Abstract
COVID-19 and pulmonary tuberculosis (PTB) coinfection is associated with increased mortality and presents a unique diagnostic challenge to the clinician. We describe three cases of newly diagnosed PTB in COVID-19 patients treated at our centre and their clinical and radiological features. The challenges associated with diagnosis and management are also explored. Patient 1 was a case of smear positive, endobronchial tuberculosis incidentally diagnosed due to CT changes, and eventually made good recovery. Patient 2 was a case of COVID-19 who succumbed but was diagnosed posthumously due to a positive sputum culture for tuberculosis. Patient 3 showed radiographic features of PTB and was treated empirically for TB. In conclusion, COVID-19 and PTB coinfection should be suspected in the presence of constitutional symptoms, prior immunocompromised states, prolonged respiratory symptoms or fever, or unresolved radiological abnormalities, more so in regions where TB is endemic.
Keywords: COVID-19, Pulmonary tuberculosis, Coinfection, Endobronchial tuberculosis, Case series
List of abbreviations
- TB
tuberculosis
- PTB
pulmonary tuberculosis
- CT
computed tomography
- WHO
World Health Organization
- NPOP
nasopharyngeal and oropharyngeal
- CTPA
computed tomography pulmonary angiogram
- HRCT
high resolution computed tomography
- GGO
ground glass opacities
- ATT
anti-tuberculous therapy
- IGRA
interferon-gamma release assay
1. Introduction
COVID-19 was declared a pandemic in 2020, whereas tuberculosis (TB) was declared a global health emergency by the World Health Organization (WHO) in 1993 [1]. Both diseases manifest as respiratory illnesses with varying severities and display similar symptoms [2], reflecting Hickam's dictum which states that multiple symptoms and signs may be due to more than one disease [3]. TB is endemic in the state of Sarawak, Malaysia, with a 2016 incidence of 104.23 per 100,000 population, far above the national average of 81.3 per 100,000 population [4]. Pulmonary TB (PTB) and COVID-19 coinfection has been well-reported [2,[5], [6], [7], [8], [9]] with meta-analysis showing increased risk of mortality [10]. We describe three cases of newly diagnosed PTB in COVID-19 patients treated at our tertiary centre, discuss their varied clinical and radiological presentations, and elucidate the challenges associated with diagnosis and management of PTB and COVID-19 coinfection. All three patients were infected with COVID-19 before the introduction of COVID vaccines.
1.1. Case presentation
1.1.1. Patient 1
A 61-year-old woman with a history of total abdominal hysterectomy and salpingectomy for cervical carcinoma initially visited the surgical clinic with complaints of abdominal bloating and loss of weight for a month, associated with a recent dry cough for one week. She denied shortness of breath, fever, haemoptysis, sore throat, anosmia or dysgeusia. Computed tomography (CT) of the abdomen and pelvis showed no intrabdominal abnormalities, however, ground glass opacities were observed at bilateral lower zones of the lung. Rapid nasopharyngeal and oropharyngeal (NPOP) antigen assay for COVID-19 was positive, and she was admitted to the isolation ward.
On admission, she was apyrexial and normotensive, but was noted to be tachycardic (heart rate of 128 beats per minute), tachypnoeic (respiratory rate of 22 breaths per minute) and had an oxygen saturation of 89% under room air. Arterial blood gas taken under room air revealed type 1 respiratory failure (pH 7.46, PO2 67 mmHg, PCO2 30 mmHg, HCO3 28 mmol/L). Electrocardiogram showed atrial fibrillation with fast ventricular response, but no ischemic or myocarditis changes. Chest radiograph revealed bilateral nodular and airspace opacities (Fig. 1A).
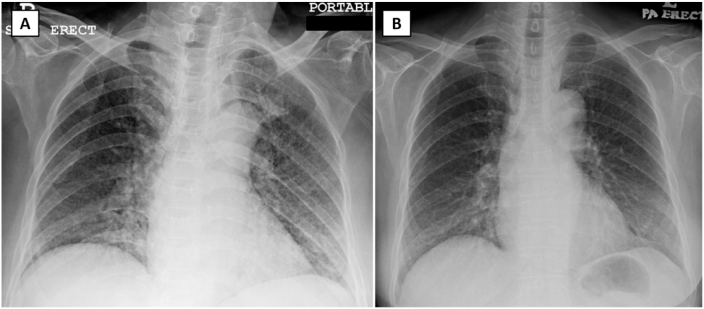
Fig. 1.
