ABSTRACT
Klebsiella pneumoniae is a Gram-negative, opportunistic pathogen that commonly causes nosocomial pneumonia, urinary tract infection, and septicemia. Our recent work utilizing a murine model of respiratory tract infection with classical K. pneumoniae demonstrated leukocyte aggregates in the lungs of mice at 28 days postinfection. Here, we sought to characterize the composition and development of these structures. Histopathological analyses of murine lungs revealed immune cell clusters surrounding the pulmonary vasculature and airways by 14 days postinfection, resembling inducible bronchus-associated lymphoid tissue (iBALT). Further investigation of these structures demonstrated central B cell aggregates with concomitant dispersed T cells. At day 28 postinfection, these lymphoid clusters expressed germinal center markers and CXCL12, qualifying these structures as iBALT with nonclassical B cell follicles. Investigations in mutant mice revealed that those lacking B and/or T cells were not able to form fully defined iBALT structures, although some rudimentary B cell clusters were identified in mice lacking T cells. The longevity of K. pneumoniae-induced BALT was assessed for up to 120 days postinfection. Lymphoid aggregates significantly decreased in size and quantity by 90 days after K. pneumoniae infection; however, aggregates persisted in mice that were restimulated with K. pneumoniae every 30 days. Finally, infections of mice with an array of classical K. pneumoniae clinical isolates demonstrated that the development of these structures is a common feature of K. pneumoniae lung infection. Together, these data confirm that murine lungs infected with K. pneumoniae develop iBALT, which may play a role in pulmonary immunity to this troublesome pathogen.
KEYWORDS: Klebsiella pneumoniae, iBALT, induced bronchus-associated lymphoid tissue, pneumonia, T cells, B cells
INTRODUCTION
Klebsiella pneumoniae is one of the most prevalent hospital-acquired pathogens (1), is a leading cause of nosocomial pneumonia, and has been identified as a major cause of secondary bacterial pneumonia in patients with coronavirus disease 2019 (COVID-19) (2–5). Two distinct pathotypes of K. pneumoniae are currently circulating, termed classical K. pneumoniae and hypervirulent K. pneumoniae (6). Classical strains often cause nosocomial infections and are common in the United States and Europe (7). In contrast, hypervirulent K. pneumoniae strains are most commonly found in Southeast Asia and cause community-acquired infections in otherwise healthy hosts (6). The increasing antibiotic resistance among all K. pneumoniae isolates presents an immense challenge in treating these infections, leading the Centers for Disease Control and Prevention to classify antibiotic-resistant K. pneumoniae as an urgent public health threat (8).
Despite the clinical importance of this pathogen, only a few studies have attempted to define the pulmonary immune response to classical K. pneumoniae. While past investigations leveraged a heat-killed hypervirulent isolate (9, 10), our laboratory recently developed a murine model utilizing a live, classical isolate of K. pneumoniae, enabling the further study of adaptive immune responses following survival of K. pneumoniae pulmonary infection (11). In this model, distinct collections of leukocytes are apparent in the lungs of mice 28 days after surviving pulmonary infection (11). Subsequent reinfection of these survivors with K. pneumoniae led to significantly less morbidity and mortality than with challenge of naive mice previously mock infected with phosphate-buffered saline (PBS). Based on the presence of these leukocyte collections near large airways in K. pneumoniae-exposed mice, we hypothesized that these aggregates may represent inducible bronchus-associated lymphoid tissue (iBALT).
iBALT is a tertiary lymphoid structure that forms subsequent to infection or inflammation in the lung (12). The presence of iBALT is theorized to facilitate immune responses by providing a local niche for the development and maintenance of T and B cell memory populations (12–17). The defining features of these tertiary lymphoid organs classically include B cell follicles containing CXCL13-secreting follicular dendritic cells (FDCs), peripheral high endothelial venules (HEVs), and T cells (13, 15, 18). Alternatively, nonclassical follicles have been described that lack FDCs but instead rely on CXCL12-positive (CXCL12+) fibroblast-like stromal cells (19). Additionally, mature aggregates of both types commonly form germinal centers with proliferating cell nuclear antigen (PCNA)-positive B cells (13, 18). Interleukin-17 (IL-17) signaling has been demonstrated to be imperative for the formation of both classical and nonclassical follicles (18, 19).
Here, we sought to determine if the observed lymphoid structures induced by classical K. pneumoniae infection satisfied iBALT criteria and aimed to characterize their development, composition, and durability. Through histological staining, the use of lymphocyte-deficient murine models, and durability studies with and without repeat challenges, we determined that these aggregates fully formed as early as 14 days postinfection (dpi), were consistent with nonclassical iBALT follicles, and lasted for several months while decreasing in size and number without restimulation.
RESULTS
Lymphoid aggregates form by 14 days after K. pneumoniae infection.
