Abstract
A PCR-based technique using new fluorescent probes, called molecular beacons, was developed to detect the antifolate resistance-associated S108N point mutation in Plasmodium falciparum. One hundred African clinical isolates were tested by the new method in comparison with the PCR-restriction fragment length polymorphism method. This new molecular technique appears to be a promising tool for epidemiological studies.
The spread of chloroquine-resistant Plasmodium falciparum strains in many areas of malaria endemicity has required the use of other drugs for malaria treatment and prophylaxis (3). Pyrimethamine and proguanil, which are specific competitive inhibitors of dihydrofolate reductase (DHFR) (9), are currently given by clinicians in combination with other antimalarial agents, such as sulfadoxine, chloroquine, and, recently, atovaquone (11). Because antifolate-resistant P. falciparum strains have been reported since the 1950s, when antifolates were introduced as single antimalarial treatments (14), monitoring antifolate susceptibility appears to be essential. During the past few years, the molecular basis of antifolate resistance in P. falciparum has been largely elucidated (2, 15). It has been shown that the DHFR S108N mutation was the main molecular event leading to the resistance, followed by mutations in other positions. Besides in vitro and in vivo studies, genomic approaches have been developed to survey antifolate resistance of P. falciparum isolates. PCR followed by restriction fragment length polymorphism (PCR-RFLP) (8) or mutation-specific PCR assays (13, 18) are usually used and give reliable results. However, PCR-RFLP is labor-intensive and time-consuming, and mutation-specific PCR assays need strictly controlled conditions to be reproducible. Moreover, the handling of the amplified DNA in both these methods creates the risk of contaminating untested samples. All these drawbacks may be bypassed by the use of a new PCR-based method in which novel fluorescent probes, called molecular beacons, are used to determine the nature of point mutations. Molecular beacons are hairpin-shaped probes that undergo a spontaneous fluorogenic conformational change when they hybridize to their target (10, 23). A fluorophore and a nonfluorescent quencher are covalently attached to each end of an allele-specific oligonucleotide. In the absence of a target, the fluorophore is held close to the quencher, and fluorescence cannot occur. When the probe encounters a target molecule, it forms a hybrid that is longer and more stable than the hybrid included in the hairpin-shaped structure, resulting in the separation of the fluorophore and quencher and the restoration of fluorescence. This study describes the development and evaluation of a PCR-based technique that can very rapidly and inexpensively detect the DHFR S108N mutation of P. falciparum.
One hundred clinical isolates of P. falciparum were obtained in 1998 and 1999 from malaria-infected travellers returning to France from Africa. Fresh blood samples were processed as previously described (6). Briefly, the red blood cells in 100 μl of venous blood samples from the patients were eliminated by a selective lysis buffer (0.32 M sucrose, 10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, and 1% Triton X-100). After centrifugation (11,300 × g, 5 min), the pellets were resuspended in 100 μl of extraction buffer (10 mM NaOH, 0.5% Tween 20, and 0.5% Nonidet P-40), heated at 100°C for 10 min, and then placed in ice. After centrifugation (11,300 × g, 5 min), the amplification was performed with 2 μl of the supernatant by a PCR assay which was developed in order to obtain an amplicon of 119 bp centered on codon 108. The primers used were synthesized based on the published full-length DHFR sequence of P. falciparum (strain FCR-3) (GenBank accession number J03028) and using Primer 3 software (Steve Rozen and Helen J. Skaletsky [1996, 1997]; Primer 3 code available at