ABSTRACT
We reported the complete coding sequence of a lumpy skin disease virus (LSDV) isolated from cattle from Kinmen, Taiwan, in 2020. The nucleotide sequence of LSDV/KM/Taiwan/2020 was most closely related to strains from an outbreak in China and Vietnam in 2020 and clustered within the vaccine or vaccine-derived clade.
ANNOUNCEMENT
Lumpy skin disease virus (LSDV) is an emerging pathogen of the family Poxviridae, having spread over the past 10 years from Africa and the Middle East into southeastern Europe, the Caucasus, Russia, and, more recently, Asia (1–8). It will cause an economic impact on the cattle industry in the invaded regions (9–10). Here, we report the complete coding sequence of an LSDV isolate obtained from the first outbreak in Kinmen Island of Taiwan in 2020.
The LSDV/KM/Taiwan/2020 isolate, recovered from skin lesions of affected cattle, was grown in primary sheep testis cells following the protocol of the World Organization for Animal Health (OIE) (11). DNA was purified from cell culture supernatant harvested when cytopathic effects were observed, using a MagNA Pure compact nucleic acid isolation kit I (Roche Diagnostics, Mannheim, Germany). A paired-end sequencing library was constructed with a Nextera DNA Flex library prep kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocols. Sequencing was performed using a 500-cycle (2 × 250-bp paired-end) MiSeq reagent kit version 2 (Illumina, San Diego, CA, USA) with an MiSeq sequencer. Default parameters were applied for all programs unless specified. Bases lower than Q30 were trimmed using BBDuk implemented in Geneious Prime version 2020 (https://www.geneious.com). De novo assembly was performed using Geneious assembler with low-sensitivity setting and 3% maximum mismatches per read. The longest consensus sequence was identified as LSDV using BLASTN search. The assembled sequence was further checked via mapping. Open reading frames (ORFs) were predicted with initial codon ATG by ORF Finder. Complete genomes of selected wild and vaccine LSDV strains were aligned using MAFFT version 7 (12, 13). Maximum-likelihood phylogeny was reconstructed using IQTREE version 1.6.12 (14, 15) with 1,000 replicates of ultrafast bootstrap approximation (16) for branch support assessment. Phylogeny was visualized by FigTree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
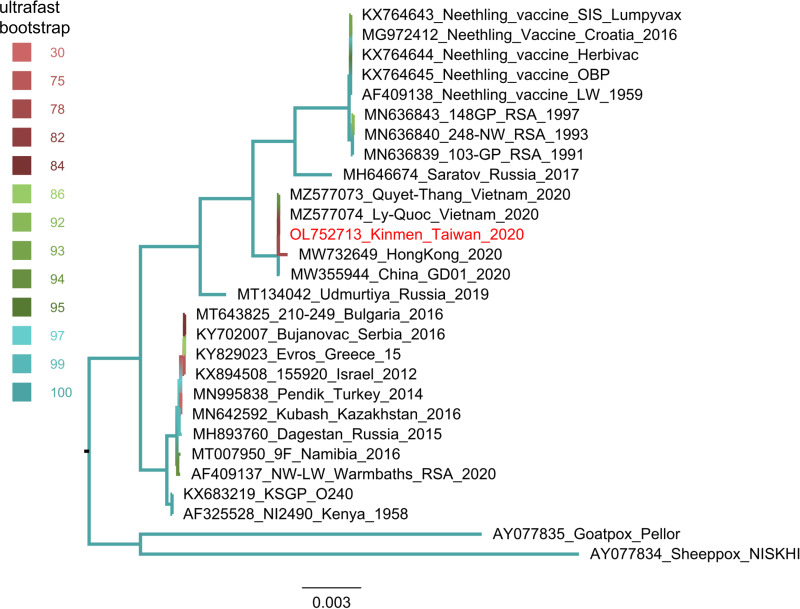
In total, 2,552,662 reads were acquired (SRA accession number SRX14182446). The assembled genome of LSDV/KM/Taiwan/2020 was 150,822 bp with 25.9% GC content (GenBank accession number OL752713). The average sequencing depth was 380.8×. The genome was 99.99% identical to four Vietnam isolates (GenBank accession numbers MZ577073 to MZ577076). Two indels and two single nucleotide polymorphisms (SNPs) were identified among the genome alignment of LSDV/KM/Taiwan/2020 and two Vietnam isolates (MZ577073 and MZ577074) (Table 1). One point mutation caused an amino acid change from leucine to serine that encoded the viral membrane protein of the entry-fusion complex component (Table 1). Maximum-likelihood phylogeny showed that the LSDV/KM/Taiwan/2020 isolate clustered with strains that were associated with outbreaks in China and Vietnam in 2020 and close to vaccine or vaccine-derived strains (Fig. 1).