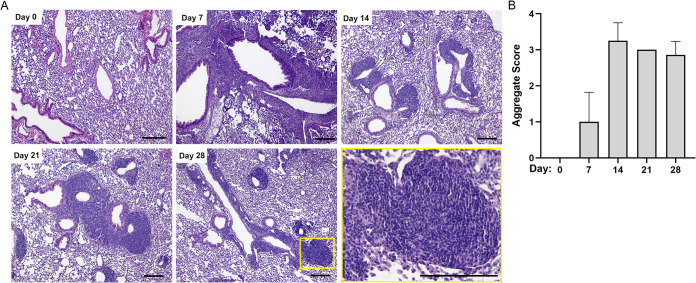
We previously observed leukocyte aggregates resembling iBALT via hematoxylin and eosin (H&E) staining of murine lungs 28 days after respiratory tract infection with a classical strain of K. pneumoniae (11). We first sought to determine the timing of the formation of these leukocyte collections. Using a preclinical murine model of classical K. pneumoniae lung infection, C57BL/6 mice were infected via oropharyngeal aspiration with 108 CFU of K. pneumoniae TOP52 (or the PBS control) and sacrificed at 7, 14, 21, and 28 dpi. Consistent with previous experiments using this model, approximately 80% of mice survived this infection and were available for histological analysis. Lungs demonstrated an acute inflammatory infiltrate at 7 dpi without apparent lymphocyte collections, but organized lymphoid aggregates were observed by 14 dpi (Fig. 1A). These aggregates were generally located adjacent to large airways and vessels, and they persisted with similar morphologies at 21 and 28 dpi. No similar lymphoid structures were observed in murine lungs prior to infection (day 0) or in PBS mock-infected murine lungs through 28 dpi (data not shown). To quantify these findings, lung sections from several different mice per time point were reviewed in a blind manner and assigned a semiquantitative lymphoid aggregate score based on the number of lymphoid aggregates and the total percent aggregate area of the lung section. On average, aggregate scores peaked at 14 dpi and remained high at 21 and 28 dpi (Fig. 1B).
FIG 1.
Lymphoid aggregate development in mice infected with K. pneumoniae. (A) H&E staining of left lung sections at 0, 7, 14, 21, and 28 dpi, including a higher magnification of the day 28 aggregate (inset and bottom right). (B) Average blindly assigned lymphocytic aggregate scores with standard deviations (score of 3 assigned for all mice at 21 dpi). Images are representative, and scores are averages from 4 to 7 mice evaluated per time point. Bars, 100 μm.
K. pneumoniae-induced lymphocytic collections contain cells characteristic of iBALT with nonclassical follicles.
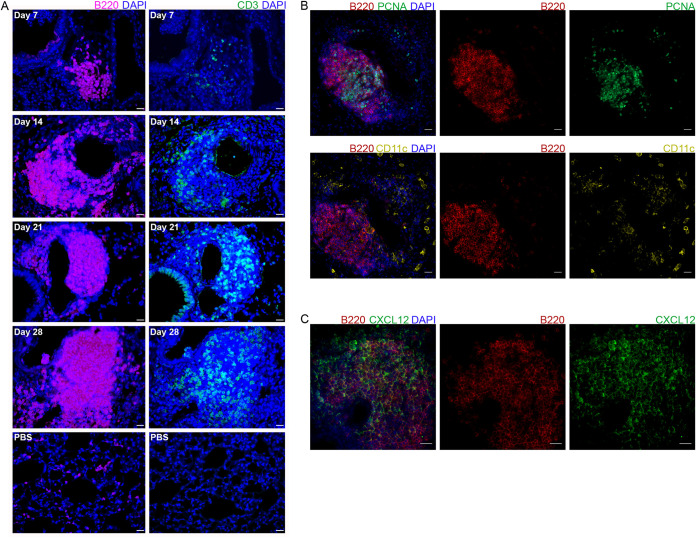
To further investigate the cellular composition and organization of these aggregates, we utilized immunohistochemistry (IHC) staining of lungs at selected intervals after K. pneumoniae infection. To determine if these aggregates are consistent with iBALT, sections from 7, 14, 21, and 28 dpi (or 28 days after PBS mock infection) were stained for B and T cells using antibodies to B220 and CD3, respectively (Fig. 2A). By as early as 7 dpi, small B cell aggregates were observed lining some airways and vessels, with very few associated T cells. By 14 dpi, B cell aggregates were larger, and T cells were seen dispersed throughout these structures. Similarly, at 21 and 28 dpi, large B cell aggregates were found throughout the lung, with T cell staining being observed throughout the aggregates. While PBS mock-infected lungs demonstrated positive staining for individual B and T cells, they did not exhibit similar aggregates through 28 dpi. These data indicate that lymphocytic aggregates begin to form by as early as 7 dpi, initially with dense B cell regions followed by T cell recruitment by 14 dpi. These aggregates persist through at least 28 dpi.
FIG 2.
Characterization of lymphoid aggregate development and composition in mice infected with K. pneumoniae. (A) B cell (B220) and T cell (CD3) IHC staining of murine lungs at 7, 14, 21, and 28 dpi (and the PBS control). (B) Germinal center B cell (PCNA) (top) and conventional dendritic cell (CD11c) (bottom) IF staining of lymphoid aggregates in mice at 28 dpi. (C) CXCL12 IF staining of lymphoid aggregates in mice at 28 dpi. Images are representative of results from 4 to 6 mice evaluated per time point. Bars, 20 μm.
