Summary
Background
The present study aimed to evaluate the persistent immunogenicity offered by a third dose of BNT162b2 against Delta and Omicron variants, in nursing home (NH) residents.
Methods
In this monocenter prospective observational study, anti-spike IgG levels, S1 domain reactive T cell counts, serum neutralizing antibody titers against Delta and Omicron variants were compared before and up to three months after the BNT162b2 booster dose, in NH residents without COVID-19 (COVID-19 naive) or with COVID-19 prior to initial vaccination (COVID-19 recovered).
Findings
106 NH residents (median [interquartile range] age: 86·5 [81;91] years) were included. The booster dose induced a high increase of anti-spike antibody levels in all subjects (p < 0.0001) and a mild transient increase of specific T cells. Before the booster dose, Delta neutralization was detected in 19% (n = 8/43) and 88% (n = 37/42) of COVID-19 naive and COVID-19 recovered subjects, respectively. Three months after the booster dose, all NH residents developed and maintained a higher Delta neutralization (p < 0·0001). Before the booster dose, Omicron neutralization was detected in 5% (n = 2/43) and 55% (n = 23/42) of COVID-19 naive and COVID-19 recovered subjects, respectively, and three months after, in 84% and 95%, respectively. Neutralizing titers to Omicron were lower than to Delta in both groups with a 35-fold reduction compared to Delta.
Interpretation
The booster dose restores high neutralization titers against Delta in all NH residents, and at a lower level against Omicron in a large majority of participants. Future studies are warranted to assess if repeated BNT162b2 booster doses or new specific vaccines might be considered for protecting such fragile patients against Omicron and/or future SARS-CoV-2 variants.
Funding
French government through the Programme Investissement d'Avenir (I-SITE ULNE/ANR-16-IDEX-0004 ULNE) and the Label of COVID-19 National Research Priority (National Steering Committee on Therapeutic Trials and Other COVID-19 Research, CAPNET).
Keywords: BNT162b2 vaccine, Boost, SARS-CoV-2, Delta, Omicron, Older people, immunogenicity
Research in context.
Evidence before this study
The emergence of SARS-CoV-2 Omicron may expose immunocompromised subjects to a new variant with an increased transmissibility and potential immune evasion to current vaccines. Recent reports demonstrated that two doses of a mRNA-based vaccine elicit poor neutralization to Omicron compared to Delta, while a third dose broadens neutralizing antibody responses against Omicron in healthy adults.
Added value of this study
Our work shows that a third dose of BNT162b2 remains effective at least three-month post-vaccination to neutralize Delta in nursing home residents, but that Omicron is less sensitive to neutralization.
Implications of all evidence available
Considering the partial immune escape to BNT162b2, further studies are necessary to assess if new specific vaccines or repeated BNT162b2 booster doses should be considered in the medium term.
Alt-text: Unlabelled box
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019 in China and has since then caused the coronavirus disease 2019 (COVID-19) pandemic. Nursing home (NH) residents display both a higher risk of severe COVID-19 and an age-related immune alteration (also called immunosenescence).1 Previous reports demonstrated a low postvaccination antibody and cellular response against SARS-CoV-2 in older people compared to younger.2,3 We notably reported that the antibody response and the functional T-Cell response to SARS-CoV-2 were impaired three months after initial SARS-CoV-2 BNT162b2 mRNA vaccination series (two doses) in NH residents, including the neutralizing response against the B.1.1.7 (Alpha) variant which was dominant in the first months of 2021.3 This data suggested that a third dose should be considered to improve protective immunity in this at-risk population. Since the summer of 2021, numerous national health authorities have recommended such a booster vaccination in older people and other immunocompromised subjects, but the Delta variant was already dominant at this time, and the efficiency of such vaccinal schemes against the current dominant variants of concern (VoC) B.1.617.2 (Delta) and B.1.1.529 (Omicron) needs to be assessed.
Indeed, the SARS-CoV-2 spreading has been controlled thanks to vaccines and despite the emergence of successive virus variants. Recently, Omicron has been detected in South Africa, Botswana and in a traveler from South Africa in Hong Kong in November 2021.4 Compared to the previous VoC, Omicron displays numerous additional mutations modifying epitope sites within the receptor binding domain (RBD) of the spike protein.5,6 Consequently, several reports logically demonstrated that neutralizing activity against Omicron was absent or very low for monoclonal antibodies, and for sera from convalescent or from double vaccinated people.5,7, 8, 9 Conversely, a third dose of mRNA based vaccine elicited humoral immunity capable of cross-neutralizing new variants.5,7 However, specific studies are warranted to assess immunogenicity of a booster strategy in immunocompromised and/or subjects at risk for severe COVID-19. The present study aimed to evaluate the persistent immunogenicity offered by a third dose of BNT162b2 against Delta and Omicron variants, in boosted NH residents without or with COVID-19 prior to initial vaccination.
