC Neurol 5, 11 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wider C, Dachsel JC, Soto AI, Heckman MG, Diehl NN, Yue M, Lincoln S, Aasly JO, Haugarvoll K, Trojanowski JQ, Papapetropoulos S, Mash D, Rajput A, Rajput AH, Gibson JM, Lynch T, Dickson DW, Uitti RJ, Wszolek ZK, Farrer MJ, Ross OA, FGF20 and Parkinson’s disease: no evidence of association or pathogenicity via alpha-synuclein expression. Mov Disord 24, 455–459 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, Ornitz DM, Kopan R, FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22, 1191–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, Cantarovich D, Stanton R, Krolewski AS, The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney international 82, 589–597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI, A new equation to estimate glomerular filtration rate. Ann Intern Med 150, 604–612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RH, Molitoris BA, A statistical method for determining the breakpoint of two lines. Anal Biochem 141, 287–290 (1984). [DOI] [PubMed] [Google Scholar]
- 41.Shah BV, Levey AS, Spontaneous changes in the rate of decline in reciprocal serum creatinine: errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol 2, 1186–1191 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Yamanouchi M, Skupien J, Niewczas MA, Smiles AM, Doria A, Stanton RC, Galecki AT, Duffin KL, Pullen N, Breyer MD, Bonventre JV, Warram JH, Krolewski AS, Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney international 92, 258–266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, Doria A, Warram JH, Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes care 37, 226–234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindeman RD, Tobin J, Shock NW, Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33, 278–285 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Tuerk C, Gold L, Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990). [DOI] [PubMed] [Google Scholar]
- 46.Ellington AD, Szostak JW, In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 – S4. -Supplementary Figures.
Table S2. Global proteomic profiling data of the circulating plasma proteins in the exploratory cohort of 214 T1D individuals and in the replication cohort of 144 T2D individuals.
Table S3. Selection of potential covariates into the logistic regression model.
Table S4. Logistic regression models examining the association of 19 circulating plasma proteins and progressive renal decline in the combined Joslin cohorts with T1D and T2D.
Table S5. Ranking of proteins/clinical covariates for elimination from the multivariable logistic regression analysis using backward elimination procedure.
Table S6. Demographics and clinical characteristics of an independent validation cohort of T1D individuals with normal renal function.
Table S7: Circulating plasma concentrations of top 3 protective proteins in non-diabetic parents of two categories of T1D probands.
Table S8. Logistic regression models comparing the protective effect of ANGPT1, the risk effect of ANGPT2 and the effect of ANGPT1/ANGPT2 ratio on the risk of progressive renal decline in the combined Joslin cohorts.
Table S1. A complete list of all proteins (N=1,129) measured on the SOMAscan platform.