To determine if these lymphocytic aggregates have characteristics historically required to define iBALT with classical or nonclassical follicles, we performed immunofluorescence (IF) staining on the lungs of infected mice at 28 dpi to identify proliferating germinal center B cells (PCNA+) and dendritic cells (CD11c+) (Fig. 2B). At 28 dpi, PCNA+ B cells were located centrally within lymphocytic aggregates, indicating germinal centers. Dendritic cells, however, were largely scattered throughout the lung, without definitive central straining within the follicle. Furthermore, staining for the FDC marker CD21 and the chemokine CXCL13 was negative within these aggregates, indicating that they do not represent classical B cell follicles (data not shown). Staining for the chemokine CXCL12 demonstrated diffuse positivity throughout the aggregates (Fig. 2C). Together, these data confirm that the lymphocytic aggregates observed in mice infected with classical K. pneumoniae represent the nonclassical follicles (19) described in select subtypes of iBALT.
B cells, but not T cells, can organize independently in the early steps of iBALT formation.
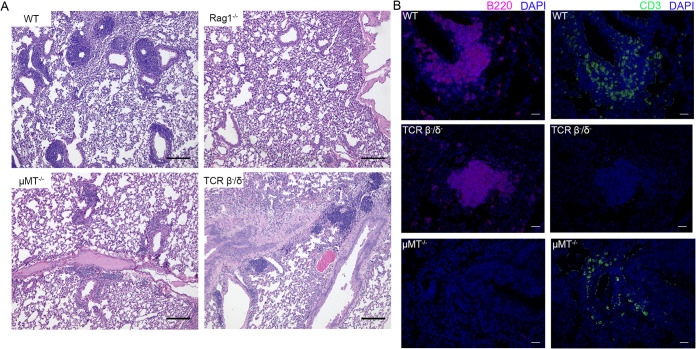
As distinct B and T cell regions were observed in K. pneumoniae-induced lymphocytic aggregates and are considered essential for early iBALT formation (17), we hypothesized that organized lymphoid aggregates would not be observed in mice lacking either or both of these cell types. To test this hypothesis, we infected RAG1−/− mice (lacking mature B and T cells) with K. pneumoniae via oropharyngeal aspiration. After 28 days, mice were sacrificed, and lung sections were analyzed via H&E staining and IHC (Fig. 3A). As predicted, no lymphoid aggregates were observed in RAG1−/− mice. We further investigated iBALT formation when individual lymphocyte subsets were absent. We infected T cell receptor β-negative/δ-negative (TCRβ−/δ−) and μMT−/− mice, lacking mature T and B cells, respectively, with classical K. pneumoniae. H&E staining demonstrated an absence of dense lymphocytic clusters in μMT−/− mice at 28 dpi, and IHC staining in these mice demonstrated scattered T cells without any well-defined structures. Meanwhile, TCRβ−/δ− mice demonstrated some clusters of lymphocytes, although these were scarcer and smaller than those seen in C57BL/6 mice. IHC staining of TCRβ−/δ− mouse lungs confirmed that these structures were B cell clusters without accompanying T cells (Fig. 3B). Combined, these data suggest that B cells, but not T cells, can organize independently in the early steps of iBALT formation after classical K. pneumoniae infection.
FIG 3.
Evaluation of lymphoid aggregate formation in mutant mice infected with K. pneumoniae. (A) H&E staining of murine left lung sections at 28 dpi in wild-type (WT), Rag1−/−, μMT−/−, and TCRβ−/δ− mice. (B) B cell (B220) and T cell (CD3) IHC staining of murine lungs at 28 dpi in WT, μMT−/− and TCRβ−/δ− mice. Images are representative of results from 3 to 6 mice evaluated per strain. Bars, 100 μm (A) and 20 μm (B).
iBALT structures decrease in size and number in the absence of repeat K. pneumoniae exposure.
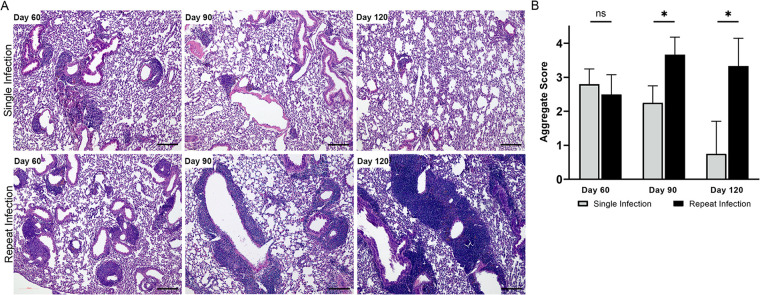
To understand the longevity of K. pneumoniae-induced BALT, we compared mice reinfected with K. pneumoniae every 30 days and mice infected with K. pneumoniae only on day 0, over a period of 120 days. A subset of mice was sacrificed every 30 days until the conclusion of the experiment. H&E staining of the lungs (Fig. 4A) showed decreases in aggregate sizes and numbers over time in mice infected only once with K. pneumoniae compared to the repeatedly challenged population. Sections were reviewed in a blind manner and assigned a semiquantitative lymphocyte aggregate score (Fig. 4B). Mice repeatedly stimulated with K. pneumoniae had significantly higher aggregate scores at 90 and 120 dpi than mice that underwent only a single infection. These data indicate that iBALT structures induced by K. pneumoniae infection are largely retained for at least 60 dpi but begin to recede by 90 dpi in the absence of restimulation.