Methods
Study design and participants
This was a prospective single-center study conducted at the Lille University Hospital, in the North of France. NH residents were included in the study before receiving the first 2 doses of BNT162b2 mRNA vaccine if they fulfilled the following inclusion criteria: age >65 years, consent to be vaccinated with BNT162b2 mRNA vaccine and eligibility for the third dose (“booster” vaccine), in absence of contraindication and/or medical reason which could limit the follow-up and compliance to the study. Exclusion criteria were any recent, current or persistent infectious disease, any neoplasia diagnosis in the last five years, or treatment with steroids and/or immunosuppressants. . Participant characteristics collected at baseline (i.e before the first dose of BNT162b2 vaccine) included other associated diseases, and assessment of prior SARS-CoV-2 infection, determined by polymerase chain reaction (PCR) and/or high antibody titer to SARS-CoV-2 spike S domain: participants with a history of positive PCR in 2020 and/or who tested positive for anti-S antibodies before inclusion were considered as “COVID-19-recovered”, and the other participants as “COVID-19-naive”. As repeated PCR were performed in case of any viral symptom and/or in case of COVID-19 diagnosis in the same ward (cluster), no COVID-19 diagnosis was only based on any clinical symptoms. Geriatric Nutritional Risk Index was calculated according to the Lorentz formula: GNRI = (1·489 x albumin, g/l) + (41·7 x present/ideal body weight), with the ideal weight calculated according to the Lorentz formula.10 Frailty was assessed with the Clinical Frailty Scale as proposed by Rockwood et al.,11 and using the Fried frailty phenotype criteria.12
The vaccination protocol was strictly performed in accordance with the guidelines of the French Health authorities. Each dose of BNT162b2 contained 30 μg (COMIRNATY, Pfizer/BioNTech). The first NH residents received the initial vaccination (i.e. Dose 1 and Dose 2) in December 2020 and January 2021, with a time interval of 21 to 28 days between both doses. In case of recent diagnosis of COVID-19, the initial vaccination was delayed by at least three months after the infection (but no delay was observed between the first and the second dose). Data at baseline and three-month post-vaccination have been previously described.3 The booster dose was administered to NH residents between September and November 2021, as soon as recommended by the French Health Authorities and from at least six months after the initial vaccination. Data before and after the booster vaccine are reported in the present study.
Anti-SARS-CoV-2 antibodies
Anti-SARS-CoV-2 spike specific immunoglobulin G (IgG) were assessed in serum samples using the LIAISON® SARS-CoV-2 TrimericS IgG assay (Diasorin S.p.A, Saluggia, Italy). The cut-off value established by the manufacturer according to the neutralizing activity from a WHO International Standard was 33·8 binding antibody units (BAU)/ml. The maximum IgG level that could be determined with appropriate precision after dilution was 2080 BAU/ml.
SARS-CoV-2 neutralization assay
Neutralizing antibodies were investigated using a live virus neutralization assay. B.1.617.2 (Delta) and B.1.1.529 (Omicron) lineages SARS-CoV-2 strains were previously isolated from clinical specimens and propagated in Vero E6 cells. The whole-genome sequences of the viral isolates, obtained with the COVIDSeq library preparation kit (Illumina®), were submitted to GISAID (accession reference EPI_ISL_2143633 and EPI_ISL_7696645 for Delta and Omicron variants respectively). In brief, serial 2-fold dilutions (starting from 1:10) of the heated serum (56 °C for 30 min) were incubated for 1 h at 37 °C with a viral solution containing 100 TCID50 of SARS-CoV-2 and then added to Vero E6 cell monolayers in a 96-well plate. The cytopathic effect was recorded after three days, and the serum virus neutralization titer (NT50) was defined as the reciprocal value of the highest dilution that showed at least 50% protection of cells. A sample with a titer ≥ 20 was defined as positive. Negative results (NT50 < 20) were set to 1 for statistical analyses and graphics.