http://www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi). To genotype the alleles, two molecular beacons were designed, one specific for the wild-type allele S108 and 5′ labeled with a green fluorophore (fluorescein) and the other specific for the mutant allele S108N and 5′ labeled with a red fluorophore (tetramethylrhodamine). Both oligonucleotides were 3′ labeled with the quencher 4-(4′-dimethylaminophenylazo)benzoic acid (DABCYL). The PCR conditions were as follows. A mixture of 15 pmol each of sense primer 5′-TGTGGATAATGTAAATGATATGCC-3′, hybridizing to positions 303 to 326 of the DHFR gene, and antisense primer 5′-CATTTATCCTATTGCTTAAAGGTT-3′, hybridizing to positions 421 to 398, 200 μM concentrations of each deoxynucleoside triphosphate, buffer (15 mM Tris-HCl [pH 8.0], 50 mM KCl, 6 mM MgCl2), 2.5 U of ampliTaq Gold DNA polymerase, and 10 pmol each of fluorescein-5′-GCGACGAAGAACAAGCTGGGAAAGGTCGC-3′-DABCYL (wild-type beacon) and tetramethylrhod amine-5′-GCGACGAAGAACAAACTGGGAAAGGTCGC- 3′-DABCYL (mutant beacon) (Eurogentec, S.A., Seraing, Belgium), which hybridize to positions 380 to 362 of the respective allelic forms of the DHFR gene, was incubated in a 50-μl volume for 10 min at 95°C followed by 40 reaction cycles (94°C, 1 min; 55°C, 1 min; 72°C, 1 min). A further step was added to enable the final hybridization of the probes (95°C, 1 min; 55°C, 5 min; 20°C, 1 s). After completion of the PCR, sealed tubes were placed on a 96-well Costar microplate in a Fluostar 403 (BMG labotechnologies GmbH) spectrofluorometer. Fluorescence measurements were carried out at an excitation wavelength of 480 nm, an emission wavelength of 520 nm for fluorescein, and emission wavelengths of 544 and 590 nm for tetramethylrhodamine. For each well, a ratio was calculated according to the following formula (Biolise 2.01 software): ratio = (flu 1 − 0.85 blank 1)/(flu 2 − 0.85 blank 2), where flu 1 is the fluorescence data value measured at 520 nm, blank 1 is the fluorescence data value of a PCR-negative control (contains no DNA) measured at 520 nm, flu 2 is the fluorescence data value measured at 590 nm, and blank 2 is the fluorescence data value of a PCR-negative control (contains no DNA) measured at 590 nm.
The determination of the thresholds (or cutoffs) was previously done on known samples of a limited series (data not shown). S108N isolates had a ratio of <0.40 and S108 isolates had a ratio of >3. Between these two values were those for mixed isolates (S108-S108N). To further evaluate the mixed isolates zone, a study of artificial mixtures of the clones 3D7 (S108) and W2 (S108N) was performed. P. falciparum clones 3D7 and W2 were originally obtained from D. Walliker (University of Edinburgh, Scotland, United Kingdom). Stock cultures of 3D7 and W2 clones were grown as described previously (22). Cultures having a parasitemia of 1% were mixed in order to have the following ratios of W2 to 3D7: 1:1, 1:10, 1:50, 1:100, 1:1,000, 10:1, 50:1, 100:1, and 1,000:1. For all ratios, results were obtained from simultaneous assays based on triplicate determinations (Table 1).
TABLE 1.
DHFR point mutation at position 108 determined by molecular beacons and PCR-RFLP in artificial mixtures of P. falciparum clones
| Sample | Median fluorescence ratioa (range) | Amino acid encoded by codon 108 detected with:
|
|
|---|---|---|---|
| Fluorescence | PCR-RFLP | ||
| Clones | |||
| W2 alone | 0.15 (0.15–0.15) | Asn | Asn |
| 3D7 alone | 28.49 (25.43–33.03) | Ser | Ser |
| Artificial mixtures (ratios of W2 to 3D7) | |||
| 10:1 | 0.67 (0.64–0.69) | Ser/Asn | Ser/Asn |
| 50:1 | 0.28 (0.26–0.30) | Asn | Asn |
| 100:1 | 0.18 (0.17–0.19) | Asn | Asn |
| 1,000:1 | 0.15 (0.14–0.16) | Asn | Asn |
| 1:1 | 1.29 (1.25–1.32) | Ser/Asn | Ser/Asn |
| 1:10 | 2.29 (2.25–2.33) | Ser/Asn | Ser/Asn |
| 1:50 | 7.14 (6.53–7.74) | Ser | Ser |
| 1:100 | 36.31 (31.05–40.53) | Ser | Ser |
| 1:1,000 | 32.62 (30.36–35.38) | Ser | Ser |
Ratios express fluorescence measurements at 520 and 590 nm.