FIG 4.
Lymphocytic aggregate durability with and without K. pneumoniae reinfection. (A) H&E staining of left lung sections 60, 90, and 120 days following either a single K. pneumoniae infection at day 0 (top) or repeat infections every 30 days (bottom). (B) Average blindly assigned lymphocytic aggregate scores with standard deviations. Images are representative, and scores are averages from 4 to 6 mice evaluated per time point. Bars, 100 μm. ns, not significant; *, P value of <0.05.
Formation of lymphoid aggregates is a common response to classical K. pneumoniae clinical strains.
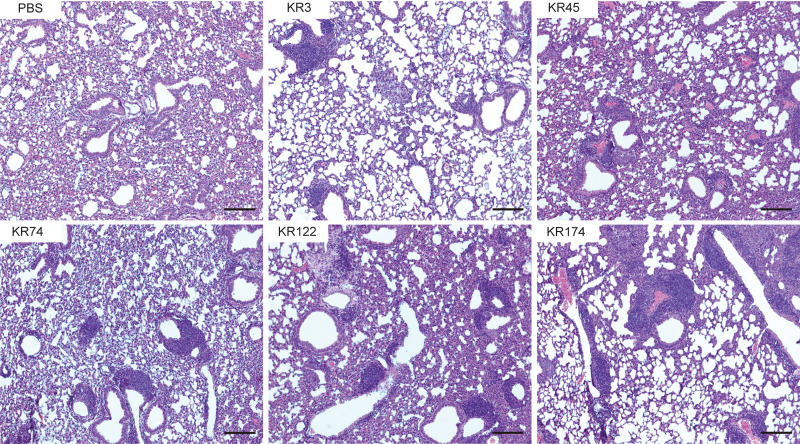
To assess the generalizability of iBALT formation following K. pneumoniae infection, we infected groups of mice using one of several distinct clinical isolates of K. pneumoniae, specifically classical lower respiratory isolates, including some with prominent antibiotic resistance determinants. Mice were infected with 5 × 107 to 9 × 107 CFU of live classical K. pneumoniae (KR3, KR45, KR74, KR122, or KR174) or PBS. At 28 dpi, H&E staining of lungs from mice infected with each classical strain demonstrated substantial lymphoid aggregates (Fig. 5), similar to those induced by TOP52 infection. These data indicate that iBALT formation is not a strain-specific effect but a common response to lung infection with classical strains of K. pneumoniae.
FIG 5.
iBALT formation in response to infection with distinct K. pneumoniae clinical isolates. Shown is H&E staining of left lung sections 28 days following mock infection (PBS) or infection with a classical K. pneumoniae lower respiratory isolate (KR3, KR45, KR74, KR122, or KR174). Images are representative of results from 2 to 5 mice evaluated. Bars, 100 μm.
DISCUSSION
K. pneumoniae currently causes a significant burden of human disease, and mounting antibiotic resistance is outpacing the development of new treatments. Protective adaptive immune responses elicited by K. pneumoniae, particularly those related to infection with classical strains, have yet to be adequately characterized. A further understanding of host immune responses to K. pneumoniae is essential for the development of host-directed treatments and vaccines. Here, we demonstrate that lung infection with classical K. pneumoniae induces the development of iBALT containing nonclassical B cell follicles. Furthermore, we show that several classical K. pneumoniae respiratory isolates induce BALT in this model.
Based on our observation of lymphoid aggregates in mice 28 days following K. pneumoniae infection (11), we sought to describe their early formation and determine if these aggregates indeed represented iBALT. H&E staining demonstrated that lymphoid aggregates develop by as early as 14 dpi. While the traditional iBALT architecture has been described as clusters of B cells surrounded by peripheral T cells (20), our IHC analyses demonstrated both B and T cells dispersed throughout the aggregates, consistent with iBALT formation observed in influenza mouse models (17, 21). Further IF analysis for PCNA demonstrated germinal center B cells; however, we did not observe central FDCs or CXCL13 staining, a hallmark of classical B cell follicles. Instead, we found high expression levels of CXCL12 throughout the aggregate, suggesting that these structures represent nonclassical follicles. There is precedent for a Gram-negative pathogen inducing nonclassical B cell follicle formation in murine lungs, as heat-killed Pseudomonas aeruginosa similarly induces aggregates that lack FDCs (15, 19). Iwanaga et al. recently reported a mucosal vaccine candidate targeting K. pneumoniae utilizing the protein OmpX (22). Interestingly, mice receiving this vaccine developed lymphoid aggregates consistent with iBALT that contained FDCs representative of classical B cell follicles. The differential induction of classical follicles by this mucosal vaccine or whole bacteria such as Chlamydophila pneumoniae (23) versus the induction of nonclassical follicles by K. pneumoniae or P. aeruginosa merits further investigation. Understanding the signals driving the formation of classical versus nonclassical follicles and potential differences in their functional significance could inform vaccination or therapeutic applications. The results reported here provide an additional novel and clinically relevant setting in which nonclassical follicle development can be utilized to further investigate these questions.