Chest radiograph of patient 1 on admission demonstrates nodular and air space opacities bilaterally (Panel A). These opacities significantly resolve on day 12 of admission (Panel B). No cavity, enlarged hilar node, or pleural effusion is seen in both chest radiographs.
Dexamethasone and lopinavir/ritonavir were administered as per local COVID-19 treatment guidelines at the time. She required invasive mechanical ventilation for three days. Endotracheal aspirates sampled for bacterial culture and respiratory virus PCR panel (QIAstat-Dx, QIAGEN, Maryland, United States) were negative. Post-extubation, she remained oxygen dependent and computed tomography pulmonary angiogram (CTPA) was performed on day 7 of admission. CTPA showed pulmonary embolism in the segmental branch of right descending pulmonary artery, generalized, diffuse peri-bronchovascular ground glass changes bilaterally, interlobular and intralobular septal thickening at bilateral upper lobes, focal consolidation at the lateral basal segment of the left lower lobe, and tree-in-bud nodules bilaterally (Fig. 2A). Sputum smear examination was positive for acid fast bacilli. Molecular testing for M. TB nucleic acids (Cepheid Xpert MTB/RIF, https://www.cepheid.com) revealed no rifampicin resistance. She was commenced on anticoagulant and anti-tuberculous therapy (ATT) consisting of rifampicin, isoniazid, pyrazinamide, and ethambutol, and finally weaned off to room air on day 32 of admission. Follow-on high resolution computed tomography (HRCT) of the thorax at day 40 of admission showed resolution of ground glass changes and absence of septal thickening. However, there were increased diffuse centrilobular lung nodules with traction bronchiectasis at bilateral lower lobes, consistent with endobronchial TB (Fig. 2B). She was discharged well and completed a six-month course of ATT. Chest radiograph taken 3 months post-discharge showed resolving opacities (Fig. 1B).
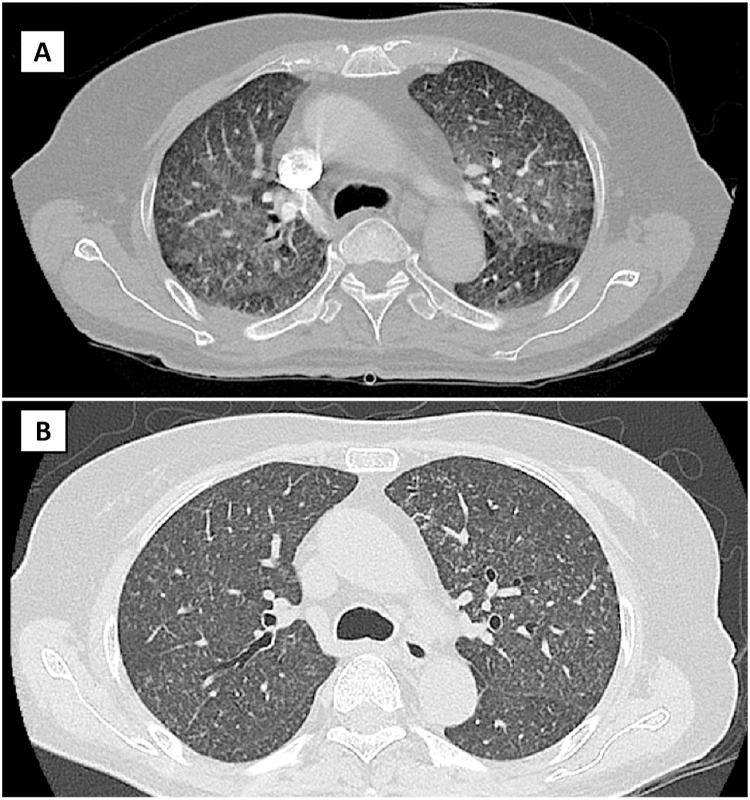
Fig. 2.
Representative slices (axial view) of CT thorax for patient 1 demonstrates ground-glass changes in both upper lobes with associated nodular changes bilaterally on day 7 of admission (Panel A), followed by resolution of the ground-glass changes, leaving residual nodular changes in both lung fields on day 40 of admission (Panel B).