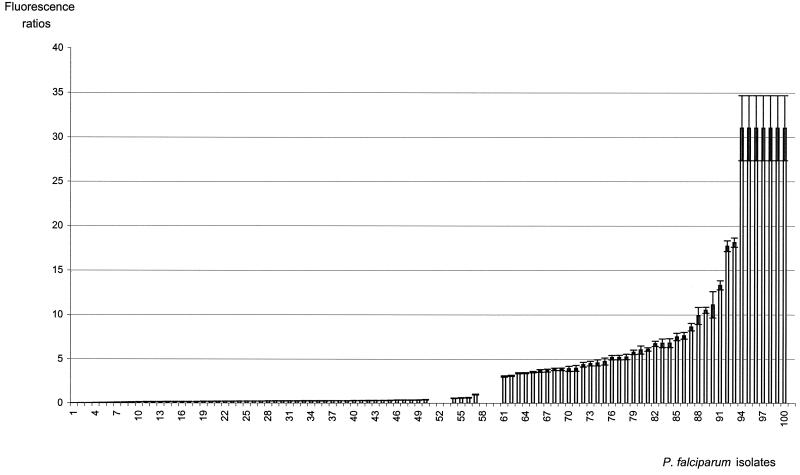
For comparison, the determination of the nature of DHFR codon 108 was determined blindly on the same isolates by an already-described PCR-RFLP method (6, 8). Fifty-nine isolates displayed the S108N mutation, either alone (n = 54) or in mixed isolates (n = 5); 41 isolates had the S108 codon alone (wild type) (Fig. 1).
FIG. 1.
Distribution of fluorescence ratios of S108, S108N, and mixed S108-S108N clinical P. falciparum isolates. Isolates 1 to 50 had the mutated codon (S108N), isolates 54 to 57 had mixed infections (S108-S108N), and isolates 61 to 100 had the wild-type codon (S108). Fluorescence ratios express fluorescence measurements at 520 and 590 nm. Data represent the means ± the standard deviations of three independent experiments. Isolates having fluorescence ratios above 32 are not shown in the figure.
The determination of the nature of codon 108 by the fluorescence method and PCR-RFLP is summarized in Fig. 1. The results showed that isolates could be clustered in three groups: those having a ratio of <0.40, those having a ratio of >3.02, and those having a ratio between these two values. No discordance was observed between the fluorescence method and PCR-RFLP about the determination of the nature of codon 108. The presented results, obtained blindly on an important series, confirm the validity of the chosen thresholds.
Molecular beacons have been successfully used for mutation detection in humans and in a very small number of microorganisms (10, 16, 17, 19, 21, 25). To our knowledge, this work is the first report of mutation analysis by molecular beacons in a parasite. The absolute specificity of the interaction of molecular beacons with their target strands has already been reported (10, 23, 24). In the present study, the resulting fluorescent signals led to an unambiguous detection of wild-type and/or mutant codon 108. Results were perfectly matched with those obtained on the same isolates by PCR-RFLP.
As expected, the few S108-S108N mixed isolates included in the studied series showed ratio values located between the S108 and the S108N cutoffs. Because fluorescence intensity depends on the concentration of a specific target molecule (7), some minority clones may not be detected, as is the case with PCR-RFLP. Data obtained with artificial mixtures showed that the molecular beacons method detected minor alleles whose presence in the mixtures was equal to or greater than about 10%. The same proportion was found with the use of allele-specific oligonucleotide polymerase reactions and with PCR-RFLP (12). A further study, focused on numerous mixed isolates, should be conducted in order to assess the detection of these isolates by the fluorescence method versus PCR-RFLP. It may be stressed that the proportion of S108-S108N mixed isolates among malaria patients infected in Africa before returning to France was previously found to be less than 10% of all the isolates investigated (4, 6).
Since molecular beacons are extremely specific probes, an unexpected mutation in the target sequence could lead to the inefficacy of the assay. In that case, the problem would be similar for the PCR-RFLP method. The risk is probably low because, at present, no point mutation near codon 108 has been reported, while the plasmodial DHFR gene has been intensively studied (1, 2, 20).