The development of B cell follicles is a universal feature of iBALT, while distinct T cell areas do not develop in all contexts (12). Our experiments revealed distinct B cell clusters, with T cells being interspersed throughout the aggregate as opposed to being segregated into distinct T cell zones. We also observed B cell aggregates in the absence of T cells (TCRβ−/δ− mice), suggesting that T cells are not necessary to support the initial aggregation of B cells. During the neonatal period, IL-17 produced by CD4+ T cells is required for iBALT formation (18). P. aeruginosa-induced BALT development in adult mice is dependent on IL-17, primarily produced by γδ T cells (19). As we have demonstrated that B cell aggregation occurs in the absence of all T cells (simultaneous germ line deletion of classical and γδ T cells), early B cell aggregation may be dependent on IL-17 secretion by innate lymphoid cells or independent of IL-17 entirely during classical K. pneumoniae infection. In the latter case, IL-17 may still be required subsequently for full mature follicle formation. Interactions between T and B cells in the lung that are not strictly organized into iBALT have also been demonstrated in other models and can result in memory B cell formation (24), leaving open the possibility that the retention of lymphoid cells in the lung regardless of organization is a relevant role of these structures.
Importantly, our findings are consistent with human observations that iBALT in adults have more loosely defined T cell areas (25, 26). Mouse studies of iBALT have historically utilized neonatal mice; in fact, many models require young mice to demonstrate observable iBALT structures (18). These models may correlate with human infants, who have a propensity for the development of tertiary lymphoid structures (20). Our studies performed in 7- to 9-week-old mice address the question of iBALT formation following bacterial infection in a mature host. We additionally investigated the persistence of iBALT structures following single versus multiple infections in mature mice. iBALT diminished by 120 dpi in mice exposed to K. pneumoniae only once. These structures, however, not only remain but also gradually become more robust in mice repeatedly restimulated with K. pneumoniae every 30 days through at least 120 dpi.
Individual K. pneumoniae strains express one of over 80 distinct capsule types as well as diverse O-antigens and can infect a variety of anatomical sites (27). To investigate the generalizability of the results observed with our model strain TOP52, we infected mice with a set of clinical K. pneumoniae strains isolated from the lower respiratory tract of patients at a tertiary care hospital. At 28 dpi, mice infected with any of the classical lower respiratory isolates demonstrated lymphoid aggregates consistent with those observed in our studies of TOP52. These data indicate that iBALT formation is not dependent on the characteristics of individual strains such as capsule or O-antigen type but occurs commonly following K. pneumoniae lung infection. The panel of isolates included multiple strains carrying critical antibiotic resistance determinants (Klebsiella pneumoniae carbapenemase [KPC] or extended-spectrum β-lactamase [ESBL]), demonstrating that iBALT formation is a host response to infection by strains of the highest clinical concern. Hypervirulent K. pneumoniae isolates exhibit significantly different pathologies compared to classical strains and are commonly utilized in murine studies of K. pneumoniae pathogenesis. The low 50% lethal doses (LD50s) of hypervirulent isolates (<103 CFU) impede the study of iBALT formation using live organisms of this pathotype. Heat-killed hypervirulent K. pneumoniae could be used to assess iBALT formation, although negative results should be interpreted with caution considering the shorter duration of antigen stimulation in the absence of live infection.
iBALT is believed to prevent excessive pathology and shorten pulmonary inflammation in some settings (28). iBALT has also been shown to play a role in local immunity by decreasing the response time to repeat infections (23, 29). Regarding K. pneumoniae specifically, diverse capsular types and pathotypes among circulating strains present a challenge to vaccination strategies. A mucosal vaccination approach that establishes iBALT as a local reservoir of T and B cells could potentially enable faster adaptation during infection with a heterologous strain. If iBALT does, in fact, contribute to a pathogen-specific protective phenotype, it could represent an additional facet of the preclinical evaluation of mucosal vaccine candidates in the future (22, 30).
An intricate understanding of the formation and function of iBALT in the context of diverse infectious and inflammatory settings is needed for the successful development of therapies based on these structures. Functional studies in our model and others are limited by the current lack of methodologies to specifically disrupt iBALT formation or function. Factors such as IL-17, CXCL12, or CXCL13 that are required for iBALT development are not sufficiently unique to iBALT to effectively interrogate function. Similarly, conclusions drawn from the elimination of specific lymphoid subsets are confounded by the simultaneous loss of the effects of these cell types independent of their inclusion in aggregates. The identification of factors that could be targeted to specifically promote or disrupt iBALT, potentially through RNA sequencing or other nonbiased approaches, will likely be needed to unravel functional roles. Despite these limitations, the current study delineates key features of iBALT as part of the lung host response to infection in a novel and clinically important context. Here, we have demonstrated that iBALT with nonclassical B cell follicles forms in response to classical K. pneumoniae infection of the murine respiratory tract. These observations contribute to our understanding of the immunology of lung infection with classical K. pneumoniae and expand our appreciation of the characteristics of iBALT structures generated in response to diverse pathogens. Important questions remain, including the role of iBALT structures in protection against homologous reinfection and against heterotypic infections and exposures. Ultimately, the implications of this work in mice for the formation and durability of such aggregates in humans are paramount and a topic of future study.
MATERIALS AND METHODS
Bacterial strain and inoculum preparation.