IFNy ELISpot assay
IFNy ELISpot assay was performed as previously described.3,13 In brief, overlapping peptide pools covering the N-terminal S1 domain were used (PepTivator SARS-CoV-2, Miltenyi Biotec, Bergisch Gladbach, Germany). Peptides consisted of 15-mer sequences with 11 amino acids overlap. Microtiter plates coated with anti-IFNy antibodies (T-SPOT.TB, Oxford Immunotec) were used. The cell suspension was normalized at a final concentration of 2·5 × 106 cells/ml, and plating with SARS-CoV-2 antigens was manually performed (2·5 × 105 peripheral blood mononuclear cells added per well). Peptide pools were added at a concentration of 0·5 μg/ml. Following an incubation at 37 °C for 16–20 h in a humidified atmosphere containing 5% CO2, wells were washed and incubated with conjugate reagent for 1 h at 2–8 °C. After a washing step, wells were developed for 7 min with substrate solution. The reaction was stopped by adding distilled water. Plates were allowed to dry in an oven at 37 °C for 1 h. Spot-forming cells (SFCs) were detected using the CTL ImmunoSpot plate reader. Appropriate negative and positive controls were used.13
Statistical analyses
Categorical variables are expressed as numbers (percentages) and quantitative variables are expressed as median (interquartile range) regarding the non-Gaussian distribution of immune parameters. Normality distribution was assessed graphically and using the Shapiro-Wilk test. Immune parameters assessed before booster vaccine dose (pre-boost values) were compared to immune parameters assessed at one- and three-month after booster vaccine dose (post-boost values) in each study group (COVID-19 naive and COVID-19-recovered) separately, using the Wilcoxon’ signed rank tests. Comparisons of immune parameters between COVID-19 naive and COVID-19-recovered were done using Mann-Whitney U tests. Comparisons of neutralizing titers between Delta and Omicron assessed at three-month after booster vaccine dose in each study group was done using the Wilcoxon’ signed rank test, and the magnitude of the difference was expressed as fold change in geometric mean neutralization titers (GMNT), after exclusion of samples with neutralization titers below the limit of detection. Statistical tests were done at the two-tailed α level of 0.05. No correction for multiple testing was carried out considering the exploratory nature of the study. Data analyses and graphs were performed using the GraphPad Prism software version 9.1.2 (GraphPad Software, La Jolla, CA, USA).
Ethics
This study was performed in accordance with the Declaration of Helsinki principles for ethical research. The study was approved by the Ile-De-France V (ID‐CRB 2021-A00119-32) ethics committee. All participants (and/or their legal representative if required) received detailed information and signed a consent form before participating in the study. The study was registered in ClinicalTrials.gov, with the identifier NCT04760704.
Role of the funding source
The funder had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Results
Patient characteristics
Among the 114 NH residents included in the initial study, 106 were included in the present study. Among these 106 NH residents who received the booster dose, 47 did not have any history of COVID-19 before the initial vaccination (COVID-19 naive), and 59 had a COVID-19 diagnosis prior to any vaccination (COVID-19 recovered) (Supplementary Fig. 1). The median [interquartile range, IQR] age was 86·5 [81;91] years and the other main characteristics are summarized in Table 1. No COVID-19 diagnosis was made between the baseline (prior the first vaccination) and the sampling performed three months after the booster dose. The median [IQR] time from the first dose to the booster dose was 8 [6·9;8·3] months. No significant immediate side effect was reported. Nineteen residents died and/or were not sampled yet three months after the booster dose, at the time of analysis (Supplementary Fig. 1). All deaths occurred remotely from vaccination and were not related to any SARS-CoV-2 infection. Finally, 87 NH residents were sampled three months after the booster vaccine (median [IQR] from booster dose to three-month evaluation: 3·2 [3;3·2] months).
Table 1.
Characteristics of nursing home residents.
| Characteristics | COVID-19-naive (n = 47) | COVID-19 recovered (n = 59) |
|---|---|---|
| Age (years) (median [IQR]) | 86 [77;90] | 86 [81;90] |
| Female [n (%)] | 30 (64) | 41(69) |
| Comorbidities [n (%)] | ||
| Hypertension | 32 (68) | 41 (69) |
| Coronary heart disease | 34 (72) | 43 (73) |
| Diabetes | 13 (24) | 14 (25) |
| Chronic renal failure | 14 (30) | 16 (27) |
| COPD | 8 (17) | 18(30) |
| Dementia | 46 (98) | 56 (94) |
| Frailty (median [IQR]) | ||
| Fried frailty phenotype criteria | 4 [3;4] | 4 [3;4] |
| Clinical Frailty Scale | 7 [7;7] | 7 [7;8] |
| Nutritional status (median [IQR]) | ||
| Albuminemia (g/L) | 33 [31;36] | 35 [33;37] |
| Geriatric Nutritional Risk Index | 91 [85;95] | 92 [86;96] |
| Body mass index (kg/m²) | 25 [20;31·2] | 24 [21;27] |
| IQR = interquartile range |
Anti-S antibody levels in NH residents before and after the BNT162b2 booster dose
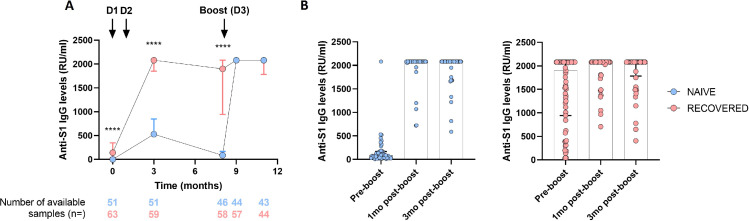
Before booster vaccination, 95 among 106 (89·6%) NH residents had detectable anti-S antibodies (i.e, greater than 33.8 BAU/ml), but COVID-19 naive residents had profoundly lower levels of anti-spike IgG than COVID-19 recovered participants (Figure 1, Table 2). After booster vaccine, both groups acquired high levels of antibodies (Figure 1, Table 2). Because a large part of the antibody levels was greater than the upper limit of measure, no comparison was allowed between pre- and post-boost.