A major advantage of the molecular beacons method is that the risk of contaminating untested samples is eliminated because assays are carried out in sealed tubes. Results are obtained very rapidly after amplification as fluorescence measurement takes only a few seconds. Real-time detection of PCR amplicons may also be performed using molecular beacons (17, 21). For field studies, detection of PCR products is feasible by a mere visual inspection of sealed tubes placed over a UV light source (17). This simplified procedure could be particularly interesting in developing countries. The cost of the mutation detection is low because molecular beacons are inexpensive. Moreover, the need for restriction enzymes and reagents for the electrophoresis step is suppressed (including the mutagen agent ethidium bromide). These features may lead, after further evaluations, to a semiautomated mutation analysis method. The additional point mutations N51I and C59R, which were associated with high resistance to pyrimethamine and cycloguanil (2), should be tested in order to monitor antifolate resistance. Other point mutations that have also been reported to be linked with drug resistance in P. falciparum, e.g., point mutations in the cg2 (5) and crt genes, may be studied by similar approaches. Since standard in vitro and in vivo drug sensitivity tests are not easy to perform on a large scale, the development of rapid molecular techniques may be of great value for epidemiological studies.
Acknowledgments
This work was supported by the French Ministry of Health (Direction de la Veille Sanitaire), Zeneca Pharma, and the Agence Universitaire de la Francophonie. S.J. is supported by a WHO/TDR Research Training grant.
We thank Bernard Grandchamp for helpful discussions throughout the course of this work. We thank Elizabeth Gabbett for linguistic assistance.
REFERENCES
- 1.Basco L K, Eldin De Pécoulas P, Le Bras J, Wilson C M. Plasmodium falciparum: molecular characterization of multidrug-resistant Cambodian isolates. Exp Parasitol. 1996;82:97–103. doi: 10.1006/expr.1996.0013. [DOI] [PubMed] [Google Scholar]
- 2.Basco L K, Eldin De Pécoulas P, Wilson C M, Le Bras J, Mazabraud A. Point mutations in the dihydrofolate reductase-thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol Biochem Parasitol. 1995;69:135–138. doi: 10.1016/0166-6851(94)00207-4. [DOI] [PubMed] [Google Scholar]
- 3.Bloland P B, Lackritz E M, Kazembe P N, Were J B, Steketee R, Campbell C C. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy and treatment policy in Africa. J Infect Dis. 1993;167:932–937. doi: 10.1093/infdis/167.4.932. [DOI] [PubMed] [Google Scholar]
- 4.Durand R, Di Piazza J P, Longuet C, Sécardin Y, Clain J, Le Bras J. Increased incidence of cycloguanil resistance in malaria cases entering France from Africa, determined as point mutations in parasites' dihydrofolate-reductase genes. Ann Trop Med Parasitol. 1999;93:25–30. doi: 10.1080/00034989958762. [DOI] [PubMed] [Google Scholar]
- 5.Durand R, Gabbett E, Di Piazza J P, Delabre J F, Le Bras J. Analysis of κ and ω repeats of the cg2 gene and chloroquine susceptibility in isolates of Plasmodium falciparum from sub-Saharan Africa. Mol Biochem Parasitol. 1999;101:185–197. doi: 10.1016/s0166-6851(99)00073-0. [DOI] [PubMed] [Google Scholar]
- 6.Durand R, Ramiliarisoa O, Sécardin Y, Eldin de Pécoulas P, Basco L K, Le Bras J. DHFR gene point mutation as a predictor of Plasmodium falciparum resistance to cycloguanil in malaria cases from Africa imported to France. Trans R Soc Trop Med Hyg. 1997;85:33–34. doi: 10.1016/s0035-9203(97)90285-6. [DOI] [PubMed] [Google Scholar]