The classical K. pneumoniae isolate TOP52 was used for all experiments unless otherwise indicated (31, 32). Lower respiratory isolates were obtained from Barnes-Jewish Hospital in St. Louis, MO, and collection was approved by the Washington University in St. Louis Institutional Review Board (IRB) (identifier 201409121). For a full list of K. pneumoniae strains used in this study, see Table 1. Murine inocula were prepared by growing 20-mL bacterial cultures statically at 37°C for 16 h in Luria-Bertani broth. Cultures were then centrifuged for 10 min at 8,000 × g, and the pellet was resuspended in sterile PBS. The dilution volume needed for the desired inoculum concentration was based on the optical density at 600 nm. The inoculum concentration was confirmed by serial dilution and plating.
TABLE 1.
K. pneumoniae strains used in this studya
| Strain | Description | Reference(s) |
|---|---|---|
| TOP52 | Classical, UTI isolate from a 26-yr-old female, used in models of UTI and pneumonia | 11, 31, 36, 37 |
| KR3 | Classical, lower respiratory BAL fluid isolate from a 61-yr-old male | This study |
| KR45 | Classical, lower respiratory BAL fluid isolate from a 57-yr-old male, KPC+ | This study |
| KR74 | Classical, lower respiratory BAL fluid isolate from a 76-yr-old female | This study |
| KR122 | Classical, lower respiratory BAL fluid isolate from a 40-yr-old female, ESBL+ | This study |
| KR174 | Classical, lower respiratory BAL fluid isolate from a 54-yr-old female, ESBL+ | This study |
UTI, urinary tract infection; BAL, bronchoalveolar lavage; KPC+, Klebsiella pneumoniae carbapenemase positive; ESBL+, extended-spectrum β-lactamase positive.
Mouse infections.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine and complied with ethical regulations for animal testing and research. Female C57BL/6J (Jackson Laboratories, Bar Harbor, ME, USA), RAG1−/− (B6.129S7-Rag1−/−/J), TCRβ−/δ− (B6.129P2-Tcrbtm1Mom Tcrdtm1Mom/J), and μMT−/− (B6.129S2-Ighmtm1Cgn/J) mice were 7 to 9 weeks old at the onset of all experiments. Each mouse was anesthetized with inhaled isoflurane and suspended on a string by its top incisors, and its oropharynx was accessed by pulling the tongue out and to the side of the mouth. For initial and subsequent infections, 50 μL of the prepared inoculum containing either 1 × 108 to 2 × 108 CFU of TOP52, 5 × 107 to 9 × 107 CFU of other clinical isolates, or sterile PBS was pipetted into the oropharynx of the mouse and aspirated on subsequent breath (11, 33, 34). Mice were sacrificed at 28 days postinoculation or at other noted time points, and histopathological analyses were performed. For long-term murine experiments that involved repeat K. pneumoniae inoculations, mice were rechallenged 30, 60, and 90 days after primary infection with 1 × 108 to 2 × 108 CFU of K. pneumoniae strain TOP52 as described above. Mice were sacrificed at set time points and processed for histopathological analysis.
Lung histology.
Murine lungs from surviving mice at various time points were perfused, and the left lung was then harvested and processed for histology. Organs for paraffin sections were washed in PBS, fixed in 10% neutral buffered formalin, dehydrated in decreasing gradients of ethanol, embedded in paraffin, and cut into 5-μm sections, and a set was stained with H&E (Nationwide Histology, Missoula, MT). Unstained sections were later used for IHC or IF analysis. Images were obtained using an Olympus BX40 microscope and Zeiss Zen 3.3 software. To assign semiquantitative lymphoid aggregate scores, H&E sections were reviewed in a blind manner and given an aggregate score from 0 to 4 according to the following scoring system: 0 indicates no aggregates, 1 indicates at least 1 aggregate, 2 indicates more than 5 aggregates, 3 indicates more than 10 well-defined aggregates composing less than 10% of the total lung section area, and 4 indicates more than 10-well defined aggregates composing more than 10% of the lung section area.
Immunohistochemistry.
Paraffin-embedded sections were deparaffinized and rehydrated prior to immunohistochemical staining. Following rehydration, antigen retrieval was performed by submerging slides in citrate buffer and placing them in a pressure cooker for 15 min. Sections were washed in Tris-buffered saline (TBS) with Tween (TBST), blocked in 5% bovine serum albumin in PBS for 1 h at room temperature (RT), and then stained with the primary antibody B220 or CD3 (catalog numbers 14-0452-82 and MA1-90582, respectively; Invitrogen) overnight at 4°C. After washing sections in TBST, Vectastain ABC-AP kits (catalog numbers AK-5004 and AK-5001; Vector Laboratories, Burlingame, CA) were used according to the manufacturer’s instructions. Sections were incubated with biotinylated secondary antibodies for 30 min at RT, washed again in TBST, and incubated for 30 min with ABC-AP reagent. Slides were then incubated with fluorescent vector red substrate (catalog number SK-5100; Vector Laboratories) for approximately 20 min. Sections were rinsed with tap water for 5 min prior to being stained with 4′,6-diamidino-2-phenylindole (DAPI) for 3 min. After the final wash, sections were mounted with Fluoromount-G, sealed with nail polish, and imaged using a Zeiss Axio Observer D1 inverted fluorescence microscope (Carl Zeiss Inc., Thornwood, NY) equipped with an X-Cite120 light-emitting diode (LED) mini-light source (Excelitis Technologies, Waltham, MA). Images were acquired using an AxioCam 503 digital camera (Carl Zeiss Inc.) and Zen 2.3 (blue version) software.