1.1.2. Patient 2
A 63-year-old woman with underlying type 2 diabetes mellitus presented to a district clinic with loss of weight and loss of appetite for the last two months, two weeks of lethargy and poor oral intake, one week of fever and productive cough with whitish sputum, and rapidly worsening shortness of breath for the past two days. She was normotensive but tachycardic (pulse rate 115 beats per minute) and tachypnoeic (respiratory rate 28 breaths per minute) and had an oxygen saturation of 96% under room air. She was hyperglycaemic (13 mmol/L) and pyretic (temperature of 38.5 °C). Auscultation of lungs revealed reduced air entry over the right lower zone, and chest radiograph revealed bilateral lower zone airspace opacities with blunting of the right costophrenic angle (Fig. 3A). She was then referred to our centre for further care. On arrival to the emergency department, arterial blood gas revealed type 1 respiratory failure despite on 40% supplementation via Venturi mask (pH 7.5 PO2 69 mmHg PCO2 29 mmHg HCO3 22 mmol/L) and she required bi-level positive airway pressure ventilation.
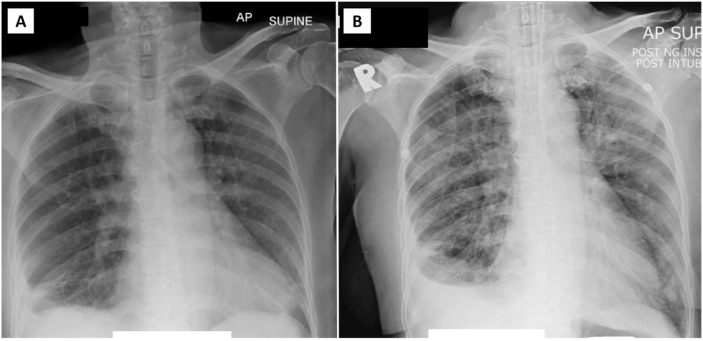
Fig. 3.
Chest radiograph of patient 2 on admission demonstrates right pleural effusion with minimal air space opacities at bilateral lower zones (Panel A) which progresses to involve the mid zone bilaterally on day 12 of admission (Panel B). There is no significant enlarged hilar node bilaterally.
NPOP sample sent for COVID-19 polymerase chain reaction testing was positive. Dexamethasone, lopinavir/ritonavir and favipiravir were administered as per local COVID-19 guidelines at the time. In view of constitutional symptoms, three successive sputum samples were submitted for smear examination, but were negative for acid fast bacilli. The sputum samples were then cultured in Lowenstein-Jensen media. Her pyrexia subsided and she was weaned off to room air by day 10 of admission. However, on day 12 of admission, she complained of chills and breathlessness, and her saturation dropped to 80% under room air. Repeated chest radiograph showed progression of airspace opacities involving mid zones bilaterally (Fig. 3B). Intravenous meropenem was commenced. She succumbed on day 17 of admission. Posthumously, mycobacterium TB complex was isolated in her sputum sample after seven weeks. First-line drug sensitivity testing confirmed susceptibility to isoniazid, rifampicin, ethambutol, and streptomycin.
1.1.3. Patient 3
A 78-year-old man with underlying hypertension presented to the emergency department with dry cough, loss of weight and intermittent fever for two months with recent progressive shortness of breath for the past two weeks. He was normotensive and apyrexial, and not tachycardic. He had a normal oxygen saturation of 98% under room air but was mildly tachypnoeic (respiratory rate of 21 breaths per minute). Clinically, he appeared cachexic. Chest radiograph showed bilateral nodular and airspace opacities with left mid zone consolidation (Fig. 4A). NPOP sample sent for COVID-19 polymerase chain reaction testing was positive, and he was admitted for isolation and treated with oral favipiravir. ATT was immediately commenced in view of suggestive chest radiograph and constitutional symptoms. Subsequently, sputum smear examination was positive for acid fast bacilli. Chest radiograph on day 14 of admission showed partial resolution of the left mid zone consolidation with residual nodular opacities (Fig. 4B). He was discharged well to continue his TB treatment as an outpatient. He did not require oxygen supplementation throughout his admission.