Figure 1.
Anti-S1 antibody levels before, 1-month and 3-month after booster BNT162b mRNA vaccine dose in NH residents.
Individual values and median [interquartile range (IQR)] are shown. (A) Anti-S1 IgG levels in NH residents without (naive, blue) or with (recovered, red) prior COVID-19 before initial vaccination according to time (in months), from before initial vaccination (Dose 1, D1; Dose 2, D2; time interval between D1-D2: 21 to 28 days), to 3-month post booster vaccine dose. Mann-Whitney U test was performed to compare naive and recovered residents. ****p-values < 0·0001 (B) Comparison of antibody levels before (pre-boost) and at 1- and 3-month post-booster vaccine dose in COVID-19 naive NH residents (blue) and in COVID-19 recovered residents (red). No statistical comparison was performed because a large part of antibody levels was greater than the upper limit of detection.
Table 2.
Immunological parameters.
| Subjects and Immunological parameters | Unit | Before initial vaccination | 3 months after initial vaccination | Before booster dose | 1 month after booster dose | 1 month after versus before booster dose, p value | 3 months after booster dose | 3 months after versus before booster dose, p value |
|---|---|---|---|---|---|---|---|---|
| COVID-19 naive NH residents | ||||||||
| Anti-Spike antibodies | BAU/mL, median [IQR] | 0 [0;2·59] N = 51 |
529 [283;849] N = 51 |
87·4 [36·5;168·5] N = 46 |
2080 [2080;2080] N = 44 |
na* | 2080 [2080;2080] N = 43 |
na* |
| S1 specific reactive T cells | IFNg SFCs, median [IQR] | 1 [0;2] N = 51 |
13 [4·5;26·5] N = 49 |
10 [5;21] N = 46 |
19 [8·5;53] N = 45 |
0·0054 | 12·5 [5;27.5] N = 42 |
0·45 |
| Neutralizing antibodies against Delta | NT50, GMNT [95%CI] | na | na | 2·3 [1·3;4]** N = 43 |
na | 1825 [1150;2896] N = 43 |
<0·0001 | |
| Neutralizing antibodies against Omicron | NT50, GMNT [95%CI] | na | na | 1·2 [0·9;1·5]** N = 43 |
na | 37·3 [20·7;67·3] N = 43 |
<0·0001 | |
| COVID-19 recovered NH residents | ||||||||
| Anti-Spike antibodies | BAU/mL, median [IQR] | 141 [38·6;348] N = 64 | 2080 [1850;2080] N = 59 |
1900 [944;2080] N = 58 |
2080 [2080;2080] N = 57 |
na* | 2080 [1783;2080] N = 44 |
na* |
| S1 specific reactive T cells | IFNg SFCs, median [IQR] | 16·5 [8;33·7] N = 60 |
65 [23;153·3] N = 62 |
41 [17·5;102·5] N = 58 | 74 [17·5;102·5] N = 58 | 0·0019 | 52·5 [29·5;164·8] N = 44 |
<0·0001 |
| Neutralizing antibodies against Delta | NT50, GMNT [95%CI] | na | na | 235·4 [105·9;523·3] N = 42 |
na | 4200 [2593;6805] N = 42 |
<0·0001 | |
| Neutralizing antibodies against Omicron | NT50, GMNT [95%CI] | na | na | 8·3 [4·4;15·6]** N = 42 |
na | 101·4 [59·9;171·7] N = 42 |
<0·0001 | |
Data are median [IQR] or GMNT [95% CI] as indicated and N are number of samples available for each analysis. BAU/mL = binding antibody units (BAU)/mL. IQR=interquartile range. IFNg SFC = interferon gamma spot-forming cells/250 000 peripheral blood mononuclear cells. NT50 = neutralization titer. GMNT=geometric mean neutralization titer. 95%CI = 95% confidence interval. na: not available. N = are number of available samples for each analysis. * * no comparison was performed because a large part of antibody levels was greater than the upper limit of measure. ** Negative results (NT50 < 20) were set to 1 for statistical analyses.