Immunofluorescence.
IF analysis was performed as previously described (35), with some modifications. Briefly, paraffin-embedded sections were deparaffinized and rehydrated with decreasing gradients of ethanol, followed by antigen retrieval in a pressure cooker for 1 min in citrate buffer (catalog number H-3300; Vector Laboratories). Sections were then washed in TBS and TBS–0.25% Triton X-100 (TBSX), blocked in 5% goat serum in TBSX for 1 h at RT, and then stained with B220 and either CD11c, PCNA, or CXCL12 (catalog numbers 14-0452-82, PA5-90208, PA5-27214, and PA5-89116, respectively; Invitrogen) primary antibodies in 1% goat serum in TBSX overnight at 4°C. After washing in TBS and TBSX, sections were incubated with Alexa Fluor 488 and 594 secondary antibodies (catalog numbers A-11008 and A-21209, respectively; Invitrogen) in 1% goat serum in TBSX for 1 h at RT. Sections were rinsed with TBSX, stained with 300 nM DAPI for 4 min, washed 3 times in TBS, and finished in MilliQ water. Sections were then mounted with Fluoromount-G, sealed with nail polish, and stored at 4°C prior to imaging. Slides were imaged using the Zeiss Axio Imager M2 upright fluorescence microscope (Carl Zeiss Inc.), and images were acquired with an ORCA-flash4.0LT digital camera (Hamamatsu Photonics, Japan) and Zen 2.3 (blue version) software.
Statistical analyses.
For aggregate scoring performed in a blind manner, the Mann-Whitney U test was used to determine significance, as values were not evenly distributed. P values of <0.05 were considered significant. Analyses were performed using GraphPad Prism 9.1.2.
ACKNOWLEDGMENTS
We thank Wandy Beatty, Teri Hreha, Holly Hinrichs, and the labs of David Hunstad and Michael Thompson for assistance with IF and IHC staining; Carey-Ann Burnham and Meghan Wallace for isolate collection; and James Fitzpatrick and Javier Rangel-Moreno for helpful discussions.
This work was supported by the National Institute of Allergy and Infectious Diseases (K08-AI127714) and by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital via a microgrant from the Washington University Center for Cellular Imaging (WUCCI). Joseph J. Mackel was supported through The American Association of Immunologists Careers in Immunology Fellowship Program and The Pediatric Cardiovascular and Pulmonary Research Training Program (5T32HL125241-07).
Contributor Information
David A. Rosen, Email: rosend@wustl.edu.
Igor E. Brodsky, University of Pennsylvania
REFERENCES
- 1.Lou W, Venkataraman S, Zhong G, Ding B, Tan JPK, Xu L, Fan W, Yang YY. 2018. Antimicrobial polymers as therapeutics for treatment of multidrug-resistant Klebsiella pneumoniae lung infection. Acta Biomater 78:78–88. 10.1016/j.actbio.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Dhesi Z, Enne VI, Brealey D, Livermore DM, High J, Russell C, Colles A, Kandil H, Mack D, Martin D, Page V, Parker R, Roulston K, Singh S, Wey E, Swart AM, Stirling S, Barber JA, O’Grady J, Gant V. 2020. Organisms causing secondary pneumonias in COVID-19 patients at 5 UK ICUs as detected with the FilmArray test. medRxiv. 10.1101/2020.06.22.20131573. [DOI]
- 3.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaillancourt M, Jorth P. 2020. The unrecognized threat of secondary bacterial infections with COVID-19. mBio 11:e01806-20. 10.1128/mBio.01806-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, Zhu F, Zhu B, Cui L. 2020. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res 285:198005. 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo TA, Marr CM. 2019. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev 32:e00001-19. 10.1128/CMR.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR. 2018. Changes in prevalence of healthcare-associated infections in U.S. hospitals. N Engl J Med 379:1732–1744. 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/drugresistance/biggest-threats.html. [Google Scholar]
- 9.Amezcua Vesely MC, Pallis P, Bielecki P, Low JS, Zhao J, Harman CCD, Kroehling L, Jackson R, Bailis W, Licona-Limon P, Xu H, Iijima N, Pillai PS, Kaplan DH, Weaver CT, Kluger Y, Kowalczyk MS, Iwasaki A, Pereira JP, Esplugues E, Gagliani N, Flavell RA. 2019. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell 178:1176–1188.e15. 10.1016/j.cell.2019.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. 2011. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35:997–1009. 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twentyman J, Morffy Smith C, Nims JS, Dahler AA, Rosen DA. 2020. A murine model demonstrates capsule-independent adaptive immune protection in survivors of Klebsiella pneumoniae respiratory tract infection. Dis Model Mech 13:dmm043240. 10.1242/dmm.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randall TD. 2010. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol 107:187–241. 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiavolini D, Rangel-Moreno J, Berg G, Christian K, Oliveira-Nascimento L, Weir S, Alroy J, Randall TD, Wetzler LM. 2010. Bronchus-associated lymphoid tissue (BALT) and survival in a vaccine mouse model of tularemia. PLoS One 5:e11156. 10.1371/journal.pone.0011156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, Harmsen A. 2009. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 4:e7142. 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva-Sanchez A, Randall TD. 2020. Role of iBALT in respiratory immunity. Curr Top Microbiol Immunol 426:21–43. 10.1007/82_2019_191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE, Foster PS, King C, Steptoe RJ, Mazzone SB, Sly PD, Phipps S. 2015. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J Immunol 194:4567–4576. 10.4049/jimmunol.1400909. [DOI] [PubMed] [Google Scholar]