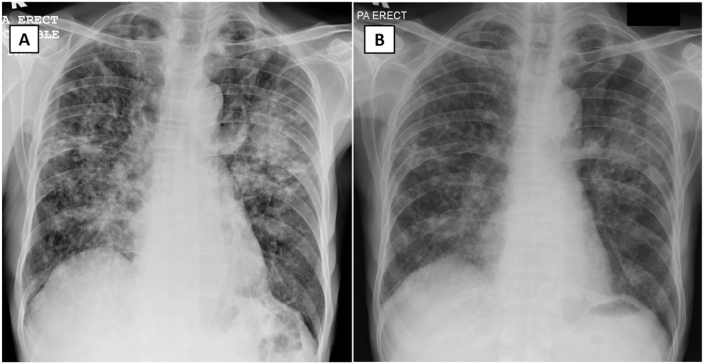
Fig. 4.
Chest radiograph of patient 3 on admission demonstrates predominantly nodular with some air space opacities bilaterally (Panel A). Some nodules coalesce to form a larger patch of consolidation at the left midzone. No cavity, air-fluid level, effusion or enlarged hilar node is seen. Repeated chest radiograph at day 12 of admission shows partial resolution of these changes (Panel B).
1.2. Discussion
Individuals with active PTB are at higher risk of contracting COVID-19 due to alterations in lung immunity driven by attenuated interferon-gamma host response to SARS-CoV-2 virus [11]. Meanwhile, reactivation of latent TB in the setting of COVID-19 infection is plausible, given that the two diseases augment each other with a transient decrease in cellular immunity [12]. Meta-analysis showed that the summary proportion of active PTB among COVID-19 patients is higher than WHO estimates for annual incidence of tuberculosis in high tuberculosis burden countries such as China, India, Nigeria, Philippines, and South Africa [10]. This is not surprising, given that both COVID-19 and PTB spread through airborne transmission of close contacts [10]. Furthermore, stay-at-home recommendations and national lockdown policies may have exacerbated crowded living conditions among underprivileged communities [13].
A large meta-analysis of 43 studies by Aggarwal et al. showed that patients with active PTB and COVID-19 carried a two-fold higher relative risk of death, which was comparable to relative risk of mortality in COVID-19 patients with diabetes, hypertension, and cardiovascular diseases [10]. In resource-limited settings, poverty and malnutrition might play an important role in increasing morbidity and mortality [9]. The proportion of PTB in our cohort of COVID-19 patients is 0.6%, or 3 out of 498 cases, presenting over a span of five months. This proportion is not generalizable as this was a single-centre study conducted in a limited time frame and did not include the entirety of COVID-19 patients admitted to non-tertiary quarantine centres or other tertiary hospitals in the state.
This case series represents varying scenarios in which TB might present in the setting of COVID-19. One of the uncommon forms of pulmonary tuberculosis is endobronchial TB, as described in the case of patient 1. While PTB commonly presents with cough (88%), loss of weight (58.1%), loss of appetite (57.3%), fever (56.4%), night sweats (30%), and haemoptysis (30%), endobronchial TB tends to present with dry cough, chest discomfort, and less commonly, wheezing or stridor in the presence of bronchial stenosis [14,15]. Further obscuring the diagnosis of TB are the shared x-ray abnormalities of endobronchial TB and COVID-19. Endobronchial TB may present as patchy parenchymal infiltrates, consolidations, segmental and lobar collapse, bronchiectasis, and consolidation [16] while COVID-19 present as ground glass opacities, coarse linear opacities, and consolidations [17].
CT may prove essential in differentiating endobronchial TB and COVID-19. Endobronchial TB tends to present with centrilobular nodules, tree-in bud appearance, consolidations, thickening of the bronchial wall and interlobular septa [14], while COVID-19 tends to show ground glass opacities (GGO), intralobular septal thickening, consolidations, and parenchymal bands [18,19]. Interestingly, in one case series, nearly half the HRCT in coinfection cases had no TB characteristic changes [9]. In the absence of CT, lung ultrasonography may prove useful - one small study demonstrated that COVID-19 subpleural lesions differed significantly from similar ones observed in bacterial pneumonia, pulmonary abscess, tuberculosis, atelectasis, and cardiogenic pulmonary edema [20]. However, whether tuberculosis changes can be differentiated from COVID-19 on LUS has yet to be demonstrated.