- 7.Ehricht R, Kirner T, Ellinger T, Foerster P, McCaskill J S. Monitoring the amplification of CATCH, a 3SR based cooperatively coupled isothermal amplification system, by fluorimetric methods. Nucleic Acids Res. 1997;25:4697–4699. doi: 10.1093/nar/25.22.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldin de Pécoulas P, Basco L K, Abdallah B, Djé M K, Le Bras J, Mazabraud A. Plasmodium falciparum: detection of antifolate resistance by mutation-specific restriction enzyme digestion. Exp Parasitol. 1995;80:483–487. doi: 10.1006/expr.1995.1060. [DOI] [PubMed] [Google Scholar]
- 9.Ferone R. Folate metabolism in malaria. Bull W H O. 1977;55:291–298. [PMC free article] [PubMed] [Google Scholar]
- 10.Giesendorf B A, Vet J A, Tyagi S, Mensink E J, Trijbels F J, Blom H J. Molecular beacons: a new approach for semiautomated mutation analysis. Clin Chem. 1998;44:482–486. [PubMed] [Google Scholar]
- 11.Looareesuwan S, Chulay J D, Canfield C J, Hutchinson D B. Malarone (atovaquone and proguanil hydrochloride): a review of its clinical development for treatment of malaria. Malarone clinical trials study group. Am J Trop Med Hyg. 1999;60:533–541. doi: 10.4269/ajtmh.1999.60.533. [DOI] [PubMed] [Google Scholar]
- 12.Mookherjee S, Howard V, Nzila-Mouanda A, Watkins W, Sibley C H. Identification and analysis of dihydrofolate reductase alleles from Plasmodium falciparum present at low frequency in polyclonal patient samples. Am J Trop Med Hyg. 1999;61:131–140. doi: 10.4269/ajtmh.1999.61.131. [DOI] [PubMed] [Google Scholar]
- 13.Parzy D, Doerig C, Pradines B, Rico A, Fusaï T, Doury J C. Proguanil resistance in Plasmodium falciparum African isolates: assessment by mutation-specific polymerase chain reaction and in vitro susceptibility testing. Am J Trop Med Hyg. 1997;57:646–650. doi: 10.4269/ajtmh.1997.57.646. [DOI] [PubMed] [Google Scholar]
- 14.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. Oxford, United Kingdom: Academic Press; 1987. [Google Scholar]
- 15.Peterson D S, Walliker D, Wellems T E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piatek A S, Telenti A, Murray M R, El-Hajj H, Jacobs W R, Jr, Kramer F R, Alland D. Genotypic analysis of Mycobacterium tuberculosis in two distinct populations using molecular beacons: implications for rapid susceptibility testing. Antimicrob Agents Chemother. 2000;44:103–110. doi: 10.1128/aac.44.1.103-110.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piatek A S, Tyagi S, Pol A C, Telenti A, Miller L P, Kramer F R, Alland D. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat Biotechnol. 1998;16:359–363. doi: 10.1038/nbt0498-359. [DOI] [PubMed] [Google Scholar]
- 18.Plowe C V, Djimde A, Bouare M, Doumbo O, Wellems T E. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 19.Poddar S K. Detection of adenovirus using PCR and molecular beacon. J Virol Methods. 1999;82:19–26. doi: 10.1016/s0166-0934(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 20.Reeder J C, Rieckmann K H, Genton B, Lorry K, Wines B, Cowman A F. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am J Trop Med Hyg. 1996;55:209–213. doi: 10.4269/ajtmh.1996.55.209. [DOI] [PubMed] [Google Scholar]
- 21.Rhee J T, Piatek A S, Small P M, Harris L M, Chaparro S V, Kramer F R, Alland D. Molecular epidemiologic evaluation of transmissibility and virulence of Mycobacterium tuberculosis. J Clin Microbiol. 1999;37:1764–1770. doi: 10.1128/jcm.37.6.1764-1770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi S, Bratu D P, Kramer F R. Multicolor molecular beacons for allele discrimination. Nat Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 24.Tyagi S, Kramer F R. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 25.Vet J A, Majithia A R, Marras S A, Tyagi S, Dube S, Poiesz B J, Kramer F R. Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc Natl Acad Sci USA. 1999;96:6394–6399. doi: 10.1073/pnas.96.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]