- 17.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10:927–934. 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 18.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. 2011. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12:639–646. 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleige H, Ravens S, Moschovakis GL, Bölter J, Willenzon S, Sutter G, Häussler S, Kalinke U, Prinz I, Förster R. 2014. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med 211:643–651. 10.1084/jem.20131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foo SY, Phipps S. 2010. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol 3:537–544. 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 21.Tan H-X, Esterbauer R, Vanderven HA, Juno JA, Kent SJ, Wheatley AK. 2019. Inducible bronchus-associated lymphoid tissues (iBALT) serve as sites of B cell selection and maturation following influenza infection in mice. Front Immunol 10:611. 10.3389/fimmu.2019.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwanaga N, Chen K, Yang H, Lu S, Hoffmann JP, Wanek A, McCombs JE, Song K, Rangel-Moreno J, Norton EB, Kolls JK. 2021. Vaccine-driven lung TRM cells provide immunity against Klebsiella via fibroblast IL-17R signaling. Sci Immunol 6:eabf1198. 10.1126/sciimmunol.abf1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jupelli M, Shimada K, Chiba N, Slepenkin A, Alsabeh R, Jones HD, Peterson E, Chen S, Arditi M, Crother TR. 2013. Chlamydia pneumoniae infection in mice induces chronic lung inflammation, iBALT formation, and fibrosis. PLoS One 8:e77447. 10.1371/journal.pone.0077447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu Van D, Beier KC, Pietzke L-J, Al Baz MS, Feist RK, Gurka S, Hamelmann E, Kroczek RA, Hutloff A. 2016. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun 7:10875. 10.1038/ncomms10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350:2645–2653. 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 26.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. 2006. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 116:3183–3194. 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi M, Hegerle N, Nkeze J, Sen S, Jamindar S, Nasrin S, Sen S, Permala-Booth J, Sinclair J, Tapia MD, Johnson JK, Mamadou S, Thaden JT, Fowler VG, Jr, Aguilar A, Terán E, Decre D, Morel F, Krogfelt KA, Brauner A, Protonotariou E, Christaki E, Shindo Y, Lin Y-T, Kwa AL, Shakoor S, Singh-Moodley A, Perovic O, Jacobs J, Lunguya O, Simon R, Cross AS, Tennant SM. 2020. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front Microbiol 11:1249. 10.3389/fmicb.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JY, Silva-Sanchez A, Carragher DM, Garcia-Hernandez MDLL, Rangel-Moreno J, Randall TD. 2020. Inducible bronchus-associated lymphoid tissue (iBALT) attenuates pulmonary pathology in a mouse model of allergic airway disease. Front Immunol 11:570661. 10.3389/fimmu.2020.570661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiley JA, Harmsen AG. 2008. Pneumocystis infection enhances antibody-mediated resistance to a subsequent influenza infection. J Immunol 180:5613–5624. 10.4049/jimmunol.180.8.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Guzman D, Le Guen P, Villeret B, Sola N, Le Borgne R, Guyard A, Kemmel A, Crestani B, Sallenave JM, Garcia-Verdugo I. 2019. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials 217:119308. 10.1016/j.biomaterials.2019.119308. [DOI] [PubMed] [Google Scholar]
- 31.Rosen DA, Hilliard JK, Tiemann KM, Todd EM, Morley SC, Hunstad DA. 2016. Klebsiella pneumoniae fimK promotes virulence in murine pneumonia. J Infect Dis 213:649–658. 10.1093/infdis/jiv440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen DA, Pinkner JS, Jones JM, Walker JN, Clegg S, Hultgren SJ. 2008. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun 76:3337–3345. 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. 2011. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186:1666–1674. 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen TB, Yan J, Luna B, Spellberg B. 2018. Murine oropharyngeal aspiration model of ventilator-associated and hospital-acquired bacterial pneumonia. J Vis Exp 2018:57672. 10.3791/57672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaqout S, Becker LL, Kaindl AM. 2020. Immunofluorescence staining of paraffin sections step by step. Front Neuroanat 14:582218. 10.3389/fnana.2020.582218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JG, Spurbeck RR, Sandhu SK, Matson JS. 2014. Genome sequence of Klebsiella pneumoniae urinary tract isolate TOP52. Genome Announc 2:e00668-14. 10.1128/genomeA.00668-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wylie KM, Wylie TN, Minx PJ, Rosen DA. 2019. Whole-genome sequencing of Klebsiella pneumoniae isolates to track strain progression in a single patient with recurrent urinary tract infection. Front Cell Infect Microbiol 9:14. 10.3389/fcimb.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]