Resolution of GGO tends to occur after day fourteen of diagnosis of COVID-19, whereas consolidation and intralobular septal thickening tend to persist [19]. By 2 months post-COVID diagnosis, a third of COVID-19 patients showed complete resolution, while nearly half had residual parenchymal bands [18]. Similarly in our patient, the repeated CT on day 40 showed resolution of her initial consolidation and septal thickening. There are no known studies on the short-term effect of TB treatment on CT abnormalities in endobronchial TB. One study demonstrated complete resolution of changes in 60% (33 out of 55) of patients after treatment course completion [21]. Nodules (inter and intra-lobular) are not a common CT feature of COVID-19 infection [18,19], hence should raise suspicion of an alternate diagnosis, such as TB. The differences between the two CTs in patient 1 may demonstrate not only the resolution of COVID disease, but also an unmasking of underlying endobronchial TB.
Patient 2 illustrates a case of severe COVID-19 complicated by hospital acquired pneumonia with posthumous diagnosis of smear-negative, culture-positive PTB. The absence of acid-fast bacilli on respiratory samples may have reflected poor sampling quality or low tuberculous load in the lungs. The use of high dose steroids has been associated with TB reactivation; our patient was given dexamethasone for the treatment of COVID-19 infection which may have contributed to a worsened outcome [22]. Given her constitutional symptoms and initial chest radiograph showing unilateral pleural effusion, follow-on rapid diagnostic methods like Xpert MTB/RIF may have contributed to a more favourable outcome in this case by ensuring early commencement of ATT. Xpert MTB/RIF has been quoted to have a detection rate of 95% [9]. Another method is interferon-gamma release assay (IGRA) (QIAGEN, https://www.qiagen.com), which has been reported to pick up TB in Xpert MTB/RIF negative cases [23]. While several countries have proposed bidirectional screening of COVID-19 and PTB, implementation is difficult in resource-limited settings [10]. In resource-limited settings, we propose sputum smear for acid-fast bacilli as preliminary screening in COVID-19 patients with high risk for PTB.
Finally, patient 3 illustrates a clinical scenario that allows for a high index of suspicion for TB, and thus, early commencement of ATT. A history of chronic loss of weight and fever is not a feature of acute COVID-19 infection and warrants further investigation for TB. The resolution of changes on chest radiograph after two weeks may reflect either resolution of COVID-19 infection or response to ATT, nevertheless, a repeat chest radiograph and sputum examination after treatment course is warranted.
2. Conclusion
In TB-endemic regions, COVID-19 and PTB coinfection should be suspected in the presence of constitutional symptoms, prior immunocompromised states, prolonged respiratory symptoms or fever, unresolved radiological abnormalities, or a prolonged dependence on oxygen supplementation. Clinicians are reminded that Hickam's Dictum remains relevant to this day.
Ethics approval and consent to participate
This case series was registered via National Medical Research Register Malaysia (NMRR ID: NMRR ID-21-02248-OBB). Written consent was obtained from the patients.
Consent for publication
Written consent was obtained from the patients for publication of this case series and accompanying images.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Funding
The authors declare that no funding was used for the publication of this manuscript.
Authors’ contributions
LEN and ZHW initiated the idea for case reporting. LEN, BSMS, AKWC, AJT and HHC prepared the final copy of the manuscript. LEN, ZHW, BSMS, AKWC, AJT and HHC were involved in the overall management of the patients. All authors have read and approved the final manuscript.
Declaration of interests
None.
Acknowledgements
The authors would like to thank the respiratory team of Sarawak General Hospital for their input on patient 1, the Faculty of Medicine and Health Sciences, University Malaysia Sabah, Malaysia for sponsoring this paper's publication fees, and the Director General of Health Malaysia for permission to publish this paper.
Contributor Information
Larry Ellee Nyanti, Email: larrynyanti@ums.edu.my.
Zhun Han Wong, Email: zhunhan@hotmail.com.
Benjamin Sachdev Manjit Singh, Email: benjaminsachdev@gmail.com.
Andrew Kean Wei Chang, Email: andrchang@gmail.com.
Ahmad Tirmizi Jobli, Email: jatirmizi@unimas.my.
Hock Hin Chua, Email: hhchua2009@gmail.com.