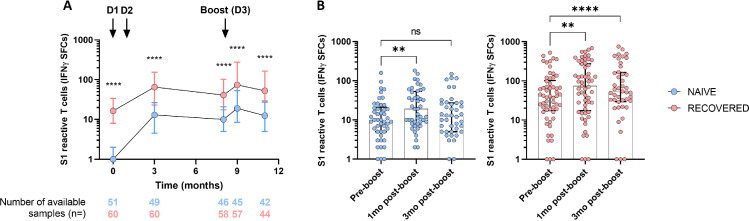
Specific memory T cells in NH residents before and after the BNT162b2 booster dose
A relative stability of T cell immune response was observed up to eight months after the initial vaccination in the whole population, with a persistent greater count in the COVID-19 recovered participants compared to COVID-19 naive participants (Figure 2, Table 2). The number of S1 reactive T cells increased transiently in both groups one month after the booster dose but went down at a similar level to pre-booster count in COVID-19 naive residents (Figure 2, Table 2).
Figure 2.
S1 specific reactive T cells before, 1-month and 3-month after booster BNT162b mRNA vaccine dose in NH residents.
Individual values and median [interquartile range (IQR)] are shown. (A) S1 specific reactive T cells in NH residents without (naive, blue) or with (recovered, red) prior COVID-19 before initial vaccination (Dose 1, D1; Dose 2, D2; time interval between D1-D2: 21 to 28 days), according to time (in months), from before initial vaccination to 3-month post booster vaccine dose. Mann-Whitney U test was performed to compare naive and recovered residents. (B) Comparison of S1 reactive T cells before (pre-boost) and 1- and 3-month post-booster vaccine dose in COVID-19 naive NH residents (blue) and in COVID-19 recovered residents (red). Wilcoxon signed rank test was used for within-subjects comparisons. ****p-value < 0·0001, ** p-value < 0·01, ns, not significant
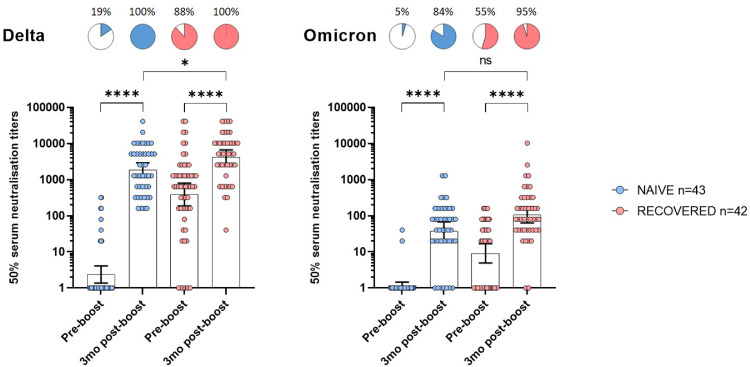
Neutralizing antibody response against Delta and Omicron three months after the booster dose
Before booster, neutralizing antibodies against Delta were found in 19% (n = 8/43) and 88% (n = 37/42) COVID-19 naive and recovered NH residents, respectively (Figure 3, panel A). Three months after the booster dose, all participants had detectable neutralizing antibodies against Delta with an increase in mean titers in both groups (p < 0·0001). COVID-19 recovered residents had significantly higher titers than COVID-19 naive participants (p = 0·01) (Figure 3, panel A -Table 2).
Figure 3.
Neutralization of the Delta and Omicron variants before and 3 months after booster BNT162b mRNA vaccine dose in NH residents.
Individual values, geometric mean neutralization titers (GMNT) and 95% confidence intervals are shown. Percentages and pie charts show the proportion of participants within each group that had detectable neutralization against the indicated variant. Serum neutralization titers of live B.1.617.2 (Delta, left) and B.1.1.529 (Omicron, right) SARS-CoV-2 strains determined for NH residents without (naive, blue) or with (recovered, red) COVID-19 before initial vaccination. Wilcoxon signed rank test was used for within-subjects comparisons. Mann-Whitney U test was performed to compare naive and recovered residents at 3 months. *p-values < 0·05; ****p-values < 0·0001; ns, not significant.
In contrast neutralizing antibodies against Omicron were found in only 5% (n = 2/43) and 55% (n = 23/42) before the booster dose in COVID-19 naive and recovered participants, respectively (Figure 3, panel B). Three months after the booster dose, Omicron-specific mean titers increased in both groups (p < 0·0001), and 84% (n = 36/43) and 95% (n = 40/42) of NH residents had detectable neutralizing antibodies in COVID-19 naive and recovered participants, respectively. Neutralization titers against Omicron were only slightly greater in COVID-19 recovered NH residents compared to naive ones (p = 0·09) (Figure 3, panel B -Table 2).