TABLE 1.
Mutations in viral genome of the LSDV/KM/Taiwan/2020, which is the first lumpy skin disease virus isolated on Kinmen Island, Taiwan, in 2020
| Region in the alignment (bp)a | Mutation type | Mutation | Note |
|---|---|---|---|
| 275–289 | 15-bp deletion | TAAGTGGAAGCCAAT | |
| 150650–150721 | 72-bp insertion | TTATTAGGTTTAATTGGCTTCTACTTAATTGGCTTCCACTTATTAGGTTTAATTGGCTTTTTATAATTAGGT | |
| 64585 | Mutation | T→C | Leu→Ser in entry-fusion complex component (viral membrane protein) |
| 150734 | Mutation | C→T |
FIG 1.
Maximum-likelihood phylogeny of lumpy skin disease virus based on complete genomic sequences. The color of branch indicates the branch support based on 1,000 replicates of ultrafast bootstrap approximation.
This work was performed at the Animal Health Research Institute, which is the national veterinary laboratory in Taiwan, and no ethical approval was required for the work carried out.
Data availability.
Raw reads were deposited in SRA under accession number SRX14182446. The assembled genomic sequence of the isolate LSDV/KM/Taiwan/2020 has been deposited in GenBank under accession number OL752713.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of Yu-Chun Chen and Chien-Hui Liao with technical assistance.
We declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Chih-Wei Huang, Email: cwhuang@mail.nvri.gov.tw.
John J. Dennehy, Queens College, CUNY
REFERENCES
- 1.Tran HTT, Truong AD, Dang AK, Ly DV, Nguyen CT, Chu NT, Hoang TV, Nguyen HT, Nguyen VT, Dang HV. 2021. Lumpy skin disease outbreaks in Vietnam, 2020. Transbound Emerg Dis 68:977–980. doi: 10.1111/tbed.14022. [DOI] [PubMed] [Google Scholar]
- 2.Lu G, Xie J, Luo J, Shao R, Jia K, Li S. 2021. Lumpy skin disease outbreaks in China, since 3 August 2019. Transbound Emerg Dis 68:216–219. doi: 10.1111/tbed.13898. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Chander Y, Kumar R, Khandelwal N, Riyesh T, Chaudhary K, Shanmugasundaram K, Kumar S, Kumar A, Gupta MK, Pal Y, Barua S, Tripathi BN. 2021. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS One 16:e0241022. doi: 10.1371/journal.pone.0241022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Food Safety Authority, Calistri P, De Clercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Marojevic D, Antoniou S-E, Broglia A. 2020. Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J 18:e06010. doi: 10.2903/j.efsa.2020.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya KP, Subedi D. 2020. First outbreak of lumpy skin disease in Nepal. Transbound Emerg Dis 67:2280–2281. doi: 10.1111/tbed.13815. [DOI] [PubMed] [Google Scholar]
- 6.Sprygin A, Pestova Y, Prutnikov P, Kononov A. 2018. Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transbound Emerg Dis 65:1137–1144. doi: 10.1111/tbed.12897. [DOI] [PubMed] [Google Scholar]
- 7.Beard PM. 2016. Lumpy skin disease: a direct threat to Europe. Vet Rec 178:557–558. doi: 10.1136/vr.i2800. [DOI] [PubMed] [Google Scholar]
- 8.Flannery J, Shih B, Haga IR, Ashby M, Corla A, King S, Freimanis G, Polo N, Tse AC-n, Brackman CJ, Chan J, Pun P, Ferguson AD, Law A, Lycett S, Batten C, Beard PM. 2021. A novel strain of lumpy skin disease virus causes clinical disease in cattle in Hong Kong. Transbound Emerg Dis doi: 10.1111/tbed.14304. [DOI] [PubMed] [Google Scholar]
- 9.Molla W, de Jong M, Gari G, Frankena K. 2017. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev Vet Med 147:100–107. doi: 10.1016/j.prevetmed.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Limon G, Gamawa AA, Ahmed AI, Lyons NA, Beard PM. 2020. Epidemiological characteristics and economic impact of lumpy skin disease, sheeppox and goatpox among subsistence farmers in northeast Nigeria. Front Vet Sci 7:8. doi: 10.3389/fvets.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Organization for Animal Health. 2021. Lumpy skin disease. In Manual of diagnostic tests and vaccines for terrestrial animals. World Organization for Animal Health, Paris, France. https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/. [Google Scholar]
- 12.Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuraku S, Zmasek CM, Nishimura O, Katoh K. 2013. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res 41:W22–W28. doi: 10.1093/nar/gkt389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw reads were deposited in SRA under accession number SRX14182446. The assembled genomic sequence of the isolate LSDV/KM/Taiwan/2020 has been deposited in GenBank under accession number OL752713.