References
- 1.Dara M., Sotgiu G., Reichler M.R., et al. New diseases and old threats: lessons from TB for the COVID-19 response. Int. J. Tubercul. Lung Dis. 2020 May 1;24(5):544–545. doi: 10.5588/ijtld.20.0151. [DOI] [PubMed] [Google Scholar]
- 2.Musso M., Di Gennaro F., Gualano G., et al. Concurrent cavitary pulmonary TB and COVID-19 pneumonia with in vitro immune cell anergy. Infection. 2021 Jan 17:1–4. doi: 10.1007/s15010-021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mani N., Slevin N., Hudson A. What Three Wise Men have to say about diagnosis. BMJ. 2011 Dec 20;343 doi: 10.1136/bmj.d7769. PMID: 22187188. [DOI] [PubMed] [Google Scholar]
- 4.Malaysian Healthcare Performance Unit . Malaysian Health at a Glance:2018. Ministry of Health Malaysia: Putrajaya; 2018. https://www.moh.gov.my/moh/penerbitan/MYHAAG2018.pdf [Google Scholar]
- 5.Sy K.T.L., Haw N.J.L., Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Inf. Disp. 2020 Nov-Dec;52(12):902–907. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 6.Motta I., Centis R., D'Ambrosio L., et al. TB, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonol. 2020 doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He G., Wu J., Shi J., et al. COVID‐19 in TB patients: a report of three cases. J. Med. Virol. 2020;92:1802–1806. doi: 10.1002/jmv.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stochino C., Villa S., Zucchi P., et al. Clinical characteristics of COVID-19 and active TB co-infection in an Italian reference hospital. Eur. Respir. J. 2020 Jul 30;56(1) doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadolini M., Codecasa L.R., García-García J.M., et al. Active TB, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur. Respir. J. 2020 doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal A.N., Agarwal R., Dhooria S., Prasad K.T., Sehgal I.S., Muthu V. Active pulmonary tuberculosis and coronavirus disease 2019: a systematic review and meta-analysis. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0259006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrone L., Petruccioli E., Vanini V., et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.02.090. In press. Epub 2021/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh A., Gupta A., Das K. Severe acute respiratory syndrome Coronavirus-2 and pulmonary TB coinfection: double trouble. Indian J. Med. Specialities. 2020 doi: 10.4103/INJMS.INJMS_72_20. [DOI] [Google Scholar]
- 13.Zumla A., Marais B.J., McHugh T.D., et al. COVID-19 and TB-threats and opportunities. Int. J. Tubercul. Lung Dis. 2020 Aug 1;24(8):757–760. doi: 10.5588/ijtld.20.0387. [DOI] [PubMed] [Google Scholar]
- 14.Ariffin F., Ahmad Zubaidi A.Z., Md Yasin M., et al. Management of pulmonary TB in health clinics in the Gombak district: how are we doing so far? Malays. Fam. Physician. 2015 Apr 30;10(1):26–33. PMID: 26425292; PMCID: PMC4567890. [PMC free article] [PubMed] [Google Scholar]
- 15.Kashyap S., Solanki A. Challenges in endobronchial TB: from diagnosis to management. Pulm Med. 2014 doi: 10.1155/2014/594806. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahzad T., Irfan M. Endobronchial TB-a review. J. Thorac. Dis. 2016 Dec;8(12):3797–3802. doi: 10.21037/jtd.2016.12.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobi A., Chung M., Bernheim A., et al. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. pmid:32302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye T., Fan Y., Liu J., et al. Research Square; 12 May 2020. Follow-up Chest CT Findings from Discharged Patients with Severe COVID-19: an 83-day Observational Study. PREPRINT (Version 1) available at: Research Square [10.21203/rs.3.rs-27359/v1. [Google Scholar]
- 19.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020 Jun;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Wang S., Liu Y., et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19) SSRN. 2020 doi: 10.2139/ssrn.3544750. [DOI] [Google Scholar]
- 21.Yanardag H., Tetikkurt C., Tetikkurt S., et al. Computed tomography and bronchoscopy in endobronchial TB. Cancer Res. J. 2003 Nov-Dec;10(8):445–448. doi: 10.1155/2003/496296. [DOI] [PubMed] [Google Scholar]
- 22.Patil S., Jadhav A. Short course of high-dose steroids for anaphylaxis caused flare up of tuberculosis: a case report. J Transl Intern. Med. 2019 Mar 29;7(1):39–42. doi: 10.2478/jtim-2019-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tham S., Lim W., Lee C., et al. Four patients with COVID-19 and TB, Singapore, April–May 2020. Emerg. Infect. Dis. 2020;26(11):2763–2765. doi: 10.3201/eid2611.202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.