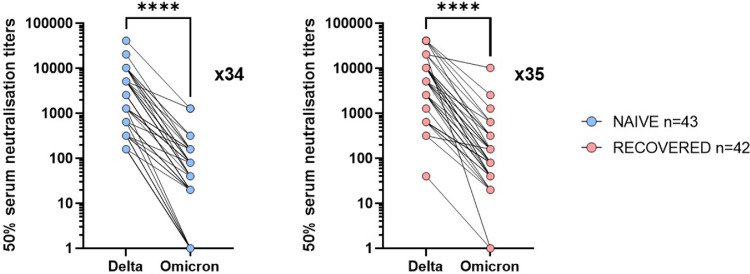
Finally, neutralizing antibody titers against Delta and Omicron were compared in the two groups of NH residents. Geometric mean neutralizing titers (GMNT) against Omicron were 34- and 35-fold reduced compared to Delta (p < 0·0001) in COVID-19 naive and COVID-19 recovered residents, respectively (p < 0·0001) (Figure 4).
Figure 4.
Fold-decrease in neutralization titers of Omicron relative to Delta 3 months after booster BNT162b mRNA vaccine dose in NH residents.
Individual values and fold-decrease in geometric mean neutralization titers (GMNT) of Omicron relative to Delta in NH residents without (naive, blue) or with (recovered, red) COVID-19 before initial vaccination is shown as a number with ‘‘x’’ symbol. Patients without detectable antibodies were not included in the GMNT reduction calculation. Wilcoxon signed rank test was used for within-subjects comparisons in titers found with Delta and Omicron variants 3 months after the boost vaccine dose. ****p-values < 0·0001.
Discussion
Our report is the first to show that NH residents, a population in which COVID-19 outbreaks were responsible for high fatality rates,1 develop and maintain high neutralizing antibodies against Delta, up to three months after a booster dose of BNT162b2 mRNA vaccine. The booster vaccine elicits neutralization activity against Omicron though at lower titers, even in NH residents with COVID-19 before the initial vaccination.
As reported by others at six months, our study shows that specific antibodies declined eight months after the initial vaccination in NH residents without prior COVID-19.14 Conversely, a large majority of COVID-19 recovered participants kept significant levels of anti-spike antibodies. These data suggest that infection-induced immunity elicits a stronger and persistent humoral immunity in association with initial vaccination. Before the booster dose, we observed that there was no correlation between the anti-spike antibody levels assessed with serological test (detected in 89·6% of NH residents) and the neutralizing antibody levels against Omicron (detected in n = 25/85, 29·4%), as well as against Delta (n = 45/85, 52·9%): this can be easily explained because serological assays used in routine practice are based on the native antigens, and this also raises the question of their relevance in a context of pandemic with new variants. Indeed, a 264 BAU/mL cut-off was shown to be correlated with protection against Alpha variant infection.15 Such cut-off still need to be determined for Delta or Omicron variants.
To assess the interest of a booster dose, some studies made a short-term evaluation of the antibody response, at two weeks after the booster dose and observed that anti-spike and anti-receptor-binding-domain antibodies increased in almost all subjects.14 As the persistence of vaccine-induced protection is a major concern in this high-risk people, we conducted our immunogenicity study up to three months after a BNT162b2 booster dose. Our data show that anti-spike antibody levels remain high in COVID-19 recovered as well as in COVID-19 naive older people.
As the T cell response is critical to support antibody response and immune memory, but as new specific T cells are usually difficult to be induced in older people, we also studied it before and after the vaccine booster. We observed that the advantage conferred by a prior infection persisted with time. By comparing COVID-19 recovered and naive participants, we observed that the specific T cell response mildly decreased with time in both groups after initial vaccination with BNT162b2 and that the booster dose induced only a mild and transient increase in specific T cells. Indeed, the COVID-19 naive NH residents’ T cell responses were not statistically higher three months after the booster dose compared to pre-booster levels. However, circulating T cell counts offer only a partial view of the whole T cell memory response, and that may explain why our data demonstrate that humoral response (against the same spike antigens) can be effectively boosted.
The worldwide expansion of SARS-CoV-2 Delta which overtook the Alpha variant in July 2021 and the current rapid expansion of Omicron questioned the risk of immune escape to mRNA SARS-CoV-2 vaccines, the latter being formulated using mRNA encoding the original wild-type SARS-CoV-2 spike protein. As neutralizing antibodies titers are considered as predictors of protection against variants (i.e severe COVID-19 infections, hospitalizations, and deaths), assessment of vaccinal strategies effectiveness must be conducted using neutralization assays.16 First, we observed that only few COVID-19 naive participants had detectable neutralizing antibodies before the booster dose contrasting with a large majority of COVID-19 recovered. As all NH residents received two doses and that no subject was diagnosed for COVID-19 between initial vaccination and the booster dose, the higher anti-delta neutralizing antibody levels in COVID-19 recovered residents can only be explained by the prior infection-induced immunity. This is in line with several studies performed in younger adults who received their primary vaccination series six to 12 months before assessment and who had a history of SARS-CoV-2 infection.5
The recent emergence of Omicron has led us to rapidly study the degree of neutralization provided by the booster in this context.16 Unlike Delta, we observed low rates of neutralization against Omicron in few COVID-19 naive and in half COVID-19 recovered participants before the booster dose, suggesting that the vaccine-induced immunity and the infection-induced immunity by variants preceding Omicron had a lower impact than observed on Delta. Consistently the booster vaccine induced only a mild increase that did not differ between COVID-19 naive residents and convalescent participants, in accordance with observations in younger adults.5,7,8,17, 18, 19, 20, 21, 22, 23 The higher number of mutations within the spike domain explains this immune escape of Omicron,5 and the similar 35-fold reduction of neutralization between Delta and Omicron titers whatever the history of prior-COVID-19.
Fortunately, Omicron, which can occur in boosted-vaccinated people, seems to be rarely responsible for severe COVID-19 even in this high risk population.24,25 Interestingly, within the days following the three-month post-boost assessments, eight subjects from the present cohort and nine additional residents in the same NH were diagnosed with asymptomatic Omicron carriage or mild COVID-19 symptoms and only one patient required low oxygen therapy (2L/min) for mild exacerbation of chronic breathing difficulties (data not shown).
Important strengths of our study are the presence of COVID-19 naive and recovered NH residents, the long-term follow up of the same cohort 12 months after the initial vaccination, and the evaluation of neutralization against two VoC three months after the booster dose, which may be relevant for older people boosted since the summer of 2021 in many countries. Our present study offers a real-time vision of the protection conferred by the booster dose during the current Omicron epidemic in subjects considered as highly fragile due to their advanced age and numerous underlying diseases.
Limitations are the small sample size of NH residents and the absence of a control group with younger subjects but findings available in healthy younger controls in other studies are consistent with our results.5 Nevertheless, we are performing a similar study in more than 100 health care workers, who were already included in the previous study,3 to compare the immunogenicity of the boost with NH residents. We are not able to compare several vaccines as in France, NH residents were only vaccinated with BNT162b2, but similar results were observed between mRNA vaccines in younger controls.5 Further studies are also warranted in older people who don't live in NH. Nevertheless, the cohort in the present study represents a vulnerable population of high interest in COVID-19 vaccine assessment. Finally, there were more missing data in COVID-19 recovered participants who died and/or who received their booster dose less than three months before the time of sampling, than in COVID-19 naive NH residents. However, we consider that this may have a limited impact on the analyzes, as results obtained within the group of subjects without prior COVID-19 are more important. Furthermore, all differences observed between pre-boost and three-month post-boost were statistically highly significant within both groups and the number of available samples three months after the booster dose was similar in both groups.
In conclusion, our report is the first to illustrate the three-month long effect of a booster BNT162b2 vaccine on neutralization of Delta and Omicron in older people: it highlights the positive effects on both Delta and Omicron neutralization, despite the relative immune escape of the latter. We can hypothesize that the combined effect of low but adequate immunization, and the lower risk of complications reported in vaccinated healthy subjects, may contribute to a lower risk of severe Omicron infection in boosted older people. However, while the SARS-CoV-2 Omicron rarely leads to severe disease in vaccinated population, a recent report demonstrated that infection can cause severe disease in unvaccinated individuals with advanced age.26 Considering the partial immune escape to BNT162b2, further studies are warranted to assess if new specific vaccines or repeated BNT162b2 booster doses could also be considered for protecting such fragile patients.
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Contributors
EKA, JD, BC-S, ML, and GL conceived and designed the study and participated in data collection, analysis, writing of the manuscript, and revision of the manuscript. EKA, JD, BC-S, ML, and GL take full responsibility for the integrity of the data and the accuracy of the data analysis. They are the guarantors. JL, AG, JT, DL, SM, FV, ArD, DH-G, JP, DaD, KF, DoD, LB, AlD, AS, DH, MH, FP, BA and YY participated in data analysis, and revision of the manuscript. All authors gave final approval for the version to be submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This work was supported by the French government through the Programme Investissement d'Avenir (I-SITE ULNE/ANR-16-IDEX-0004 ULNE) managed by the Agence Nationale de la Recherche.
This study received the Label of COVID-19 National Research Priority, awarded by the National Steering Committee on Therapeutic Trials and Other COVID-19 Research (CAPNET)
Acknowledgments
We thank Véronique Betrancourt, Virginie Dutriez, Anne Guigo, Coralie Lefebvre, Véronique Lekeux, Marie‐Thérèse Meleszka, Catherine Mortka and Anthony Rabat for their technical support and Bertrand Accart for his contribution (Centre de Ressources Biologiques). We also thank Séverine Duflos, Marie Broyez, Peggy Bouquet, Clémentine Rolland, Marion Lecorche, Abeer Shaikh Al Arab, Isabelle Tonnerre, Japhete Elenga Koanga, Laurent Schwarb, Emilie Rambaut, and all the nurses implicated in patients sampling and Mathieu Tronchon for data collection. We thank the ANRS – Maladies Infectieuses Emergentes.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100385.
Appendix. Supplementary materials
References
- 1.Thompson D.C., Barbu M.G., Beiu C., et al. The impact of COVID-19 pandemic on long-term care facilities worldwide: an overview on international issues. BioMed Res Int. 2020;2020:1–7. doi: 10.1155/2020/8870249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canaday D.H., Carias L., Oyebanji O.A., et al. Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–naive nursing home residents. Clin Infect Dis. 2021;73:2112–2115. doi: 10.1093/cid/ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demaret J., Corroyer-Simovic B., Alidjinou E.K., et al. Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front Immunol. 2022;12:778679. doi: 10.3389/fimmu.2021.778679. Accessed 20 January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu H., Krishnan P., Ng D.Y.M., et al. Probable transmission of SARS-CoV-2 Omicron variant in quarantine hotel, Hong Kong, China, November 2021. Emerg Infect Dis. 2022;28:460–462. doi: 10.3201/eid2802.212422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022 doi: 10.1016/j.cell.2021.12.033. S0092-8674(21)01496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J Med Virol. 2022;94:1728–1733. doi: 10.1002/jmv.27516. published online Dec 12. [DOI] [PubMed] [Google Scholar]
- 7.Planas D., Saunders N., Maes P., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. published online Dec 23. [DOI] [PubMed] [Google Scholar]
- 8.Carreño J.M., Alshammary H., Tcheou J., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. published online Dec 31. [DOI] [PubMed] [Google Scholar]
- 9.Cele S., Jackson L., Khoury D.S., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. published online Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouillanne O., Morineau G., Dupont C., et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Demaret J., Lefèvre G., Vuotto F., et al. Severe SARS-CoV-2 patients develop a higher specific T-cell response. Clin Transl Immunol. 2020;9:e1217. doi: 10.1002/cti2.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canaday DH, Oyebanji OA, White E, et al. Significantly elevated antibody levels and neutralization titers in nursing home residents after SARS-CoV-2 BNT162b2 mRNA booster vaccination. Infect Dis (except HIV/AIDS), 2021. 10.1101/2021.12.07.21267179. [DOI]
- 15.Feng S., Phillips D.J., White T., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cromer D., Steain M., Reynaldi A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai J., Zhang H., Zhang Y., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doria-Rose NA, Shen X, Schmidt SD, et al. Booster of mRNA-1273 strengthens SARS-CoV-2 Omicron neutralization. medRxiv 2021; 10.1101/2021.12.15.21267805. [DOI]
- 19.Edara VV, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. bioRxiv 2021; 10.1101/2021.12.20.473557. [DOI] [PMC free article] [PubMed]
- 20.Laurie M.T., Liu J., Sunshine S., et al. SARS-CoV-2 variant exposures elicit antibody responses with differential cross-neutralization of established and emerging strains including Delta and Omicron. J Infect Dis. 2022:jiab635. doi: 10.1093/infdis/jiab635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusvarghi S, Pollett SD, Neerukonda SN, et al. SARS-CoV-2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. bioRxiv 2021. 10.1101/2021.12.22.473880. [DOI]
- 22.Peiris M., Cheng S., Mok C.K.P., et al. Neutralizing antibody titres to SARS-CoV-2 Omicron variant and wild-type virus in those with past infection or vaccinated or boosted with mRNA BNT162b2 or inactivated CoronaVac vaccines. Res Sq. 2022 doi: 10.21203/rs.3.rs-1207071/v1. [DOI] [Google Scholar]
- 23.Yu X., Wei D., Xu W., et al. Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination. Cell Discov. 2022;8:4. doi: 10.1038/s41421-022-00375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espenhain L., Funk T., Overvad M., et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viana R., Moyo S., Amoako D.G., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn F., Bonander C., Moghaddassi M., et al. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities – surveillance results from southern Sweden, July 2021 to January 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.9.2200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.